

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332563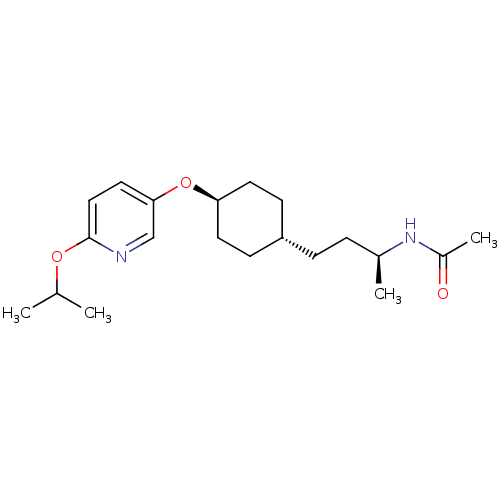 (CHEMBL1630721 | trans-(S)-N-(4-(4-(6-isopropoxypyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97610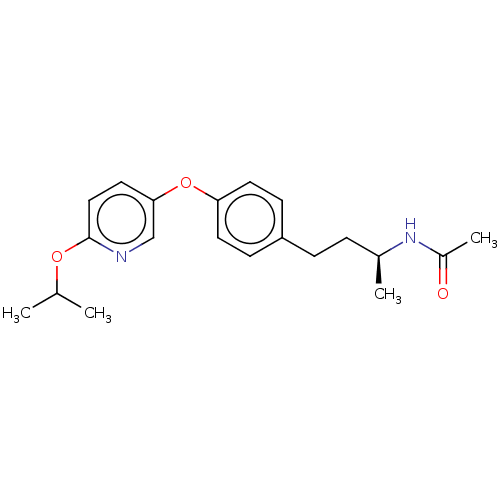 (CHEMBL1630715 | US8470841, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97600 (US8470841, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97601 (US8470841, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97609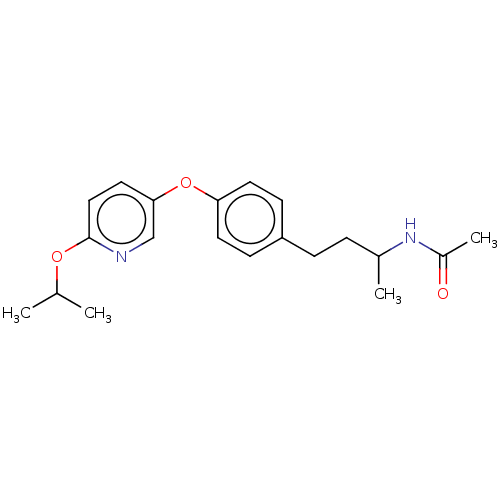 (US8470841, 33) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332564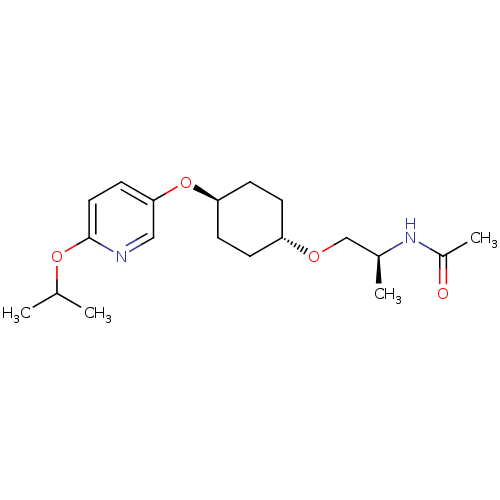 (CHEMBL1630703 | trans-(S)-N-(1-(4-(6-isopropoxypyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332562 (CHEMBL1630516 | N-{(S)-3-[2-(4-isopropoxy-phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97611 (CHEMBL1630712 | US8470841, 36) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332554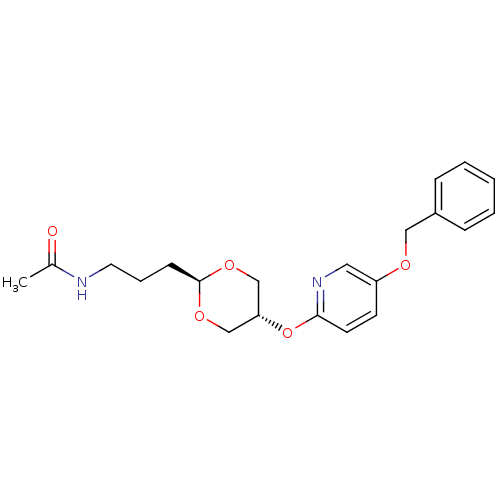 (CHEMBL1630706 | trans-N-(3-(5-(5-(benzyloxy)pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50189617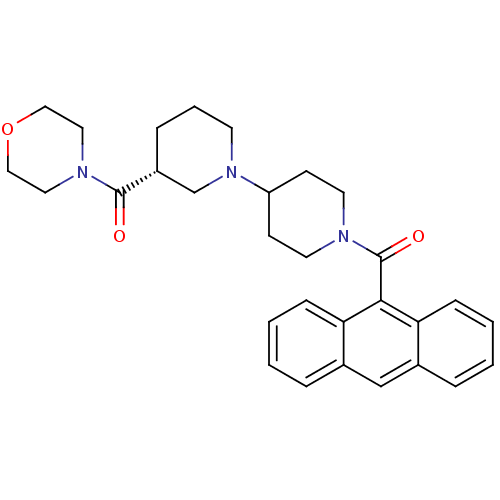 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97611 (CHEMBL1630712 | US8470841, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97622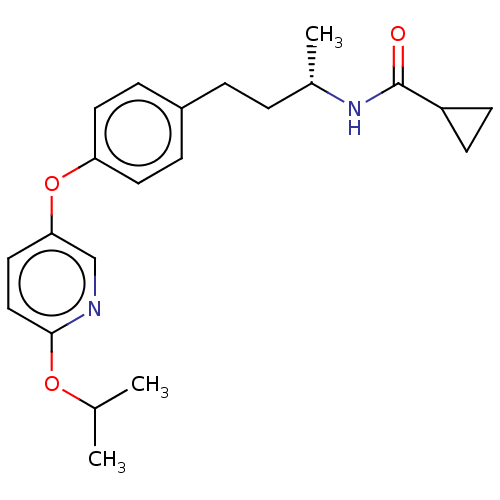 (CHEMBL1630701 | US8470841, 51) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97623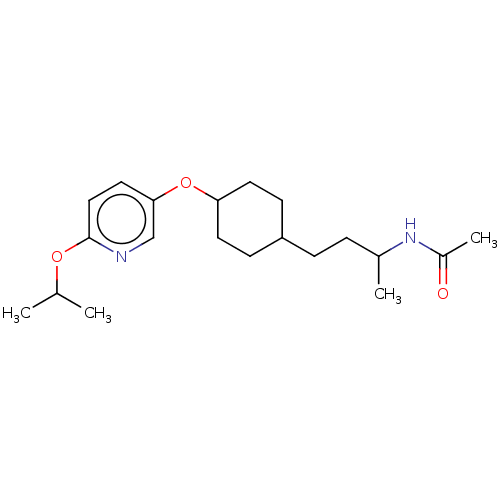 (US8470841, 52) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97622 (CHEMBL1630701 | US8470841, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97615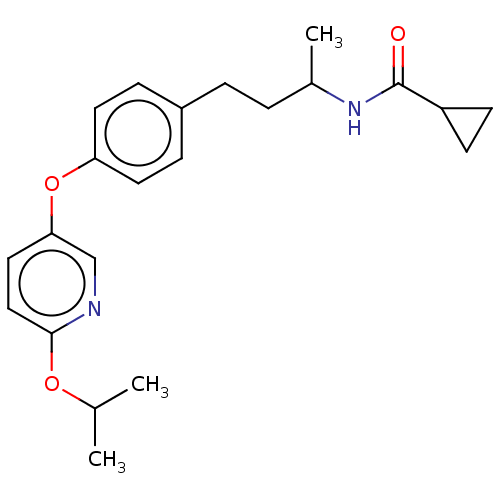 (US8470841, 44) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM64583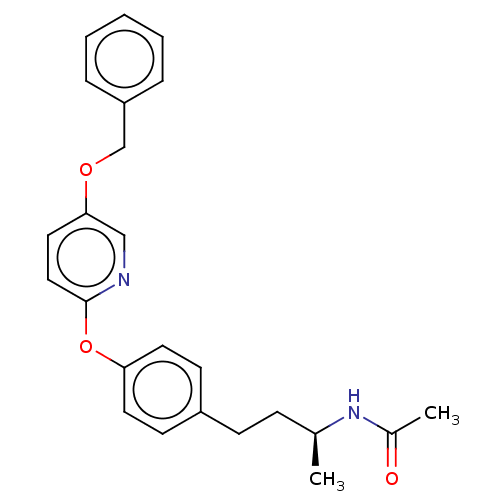 (BDBM50332551 | US8470841, 19) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM64583 (BDBM50332551 | US8470841, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97624 (US8470841, 59) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97599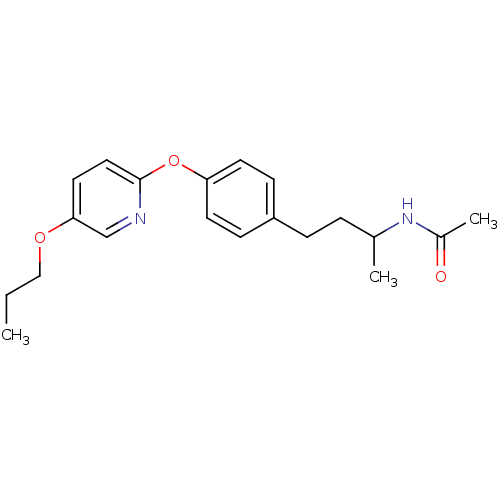 (US8470841, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97597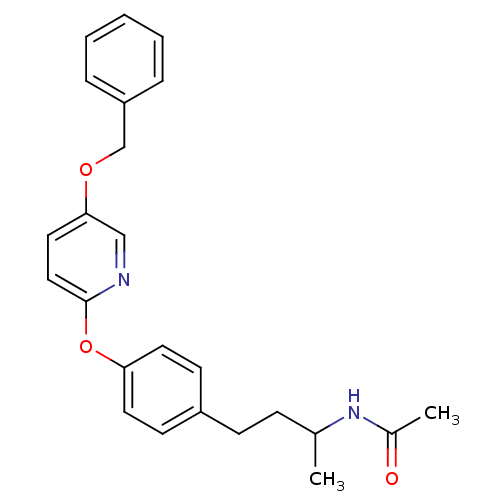 (US8470841, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332552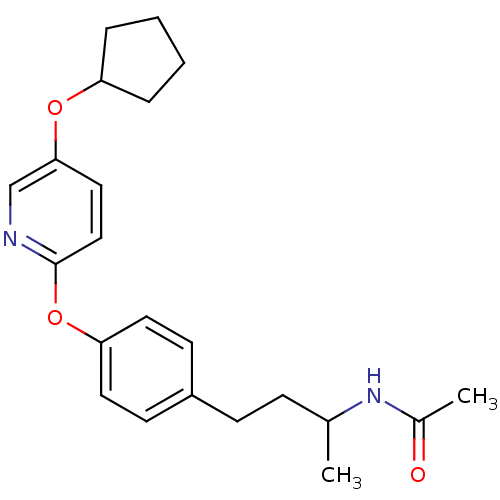 (CHEMBL1630708 | N-(4-(4-(5-(cyclopentyloxy)pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50332563 (CHEMBL1630721 | trans-(S)-N-(4-(4-(6-isopropoxypyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC1 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332552 (CHEMBL1630708 | N-(4-(4-(5-(cyclopentyloxy)pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97618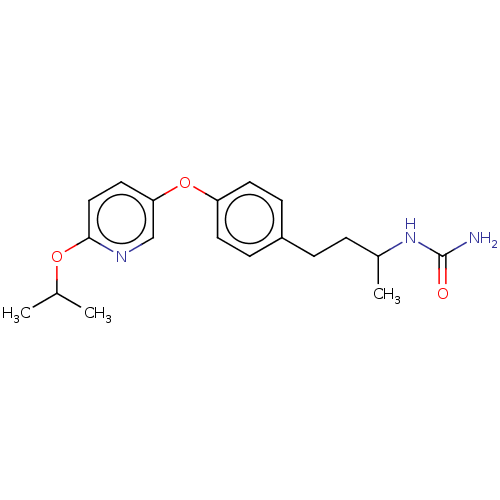 (CHEMBL1630702 | US8470841, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97618 (CHEMBL1630702 | US8470841, 47) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97598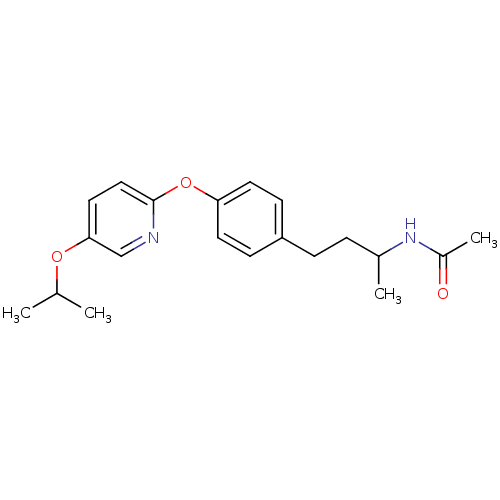 (US8470841, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97621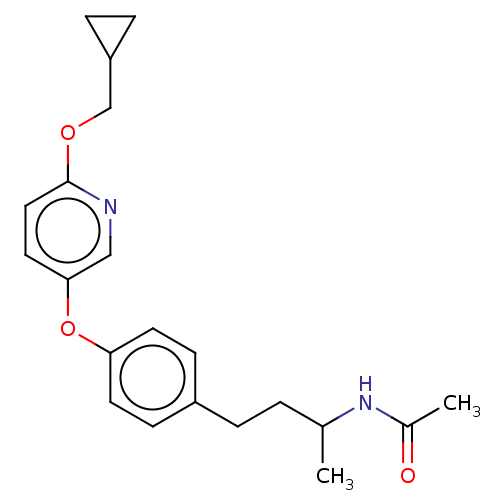 (US8470841, 50) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged rat recombinant ACC1 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332555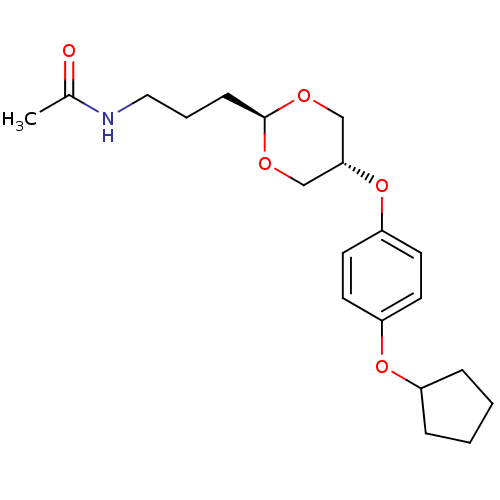 (CHEMBL1630705 | US8470841, 6 | trans-N-(3-(5-(4-(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97622 (CHEMBL1630701 | US8470841, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC1 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332549 ((S)-N-(4-(4-(5-isopropoxypyridin-2-yloxy)phenyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332549 ((S)-N-(4-(4-(5-isopropoxypyridin-2-yloxy)phenyl)bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332555 (CHEMBL1630705 | US8470841, 6 | trans-N-(3-(5-(4-(c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM64583 (BDBM50332551 | US8470841, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97617 (CHEMBL1629723 | US8470841, 46) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97617 (CHEMBL1629723 | US8470841, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332560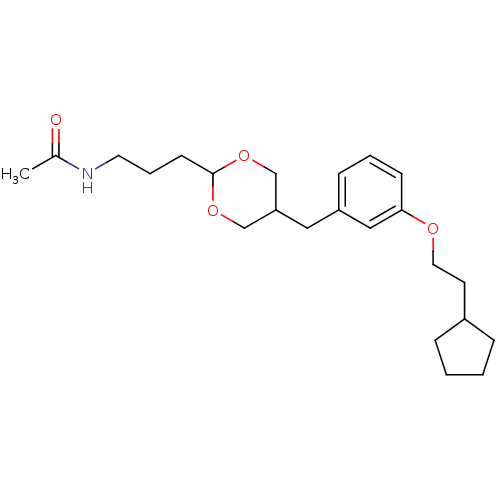 (CHEMBL1630515 | N-(3-(5-(3-(2-cyclopentylethoxy)be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM97622 (CHEMBL1630701 | US8470841, 51) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC1 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97605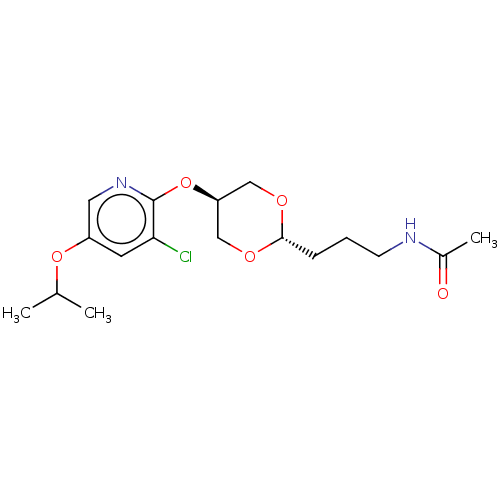 (US8470841, 25) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332556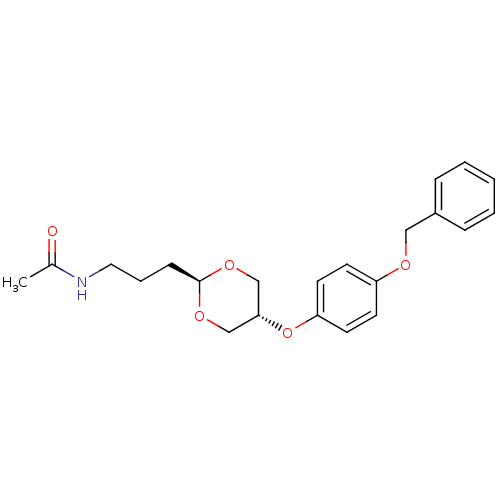 (CHEMBL1630704 | US8470841, 4 | trans-N-(3-(5-(4-(b...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332556 (CHEMBL1630704 | US8470841, 4 | trans-N-(3-(5-(4-(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50189617 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged rat recombinant ACC1 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM97622 (CHEMBL1630701 | US8470841, 51) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC1 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50332561 (CHEMBL1630517 | N-(3-(5-(6-(cyclopentyloxy)naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM97616 (CHEMBL1630720 | US8470841, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay | J Med Chem 53: 8679-87 (2010) Article DOI: 10.1021/jm101179e BindingDB Entry DOI: 10.7270/Q28K79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |