

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50433372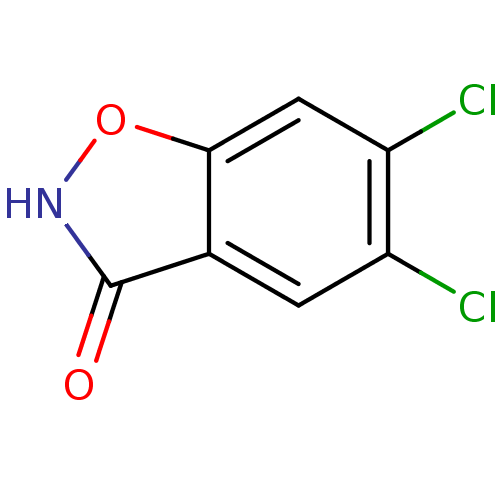 (CHEMBL2375520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725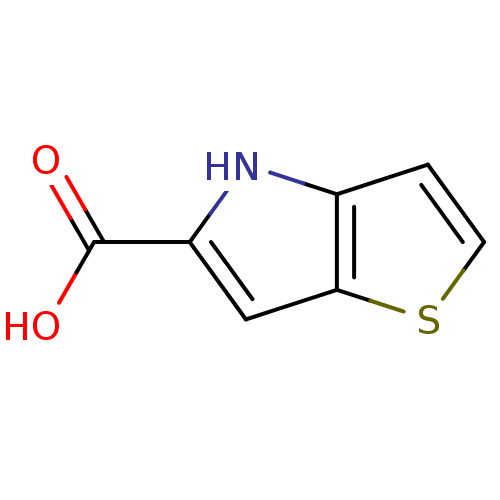 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260722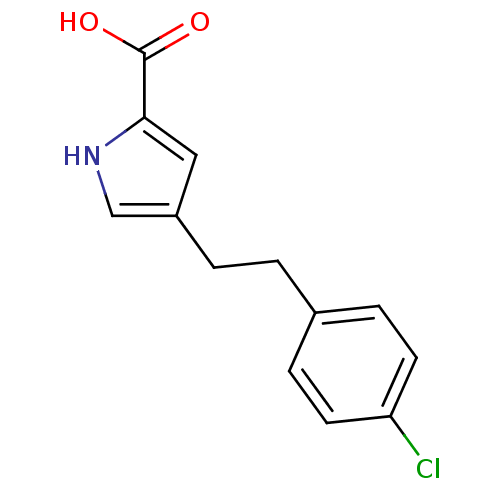 (4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50206007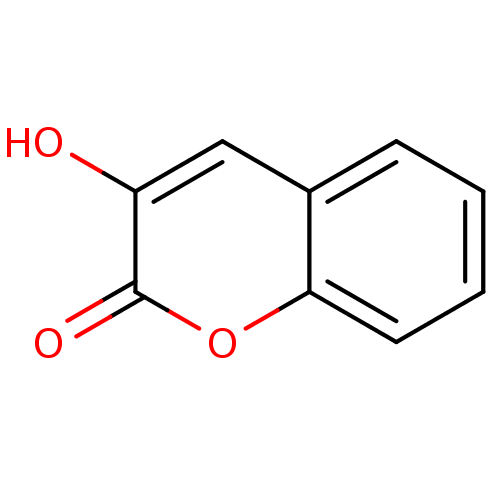 (3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50433371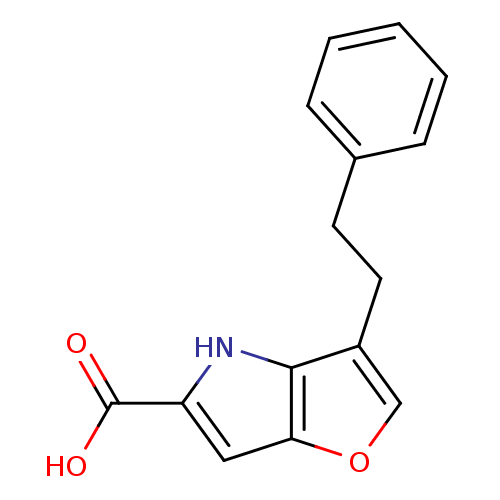 (CHEMBL2375519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM36181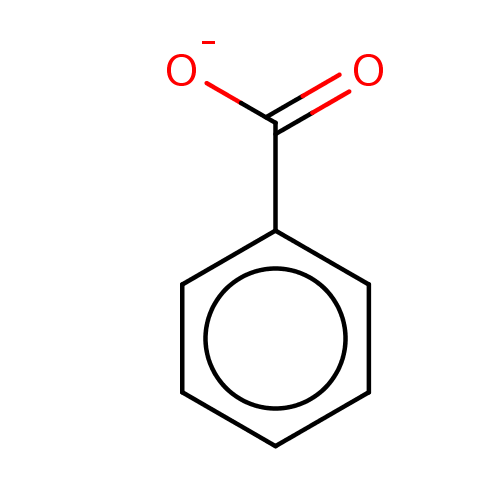 (SODIUM BENZOATE | benzoate | benzoic acid | benzoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Sus scrofa (pig)) | BDBM50004955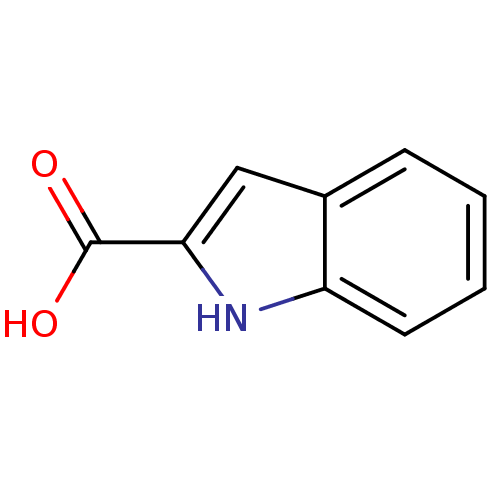 (1H-Indole-2-carboxylic acid | CHEMBL278390 | Indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of pig kidney DAAO in presence of D-alanine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128787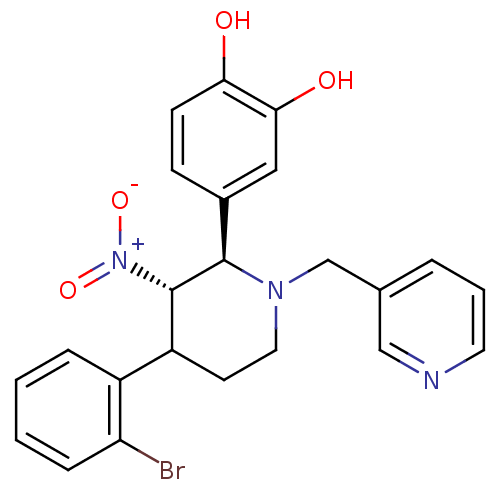 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128787 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50433372 (CHEMBL2375520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of DAAO (unknown origin) | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128790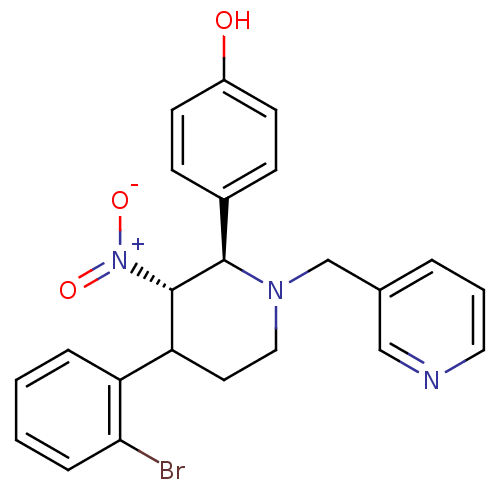 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128790 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3152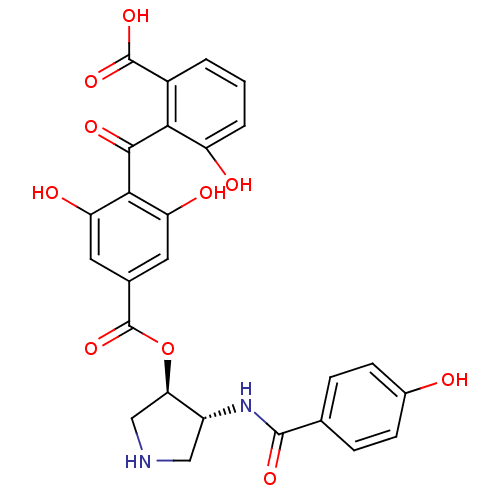 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260722 (4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128796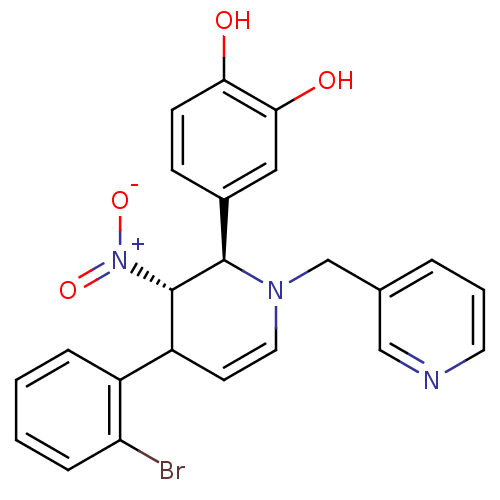 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128795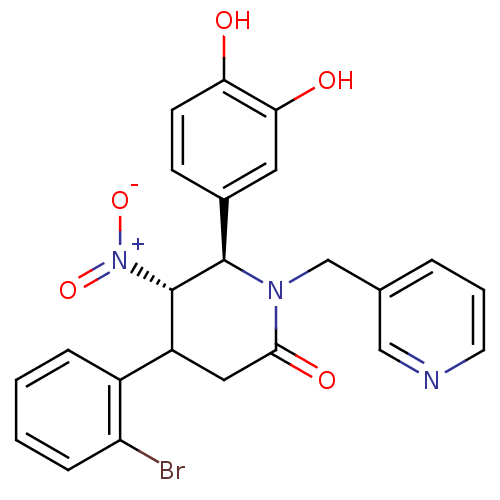 (4-(2-Bromo-phenyl)-6-(3,4-dihydroxy-phenyl)-5-nitr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50206007 (3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3235 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3230 ((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3152 (2-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128800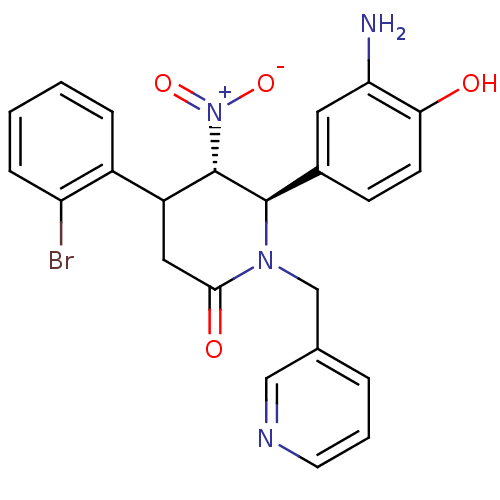 (6-(3-Amino-4-hydroxy-phenyl)-4-(2-bromo-phenyl)-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3235 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3236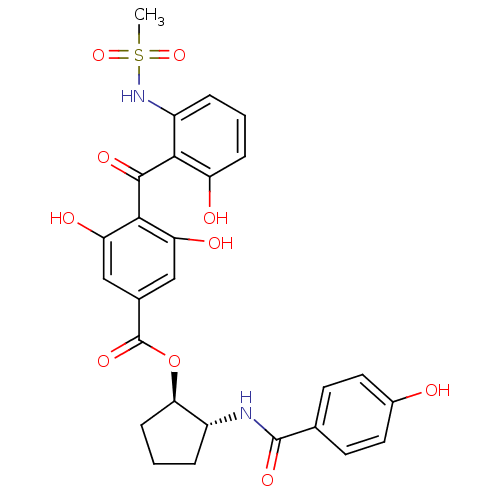 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128789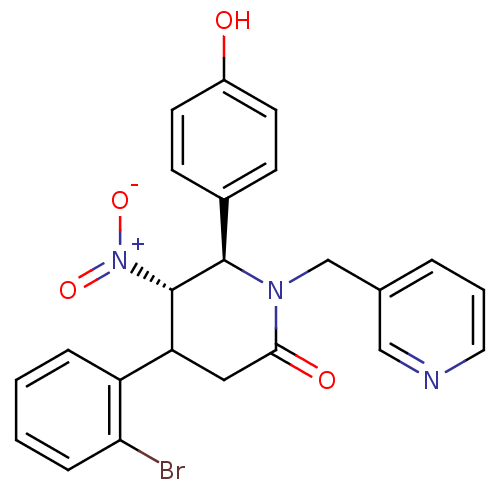 (4-(2-Bromo-phenyl)-6-(4-hydroxy-phenyl)-5-nitro-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3239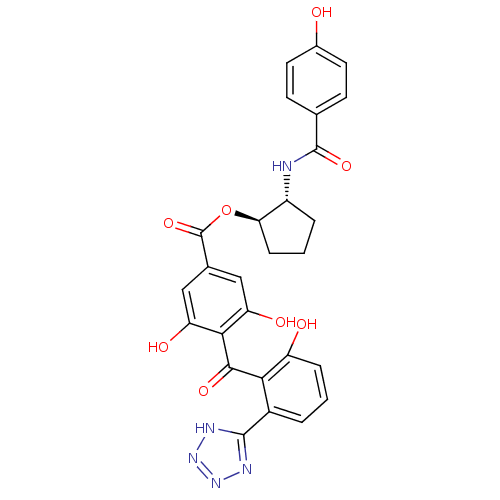 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50433371 (CHEMBL2375519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine | J Med Chem 56: 3710-24 (2013) Article DOI: 10.1021/jm4002583 BindingDB Entry DOI: 10.7270/Q2X92CPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3231 ((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 3,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3239 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3230 ((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM3220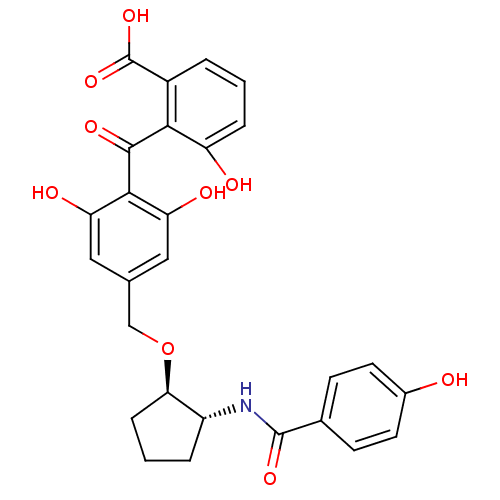 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3228 ((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3230 ((3R,4R)-4-[(4-hydroxybenzene)amido]pyrrolidin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3226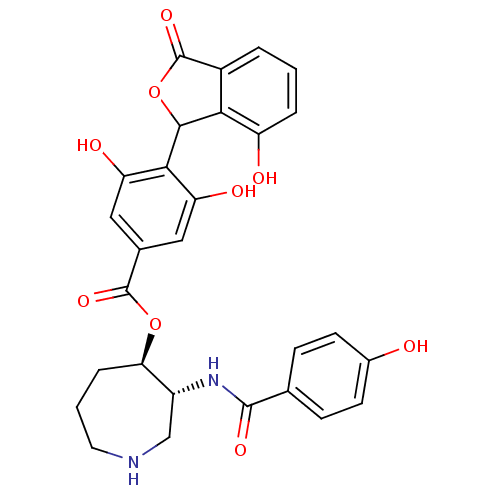 ((3R,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl 3,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128787 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128790 (4-[4-(2-Bromo-phenyl)-3-nitro-1-pyridin-3-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM3235 ((1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50128793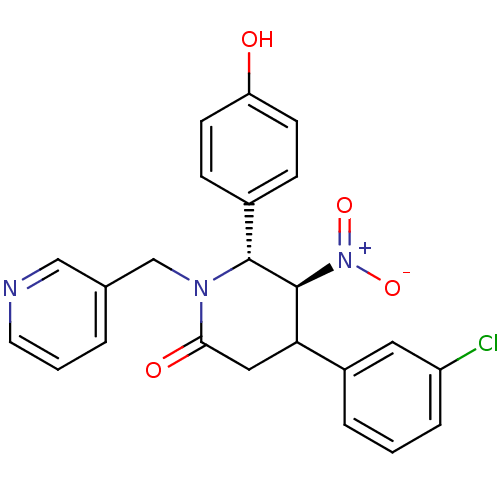 (4-(3-Chloro-phenyl)-6-(4-hydroxy-phenyl)-5-nitro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine brain farnesyltransferase (FTase) farnesylation of viral K-Ras | J Med Chem 46: 2467-73 (2003) Article DOI: 10.1021/jm020522k BindingDB Entry DOI: 10.7270/Q2RN3775 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3220 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM3227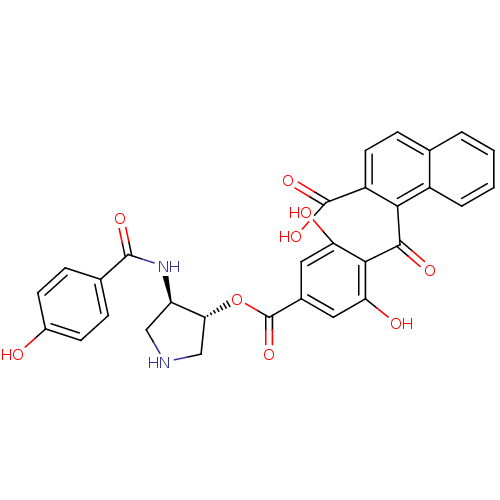 (1-{[2,6-dihydroxy-4-({[(3R,4R)-4-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 45: 2624-43 (2002) Article DOI: 10.1021/jm020018f BindingDB Entry DOI: 10.7270/Q2BG2M50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 229 total ) | Next | Last >> |