

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50047247 (1,3-Dibutyl-7-(2-oxo-propyl)-3,7-dihydro-purine-2,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473208 (CHEMBL20531) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473209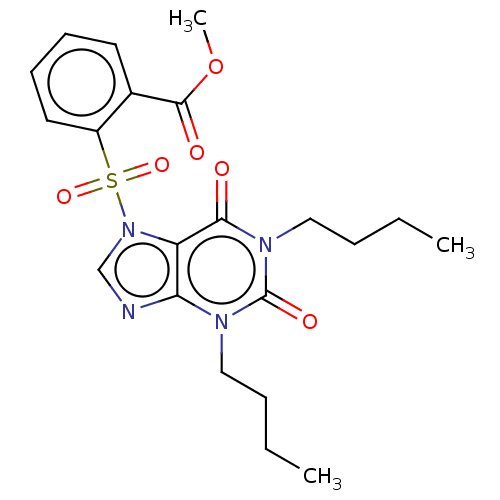 (CHEMBL20958) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473206 (CHEMBL20951) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473212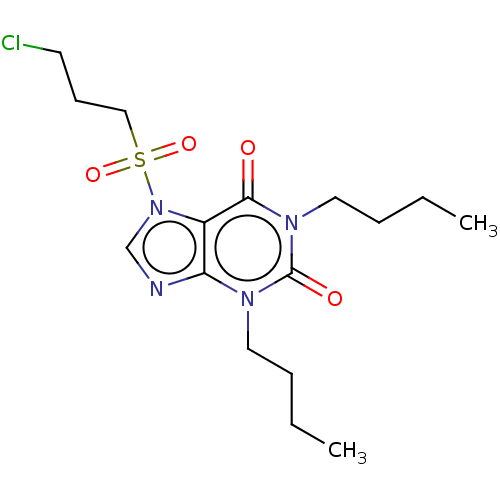 (CHEMBL418740) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473211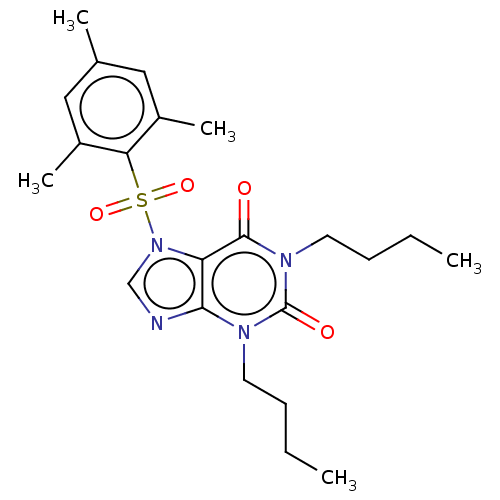 (CHEMBL282923) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076683 (CHEMBL6533 | Sodium; (2S,3S,5R,6R)-6-hydroxymethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076678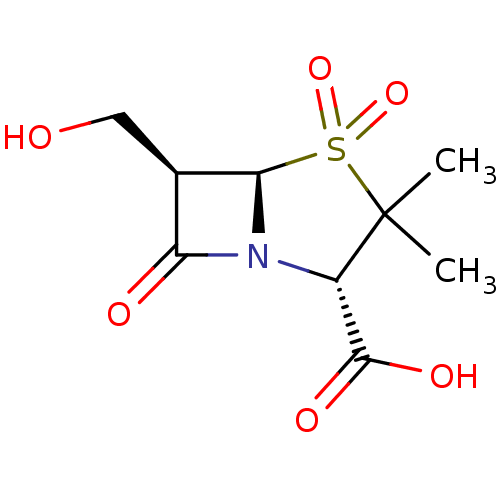 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076681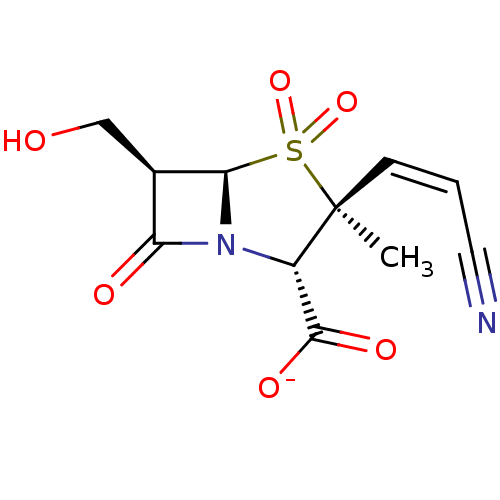 (CHEMBL6469 | Sodium; (2S,3S,5R,6R)-3-((Z)-2-cyano-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473210 (CHEMBL20822) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473207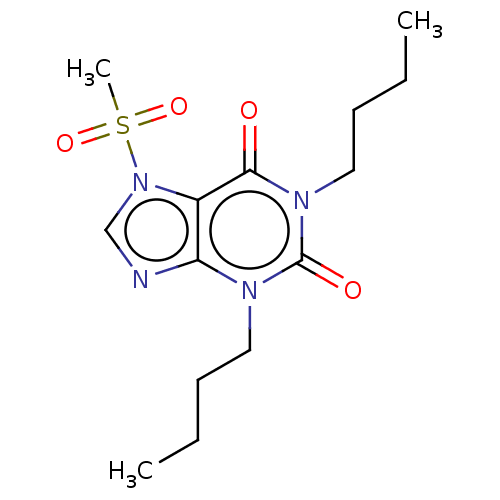 (CHEMBL418371) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited | J Med Chem 43: 1223-33 (2000) Article DOI: 10.1021/jm990558l BindingDB Entry DOI: 10.7270/Q2NK3HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50403733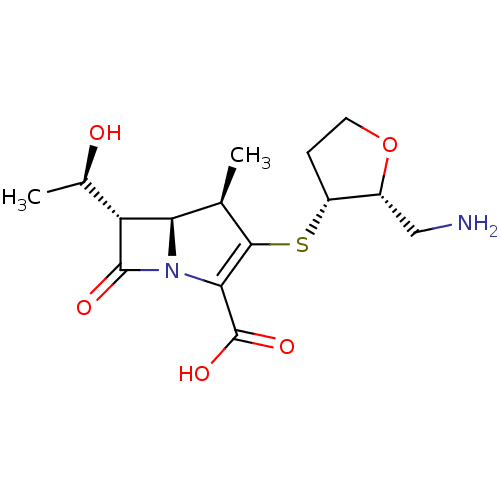 (CHEMBL1206880 | CL-191121) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50157692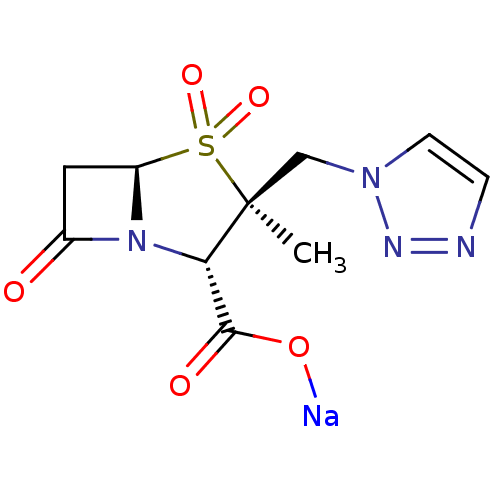 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50157692 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076682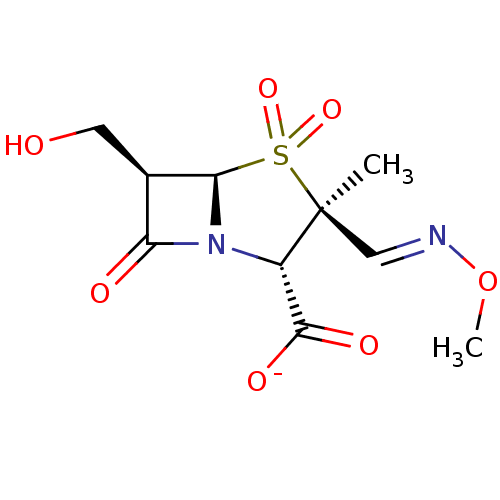 (CHEMBL6678 | Sodium; (2S,3S,5R,6R)-6-hydroxymethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076671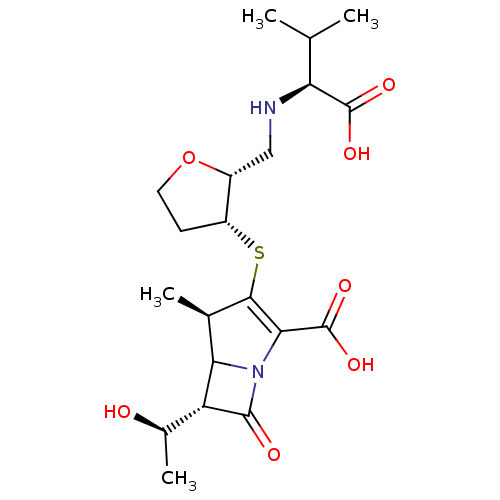 ((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076679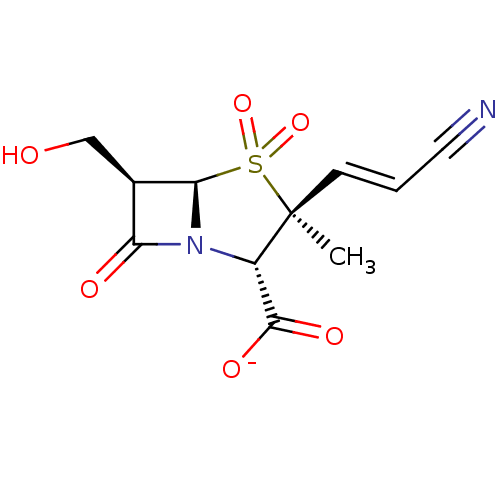 (CHEMBL269471 | Sodium; (2S,3S,5R,6R)-3-((E)-2-cyan...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076671 ((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076673 (CHEMBL175189 | Sodium; (2S,5R,6R)-6-((S)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50047247 (1,3-Dibutyl-7-(2-oxo-propyl)-3,7-dihydro-purine-2,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 4 (PDE4) | J Med Chem 45: 2342-5 (2002) Article DOI: 10.1021/jm010554s BindingDB Entry DOI: 10.7270/Q2CZ39W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50403733 (CHEMBL1206880 | CL-191121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076681 (CHEMBL6469 | Sodium; (2S,3S,5R,6R)-3-((Z)-2-cyano-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076679 (CHEMBL269471 | Sodium; (2S,3S,5R,6R)-3-((E)-2-cyan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076682 (CHEMBL6678 | Sodium; (2S,3S,5R,6R)-6-hydroxymethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076683 (CHEMBL6533 | Sodium; (2S,3S,5R,6R)-6-hydroxymethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076678 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021954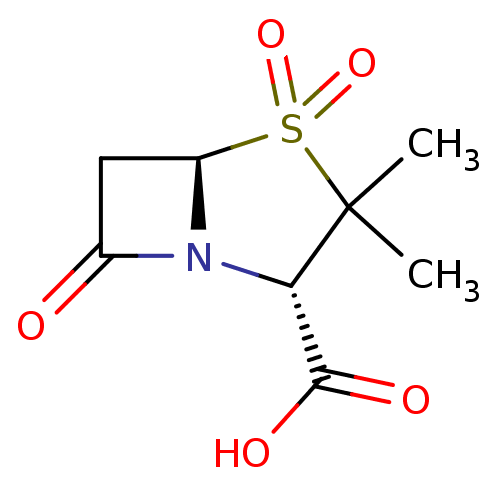 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 997-1002 (1999) BindingDB Entry DOI: 10.7270/Q2PK0FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076674 (CHEMBL177772 | Sodium; (2S,5R,6S)-6-((S)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473687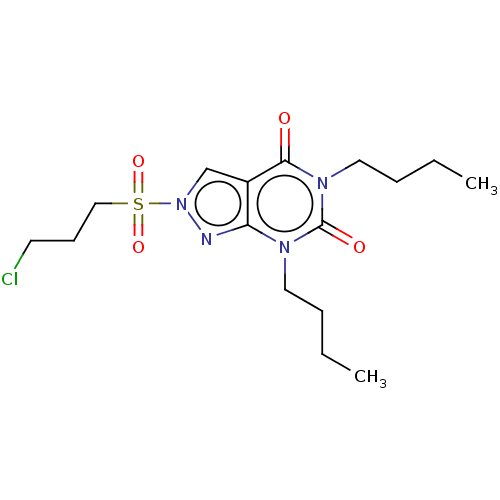 (CHEMBL76024) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 4 (PDE4) | J Med Chem 45: 2342-5 (2002) Article DOI: 10.1021/jm010554s BindingDB Entry DOI: 10.7270/Q2CZ39W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50473688 (CHEMBL77858) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 4 (PDE4) | J Med Chem 45: 2342-5 (2002) Article DOI: 10.1021/jm010554s BindingDB Entry DOI: 10.7270/Q2CZ39W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076672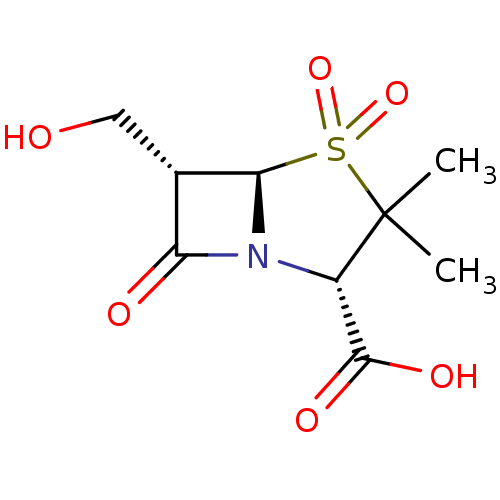 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076672 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076677 (CHEMBL177912 | Sodium; (2S,5R,6S)-6-((R)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221037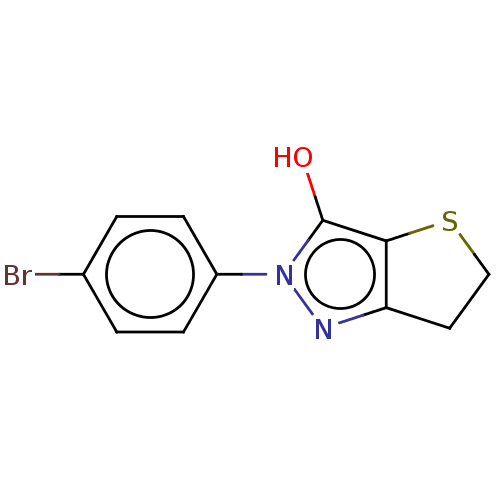 (CHEMBL87262) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221030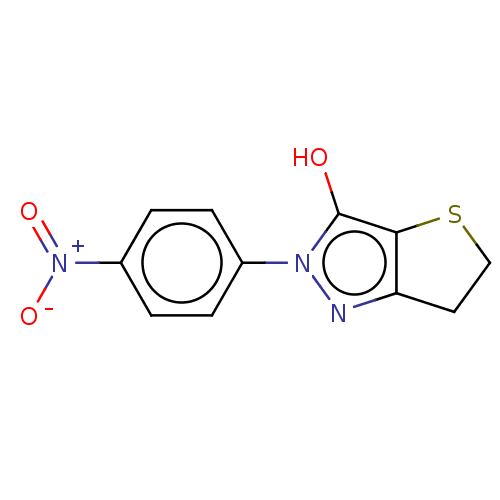 (CHEMBL313078) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221032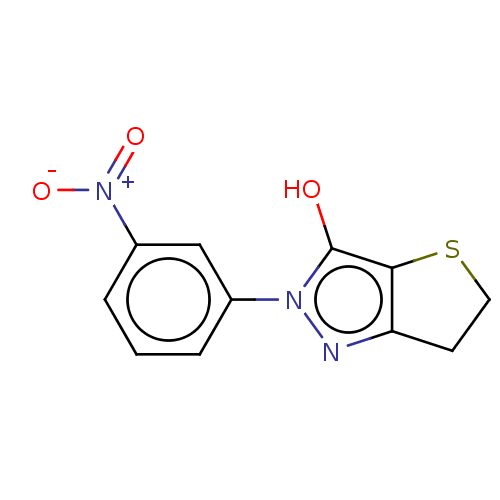 (CHEMBL86526) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramate--L-alanine ligase (Staphylococcus aureus (Firmicutes)) | BDBM50221038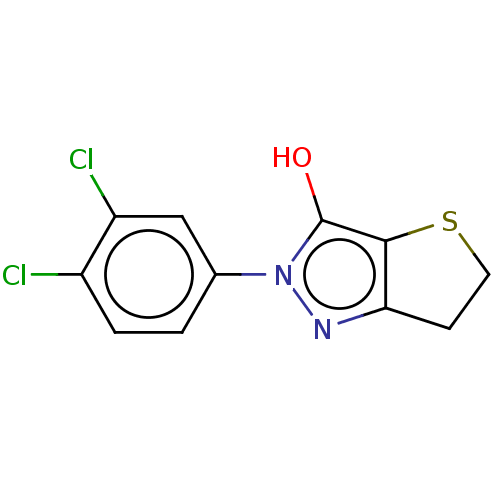 (CHEMBL87521) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramoyl-L-alanine synthetase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221018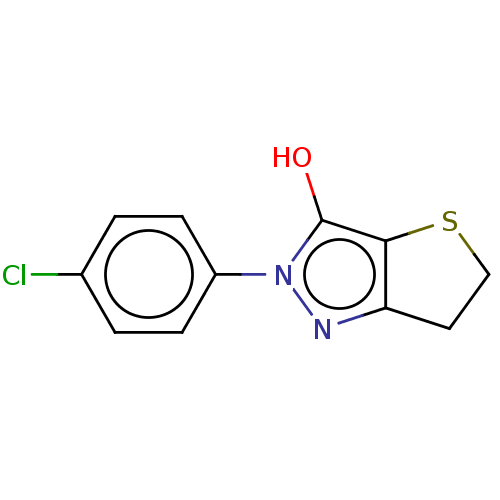 (CHEMBL85772) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Staphylococcus aureus (Firmicutes)) | BDBM50221037 (CHEMBL87262) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076675 (CHEMBL265722 | Sodium; (2S,5R,6R)-6-hydroxymethyl-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Staphylococcus aureus (Firmicutes)) | BDBM50221022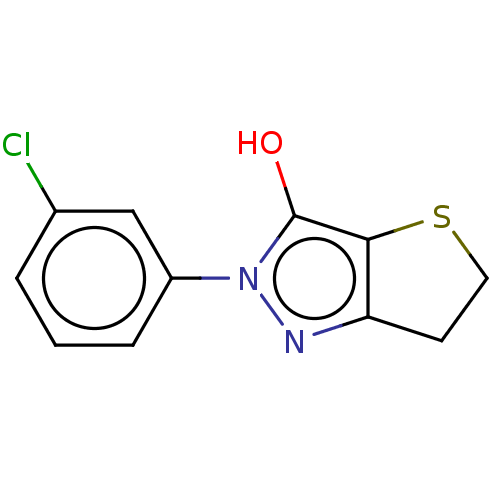 (CHEMBL86525) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221025 (CHEMBL83021) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Staphylococcus aureus (Firmicutes)) | BDBM50221032 (CHEMBL86526) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Staphylococcus aureus (Firmicutes)) | BDBM50221030 (CHEMBL313078) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramate--L-alanine ligase (Staphylococcus aureus (Firmicutes)) | BDBM50221037 (CHEMBL87262) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramoyl-L-alanine synthetase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221038 (CHEMBL87521) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylenolpyruvoylglucosamine reductase (Staphylococcus aureus (Firmicutes)) | BDBM50221020 (CHEMBL87126) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase | Bioorg Med Chem Lett 13: 2591-4 (2003) BindingDB Entry DOI: 10.7270/Q20P126D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |