

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50002660 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50373120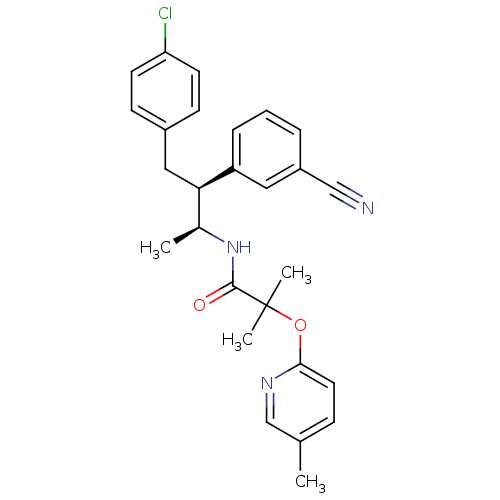 (CHEMBL260977 | MK-0364) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR-141716 from human wild type CB1R expressed in CHO cells | J Med Chem 51: 2108-14 (2008) Article DOI: 10.1021/jm7014974 BindingDB Entry DOI: 10.7270/Q27H1KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50211392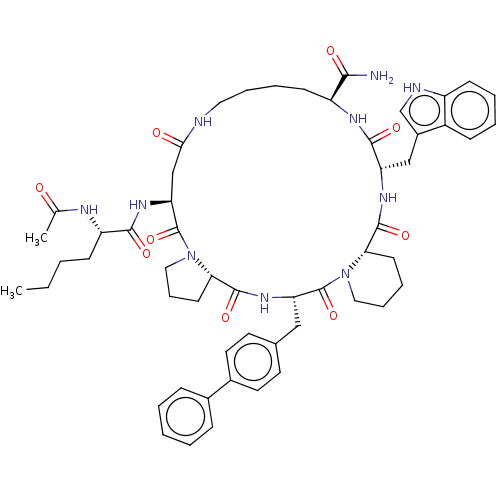 (Ac-Nle4-cyclo(Asp5-Pro6-D-4,4'-Bip7-Pro8-Trp9-Lys1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDPalphaMSH from human cloned melanocortin receptor 1b expressed in CHO cells | J Med Chem 50: 2520-6 (2007) Article DOI: 10.1021/jm0614275 BindingDB Entry DOI: 10.7270/Q2JQ10QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50211392 (Ac-Nle4-cyclo(Asp5-Pro6-D-4,4'-Bip7-Pro8-Trp9-Lys1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDPalphaMSH from human cloned MC5R expressed in CHO cells | J Med Chem 50: 2520-6 (2007) Article DOI: 10.1021/jm0614275 BindingDB Entry DOI: 10.7270/Q2JQ10QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50211392 (Ac-Nle4-cyclo(Asp5-Pro6-D-4,4'-Bip7-Pro8-Trp9-Lys1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDPalphaMSH from human cloned MC4R expressed in CHO cells | J Med Chem 50: 2520-6 (2007) Article DOI: 10.1021/jm0614275 BindingDB Entry DOI: 10.7270/Q2JQ10QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030435 (CHEMBL420543 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50316527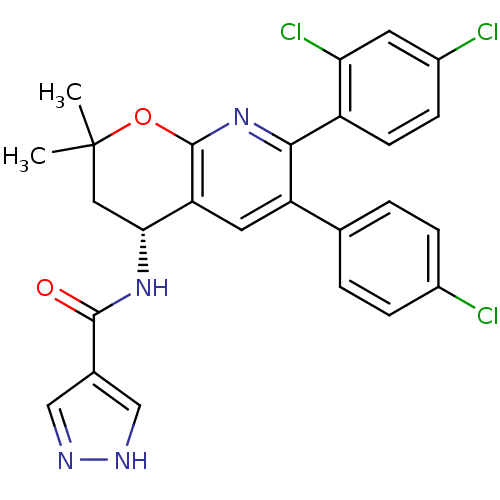 (CHEMBL1097841 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells | J Med Chem 53: 4028-37 (2010) Article DOI: 10.1021/jm100023j BindingDB Entry DOI: 10.7270/Q2TQ61QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029878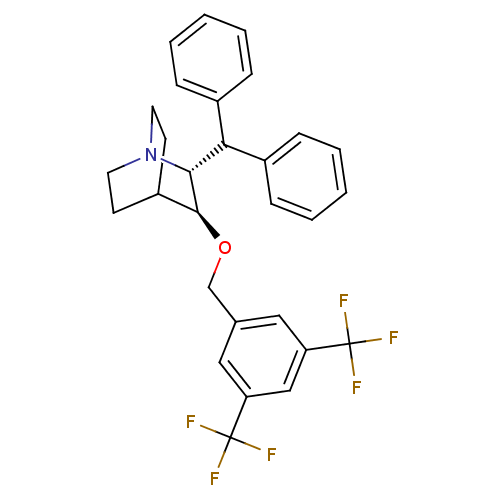 ((2R,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50320187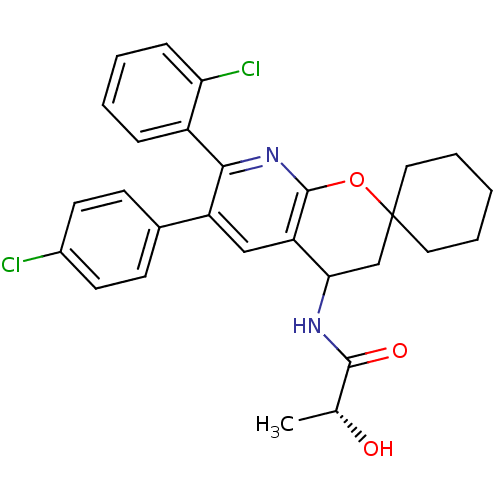 ((2R)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 20: 3750-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.071 BindingDB Entry DOI: 10.7270/Q22F7NNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50316529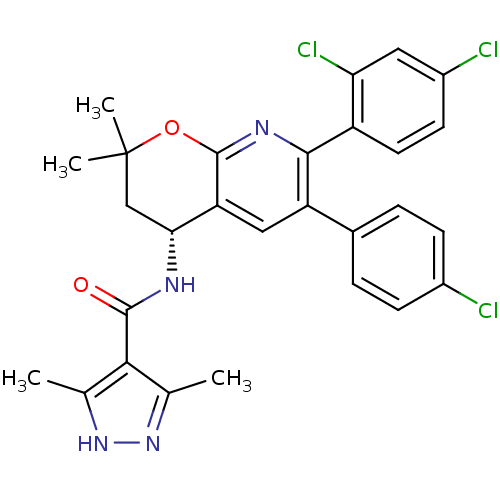 (CHEMBL1095151 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells | J Med Chem 53: 4028-37 (2010) Article DOI: 10.1021/jm100023j BindingDB Entry DOI: 10.7270/Q2TQ61QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029884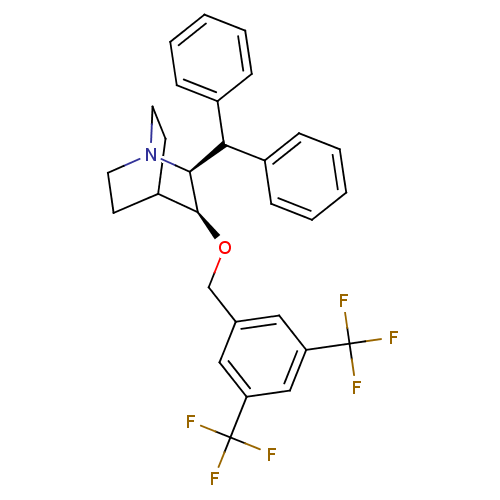 ((2S,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50316524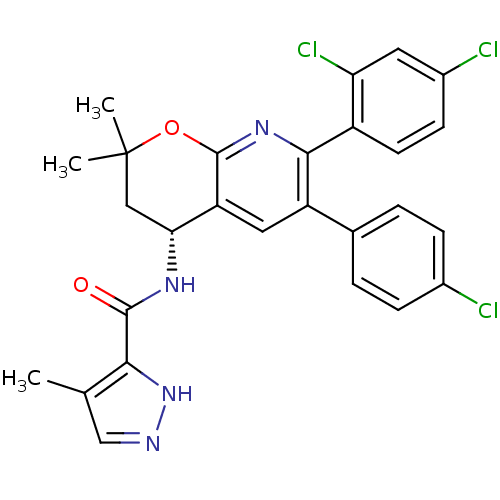 (CHEMBL1095158 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells | J Med Chem 53: 4028-37 (2010) Article DOI: 10.1021/jm100023j BindingDB Entry DOI: 10.7270/Q2TQ61QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267907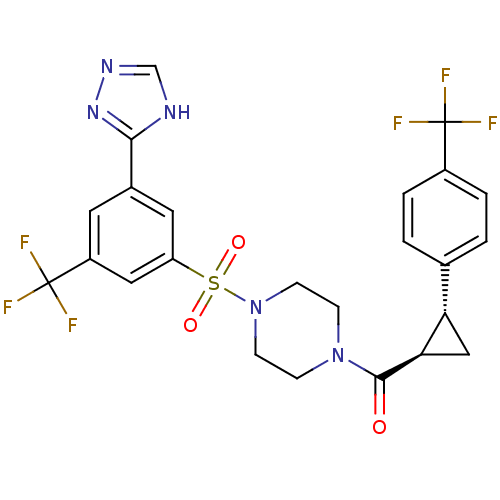 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030435 (CHEMBL420543 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against wild type human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance... | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267730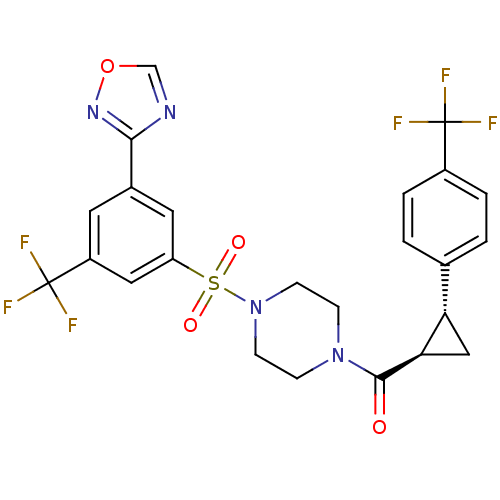 ((4-(3-(1,2,4-oxadiazol-3-yl)-5-(trifluoromethyl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030431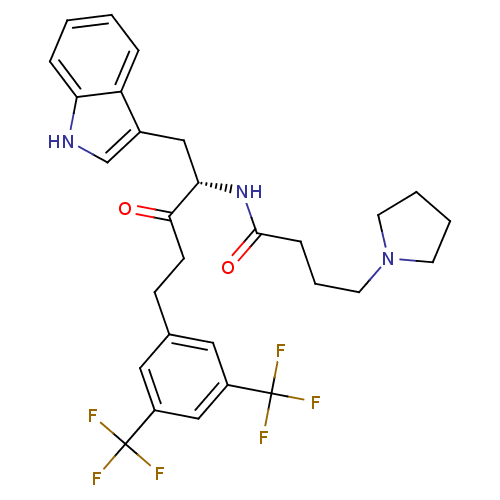 (CHEMBL176500 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267585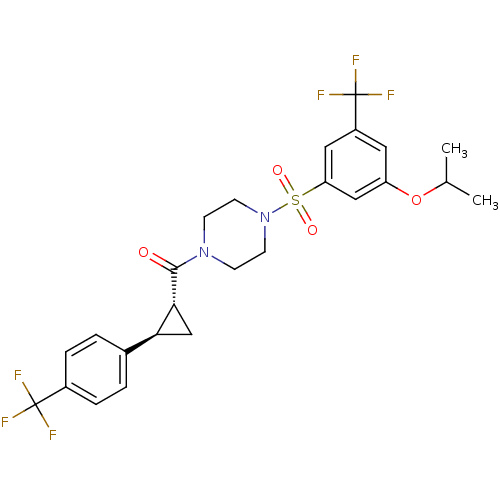 ((4-(3-isopropoxy-5-(trifluoromethyl)phenylsulfonyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at cannabinoid CB1 receptor (unknown origin) | Bioorg Med Chem Lett 19: 2591-4 (2009) Article DOI: 10.1016/j.bmcl.2009.03.005 BindingDB Entry DOI: 10.7270/Q2PK0G1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells | J Med Chem 49: 7584-7 (2006) Article DOI: 10.1021/jm060996+ BindingDB Entry DOI: 10.7270/Q2QN67KG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 5195-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.046 BindingDB Entry DOI: 10.7270/Q2028RMH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 20: 3750-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.071 BindingDB Entry DOI: 10.7270/Q22F7NNR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50320184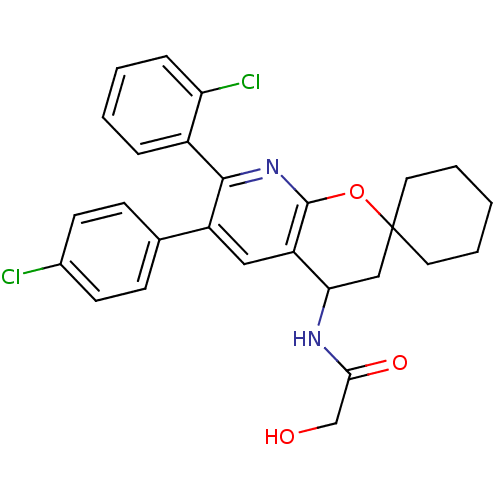 (CHEMBL1086494 | rac-N-(7'-(2-chlorophenyl)-6'-(4-c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 20: 3750-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.071 BindingDB Entry DOI: 10.7270/Q22F7NNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CB1 receptor | Bioorg Med Chem Lett 20: 1448-52 (2010) Article DOI: 10.1016/j.bmcl.2009.12.065 BindingDB Entry DOI: 10.7270/Q2028RPD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of cannabinoid CB1 receptor | J Med Chem 53: 4028-37 (2010) Article DOI: 10.1021/jm100023j BindingDB Entry DOI: 10.7270/Q2TQ61QD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50316528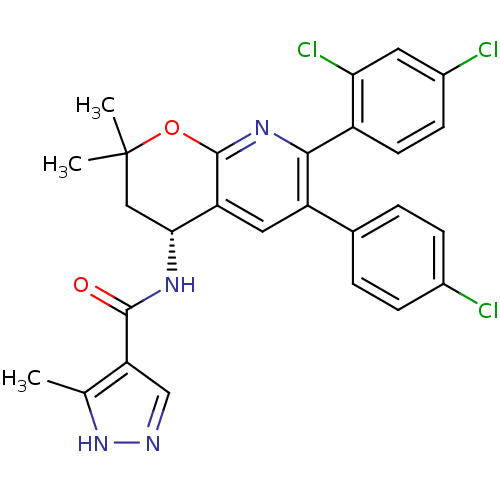 (CHEMBL1095150 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells | J Med Chem 53: 4028-37 (2010) Article DOI: 10.1021/jm100023j BindingDB Entry DOI: 10.7270/Q2TQ61QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 50: 3427-30 (2007) Article DOI: 10.1021/jm070131b BindingDB Entry DOI: 10.7270/Q2Z037VJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50205166 (CHEMBL231636 | N-((2S,3S)-4-(4-chlorophenyl)-3-(3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 2184-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.087 BindingDB Entry DOI: 10.7270/Q2HM584G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267775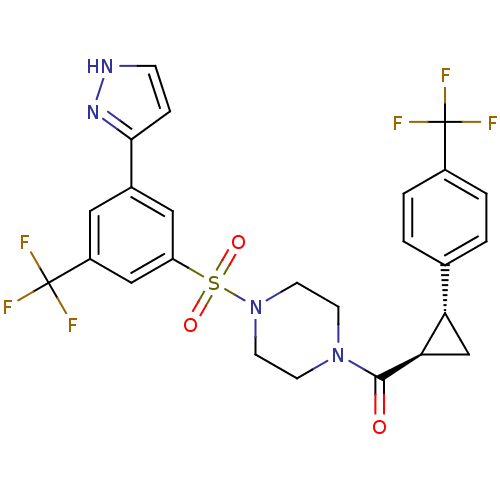 ((4-(3-(1H-pyrazol-5-yl)-5-(trifluoromethyl)phenyls...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029847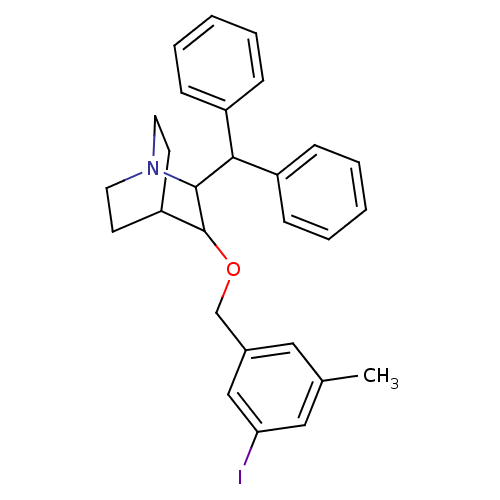 (2-Benzhydryl-3-(3-iodo-5-methyl-benzyloxy)-1-aza-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21279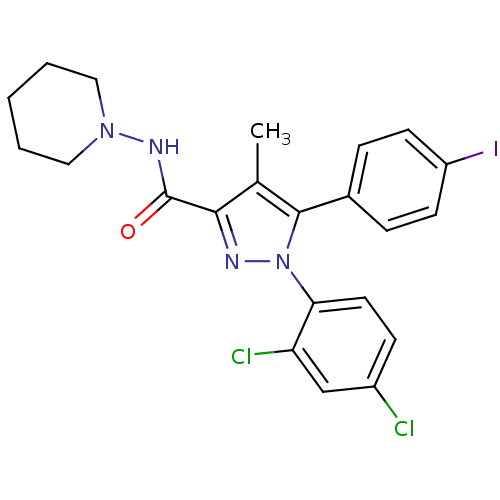 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR-141716 from human wild type CB1R expressed in CHO cells | J Med Chem 51: 2108-14 (2008) Article DOI: 10.1021/jm7014974 BindingDB Entry DOI: 10.7270/Q27H1KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030438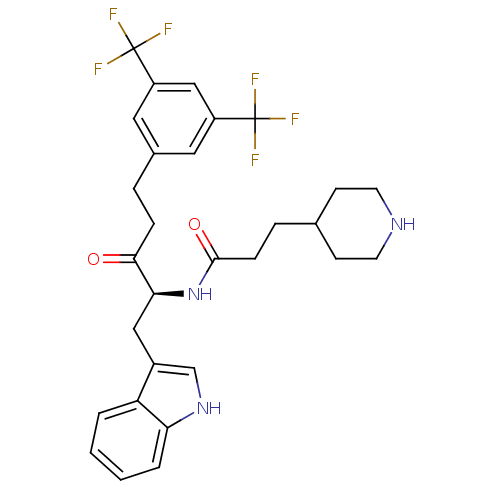 (CHEMBL426577 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50314119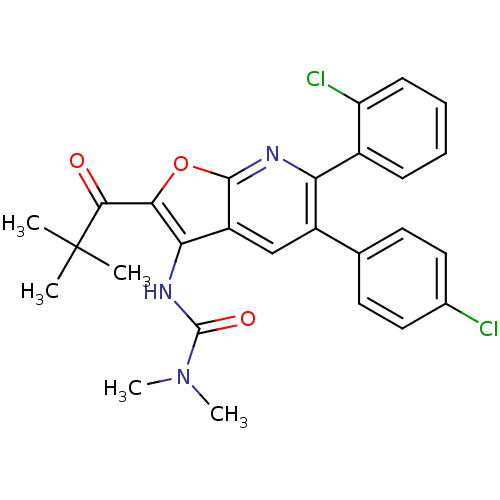 (3-(6-(2-chlorophenyl)-5-(4-chlorophenyl)-2-pivaloy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CB1 receptor | Bioorg Med Chem Lett 20: 1448-52 (2010) Article DOI: 10.1016/j.bmcl.2009.12.065 BindingDB Entry DOI: 10.7270/Q2028RPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50314122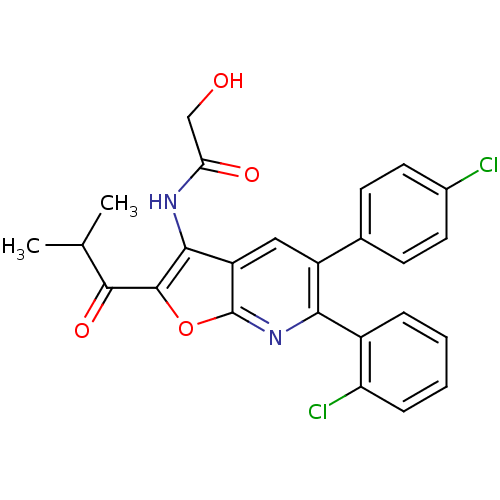 (CHEMBL1091832 | N-(6-(2-chlorophenyl)-5-(4-chlorop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CB1 receptor | Bioorg Med Chem Lett 20: 1448-52 (2010) Article DOI: 10.1016/j.bmcl.2009.12.065 BindingDB Entry DOI: 10.7270/Q2028RPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50320186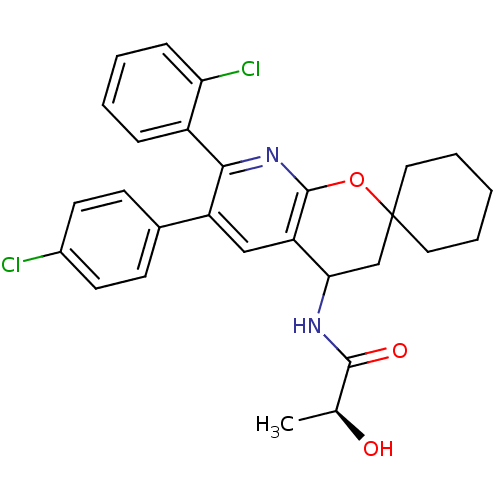 ((2S)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 20: 3750-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.071 BindingDB Entry DOI: 10.7270/Q22F7NNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030429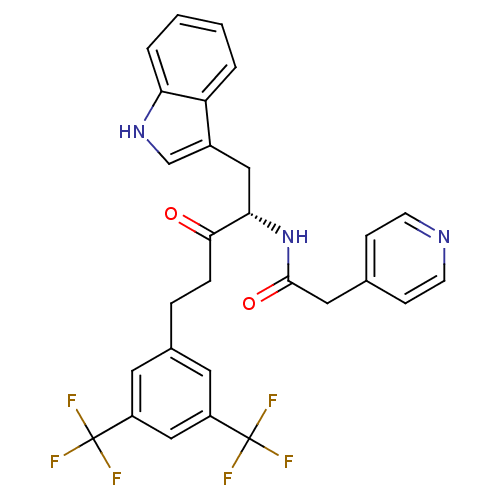 (CHEMBL173396 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against wild type human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance... | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329956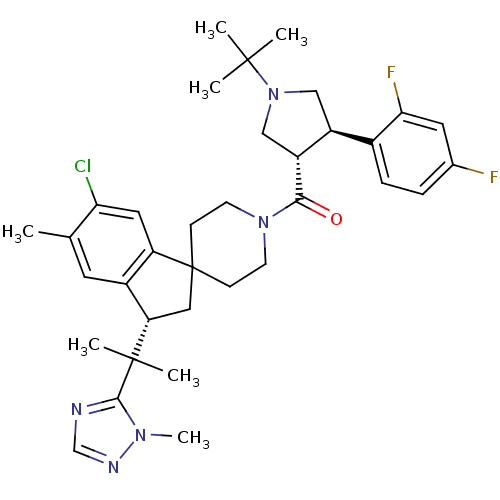 (((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329959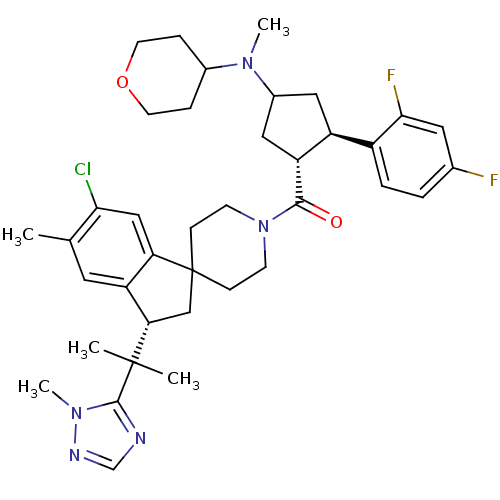 ((6-chloro-5-methyl-3-(2-(1-methyl-1H-1,2,4-triazol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50217220 (CHEMBL226590 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 50: 3427-30 (2007) Article DOI: 10.1021/jm070131b BindingDB Entry DOI: 10.7270/Q2Z037VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030439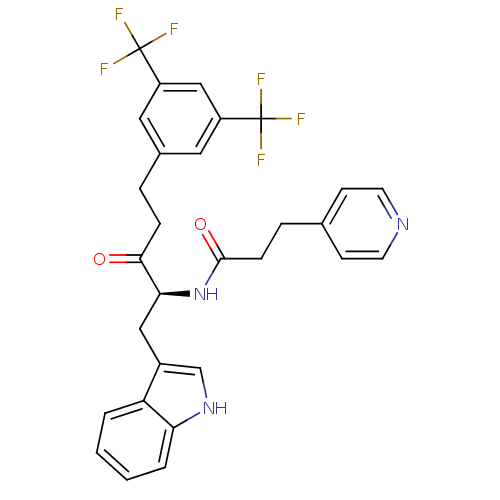 (CHEMBL175224 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267584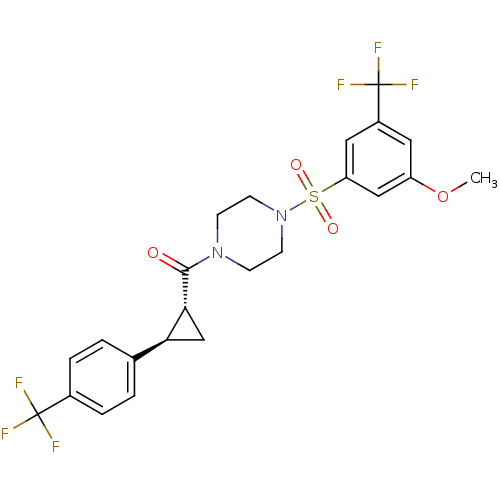 ((4-(3-methoxy-5-(trifluoromethyl)phenylsulfonyl)pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267487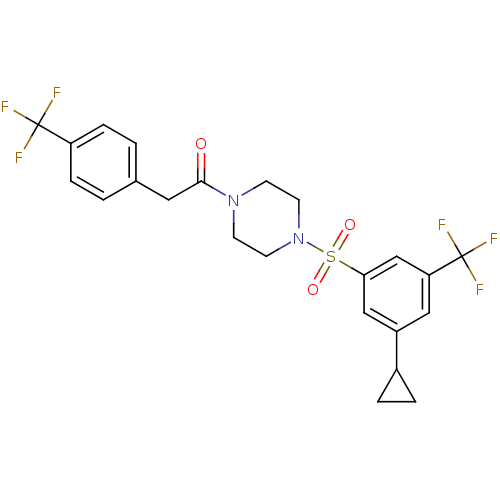 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030429 (CHEMBL173396 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50316525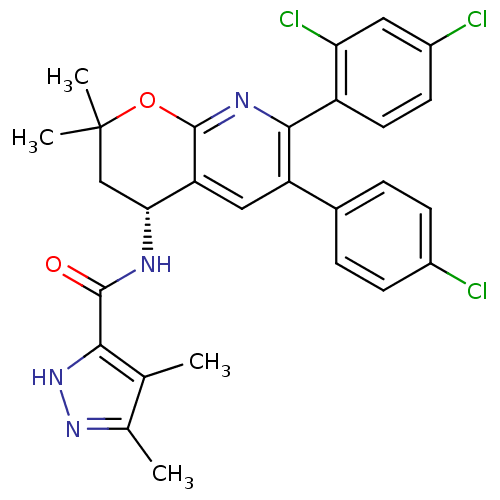 (CHEMBL1095476 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells | J Med Chem 53: 4028-37 (2010) Article DOI: 10.1021/jm100023j BindingDB Entry DOI: 10.7270/Q2TQ61QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200833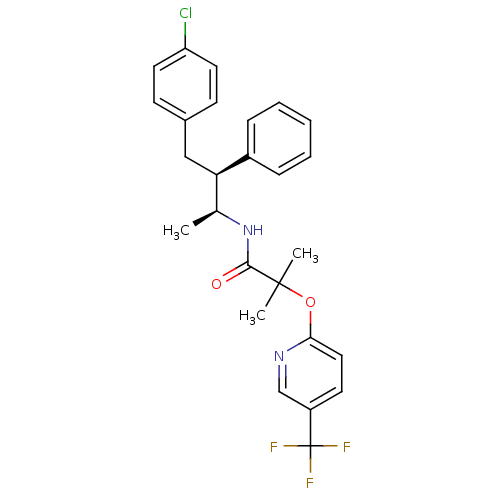 (CHEMBL373626 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells | J Med Chem 49: 7584-7 (2006) Article DOI: 10.1021/jm060996+ BindingDB Entry DOI: 10.7270/Q2QN67KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against wild type human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance... | J Med Chem 38: 934-41 (1995) BindingDB Entry DOI: 10.7270/Q2R78D8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1467 total ) | Next | Last >> |