 Found 72 hits with Last Name = 'fredenhagen' and Initial = 'a'
Found 72 hits with Last Name = 'fredenhagen' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC2 (unknown origin) expressed in SF21 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation ... |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283886
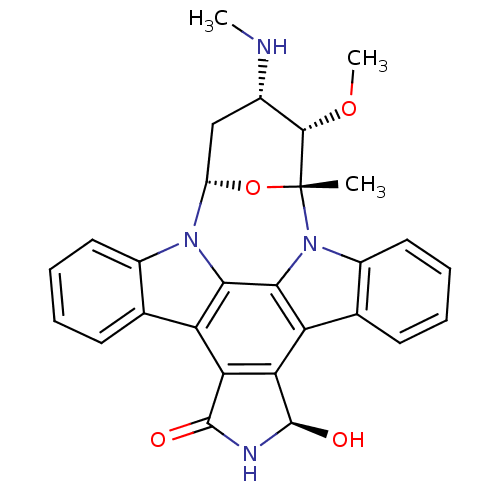
(18-hydroxy-3-methoxy-2-methyl-4-methylamino-(2R,3S...)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283895
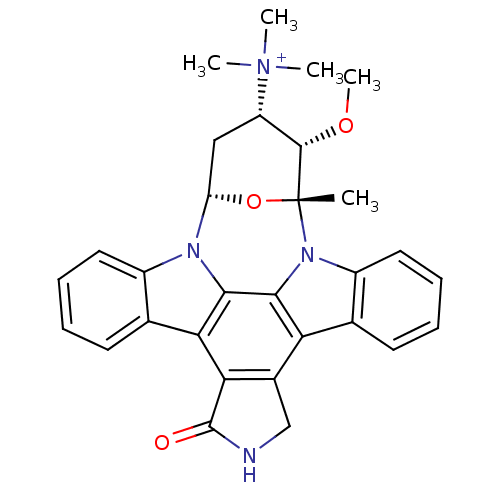
(3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa-1,7...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)[N+](C)(C)C Show InChI InChI=1S/C30H30N4O3/c1-30-28(36-5)21(34(2,3)4)14-22(37-30)32-19-12-8-6-10-16(19)24-25-18(15-31-29(25)35)23-17-11-7-9-13-20(17)33(30)27(23)26(24)32/h6-13,21-22,28H,14-15H2,1-5H3/p+1/t21-,22-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283891
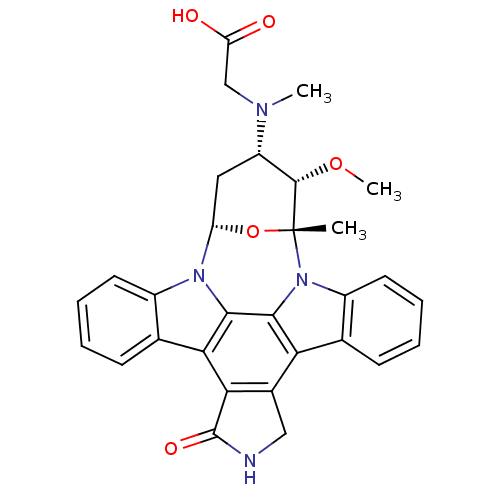
(2-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)CC(O)=O Show InChI InChI=1S/C30H28N4O5/c1-30-28(38-3)20(32(2)14-22(35)36)12-21(39-30)33-18-10-6-4-8-15(18)24-25-17(13-31-29(25)37)23-16-9-5-7-11-19(16)34(30)27(23)26(24)33/h4-11,20-21,28H,12-14H2,1-3H3,(H,31,37)(H,35,36)/t20-,21-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283899
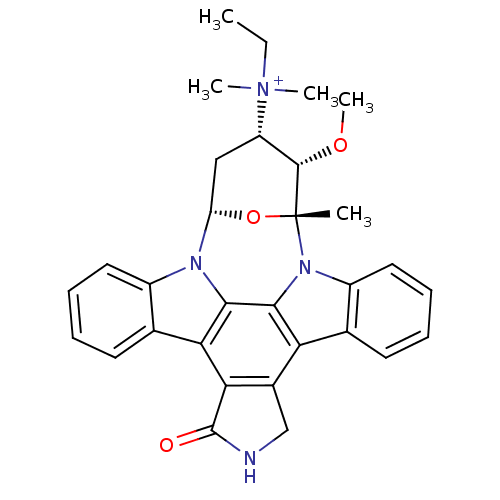
(CHEMBL340611 | ethyl[3-methoxy-2-methyl-16-oxo-(2R...)Show SMILES CC[N+](C)(C)[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C31H32N4O3/c1-6-35(3,4)22-15-23-33-20-13-9-7-11-17(20)25-26-19(16-32-30(26)36)24-18-12-8-10-14-21(18)34(28(24)27(25)33)31(2,38-23)29(22)37-5/h7-14,22-23,29H,6,15-16H2,1-5H3/p+1/t22-,23-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50103604
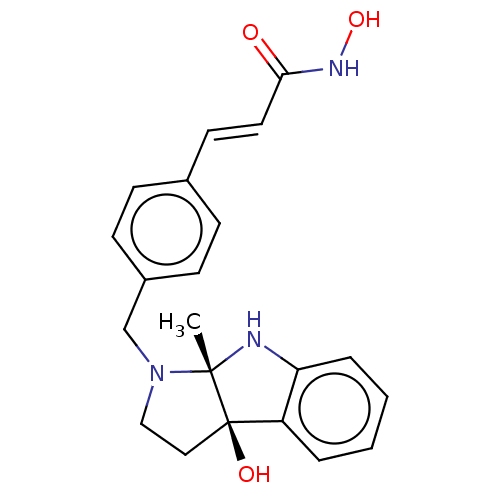
(CHEMBL3526395)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(=O)NO)cc1 |r| Show InChI InChI=1S/C21H23N3O3/c1-20-21(26,17-4-2-3-5-18(17)22-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(25)23-27/h2-11,22,26-27H,12-14H2,1H3,(H,23,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283888

(4-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C36H30N4O6/c1-36-32(45-3)25(38(2)34(42)18-12-14-19(15-13-18)35(43)44)16-26(46-36)39-23-10-6-4-8-20(23)28-29-22(17-37-33(29)41)27-21-9-5-7-11-24(21)40(36)31(27)30(28)39/h4-15,25-26,32H,16-17H2,1-3H3,(H,37,41)(H,43,44)/t25-,26-,32-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50103604

(CHEMBL3526395)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(=O)NO)cc1 |r| Show InChI InChI=1S/C21H23N3O3/c1-20-21(26,17-4-2-3-5-18(17)22-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(25)23-27/h2-11,22,26-27H,12-14H2,1H3,(H,23,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50283901
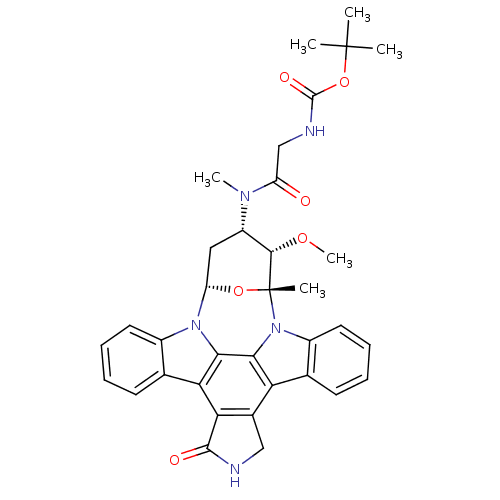
(CHEMBL335931 | Staurosporine derivative)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)CNC(=O)OC(C)(C)C Show InChI InChI=1S/C35H37N5O6/c1-34(2,3)46-33(43)37-17-24(41)38(5)23-15-25-39-21-13-9-7-11-18(21)27-28-20(16-36-32(28)42)26-19-12-8-10-14-22(19)40(30(26)29(27)39)35(4,45-25)31(23)44-6/h7-14,23,25,31H,15-17H2,1-6H3,(H,36,42)(H,37,43)/t23-,25-,31-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283892
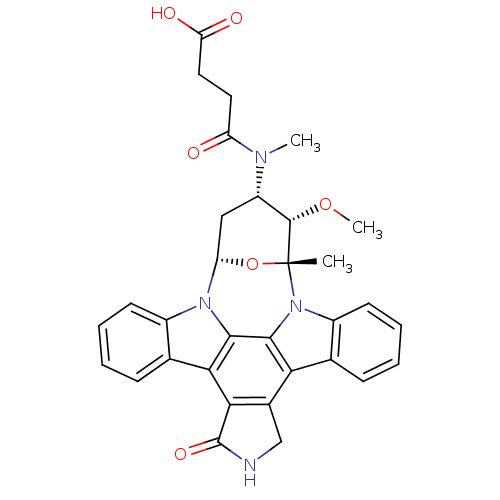
(3-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)CCC(O)=O Show InChI InChI=1S/C32H30N4O6/c1-32-30(41-3)21(34(2)22(37)12-13-24(38)39)14-23(42-32)35-19-10-6-4-8-16(19)26-27-18(15-33-31(27)40)25-17-9-5-7-11-20(17)36(32)29(25)28(26)35/h4-11,21,23,30H,12-15H2,1-3H3,(H,33,40)(H,38,39)/t21-,23-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50103604

(CHEMBL3526395)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(=O)NO)cc1 |r| Show InChI InChI=1S/C21H23N3O3/c1-20-21(26,17-4-2-3-5-18(17)22-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(25)23-27/h2-11,22,26-27H,12-14H2,1H3,(H,23,25)/b11-10+/t20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283894
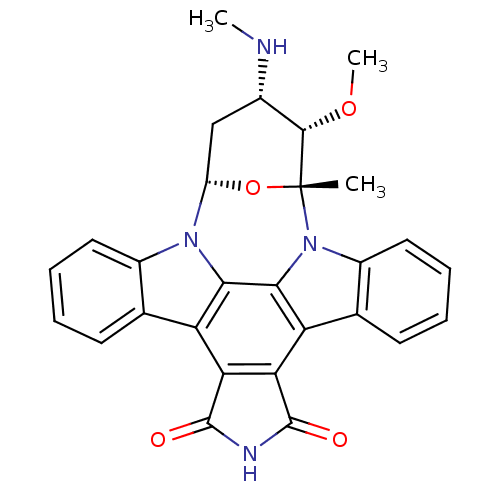
(3-methoxy-2-methyl-4-methylamino-(2R,3S,4S,6S)-29-...)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H24N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,29H,12H2,1-3H3,(H,30,33,34)/t15-,18-,25-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50283892

(3-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)CCC(O)=O Show InChI InChI=1S/C32H30N4O6/c1-32-30(41-3)21(34(2)22(37)12-13-24(38)39)14-23(42-32)35-19-10-6-4-8-16(19)26-27-18(15-33-31(27)40)25-17-9-5-7-11-20(17)36(32)29(25)28(26)35/h4-11,21,23,30H,12-15H2,1-3H3,(H,33,40)(H,38,39)/t21-,23-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283909
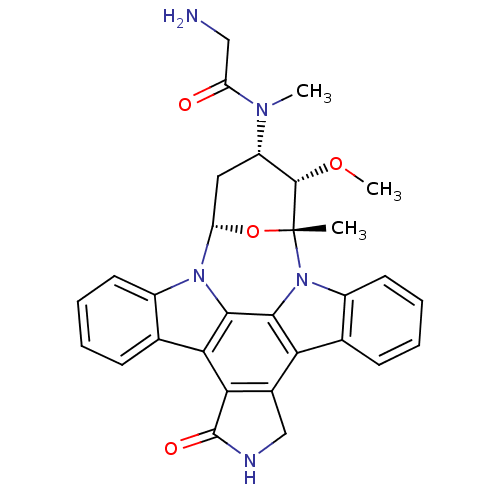
(1N-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)CN Show InChI InChI=1S/C30H29N5O4/c1-30-28(38-3)20(33(2)21(36)13-31)12-22(39-30)34-18-10-6-4-8-15(18)24-25-17(14-32-29(25)37)23-16-9-5-7-11-19(16)35(30)27(23)26(24)34/h4-11,20,22,28H,12-14,31H2,1-3H3,(H,32,37)/t20-,22-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50103604

(CHEMBL3526395)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(=O)NO)cc1 |r| Show InChI InChI=1S/C21H23N3O3/c1-20-21(26,17-4-2-3-5-18(17)22-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(25)23-27/h2-11,22,26-27H,12-14H2,1H3,(H,23,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50283900
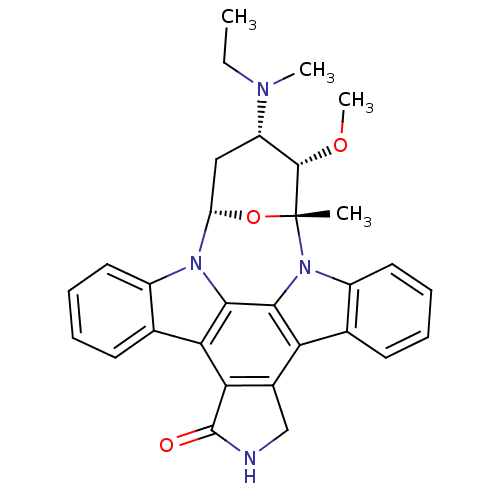
(4-ethyl(methyl)amino-3-methoxy-2-methyl-(2R,3S,4S,...)Show SMILES CCN(C)[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C30H30N4O3/c1-5-32(3)21-14-22-33-19-12-8-6-10-16(19)24-25-18(15-31-29(25)35)23-17-11-7-9-13-20(17)34(27(23)26(24)33)30(2,37-22)28(21)36-4/h6-13,21-22,28H,5,14-15H2,1-4H3,(H,31,35)/t21-,22-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283903

(18-hydroxy-5-methoxy-6-methyl-4-methylamino-(2S,4S...)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4C(=O)N[C@@H](O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25-26,29,33H,12H2,1-3H3,(H,30,34)/t15-,18-,25-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of his-strep-tagged HDAC8 (unknown origin) expressed in SF9 cells using [3H]acetylated human histone H4 peptide as substrate by scintillat... |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283900

(4-ethyl(methyl)amino-3-methoxy-2-methyl-(2R,3S,4S,...)Show SMILES CCN(C)[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C30H30N4O3/c1-5-32(3)21-14-22-33-19-12-8-6-10-16(19)24-25-18(15-31-29(25)35)23-17-11-7-9-13-20(17)34(27(23)26(24)33)30(2,37-22)28(21)36-4/h6-13,21-22,28H,5,14-15H2,1-4H3,(H,31,35)/t21-,22-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50283886

(18-hydroxy-3-methoxy-2-methyl-4-methylamino-(2R,3S...)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM31094
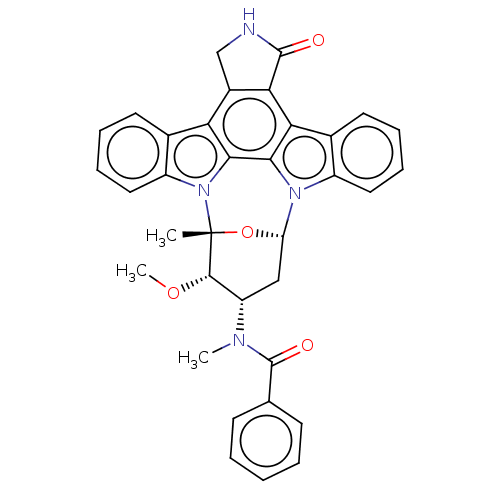
(PKC-412 | cid_24202429)Show SMILES [H][C@@]12C[C@@H]([C@H](OC)[C@@](C)(O1)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |TLB:11:10:9:4.3.2,17:33:9:4.3.2,THB:24:32:9:4.3.2,5:4:9:10.31.33.32,30:31:9:4.3.2| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50283894

(3-methoxy-2-methyl-4-methylamino-(2R,3S,4S,6S)-29-...)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H24N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,29H,12H2,1-3H3,(H,30,33,34)/t15-,18-,25-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283902
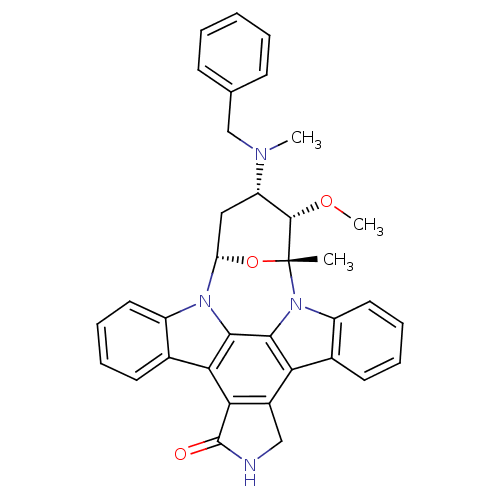
(4-benzyl(methyl)amino-3-methoxy-2-methyl-(2R,3S,4S...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)Cc1ccccc1 Show InChI InChI=1S/C35H32N4O3/c1-35-33(41-3)26(37(2)19-20-11-5-4-6-12-20)17-27(42-35)38-24-15-9-7-13-21(24)29-30-23(18-36-34(30)40)28-22-14-8-10-16-25(22)39(35)32(28)31(29)38/h4-16,26-27,33H,17-19H2,1-3H3,(H,36,40)/t26-,27-,33-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50103604

(CHEMBL3526395)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(=O)NO)cc1 |r| Show InChI InChI=1S/C21H23N3O3/c1-20-21(26,17-4-2-3-5-18(17)22-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(25)23-27/h2-11,22,26-27H,12-14H2,1H3,(H,23,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of his-strep-tagged HDAC8 (unknown origin) expressed in SF9 cells using [3H]acetylated human histone H4 peptide as substrate by scintillat... |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50103602

(CHEMBL3526396)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(N)=O)cc1 |r| Show InChI InChI=1S/C21H23N3O2/c1-20-21(26,17-4-2-3-5-18(17)23-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(22)25/h2-11,23,26H,12-14H2,1H3,(H2,22,25)/b11-10+/t20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50103602

(CHEMBL3526396)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(N)=O)cc1 |r| Show InChI InChI=1S/C21H23N3O2/c1-20-21(26,17-4-2-3-5-18(17)23-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(22)25/h2-11,23,26H,12-14H2,1H3,(H2,22,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50103602

(CHEMBL3526396)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(N)=O)cc1 |r| Show InChI InChI=1S/C21H23N3O2/c1-20-21(26,17-4-2-3-5-18(17)23-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(22)25/h2-11,23,26H,12-14H2,1H3,(H2,22,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283896

(CHEMBL131020 | N-[3-methoxy-2-methyl-16-oxo-(2R,3S...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)S(C)(=O)=O Show InChI InChI=1S/C29H28N4O5S/c1-29-27(37-3)20(31(2)39(4,35)36)13-21(38-29)32-18-11-7-5-9-15(18)23-24-17(14-30-28(24)34)22-16-10-6-8-12-19(16)33(29)26(22)25(23)32/h5-12,20-21,27H,13-14H2,1-4H3,(H,30,34)/t20-,21-,27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283906
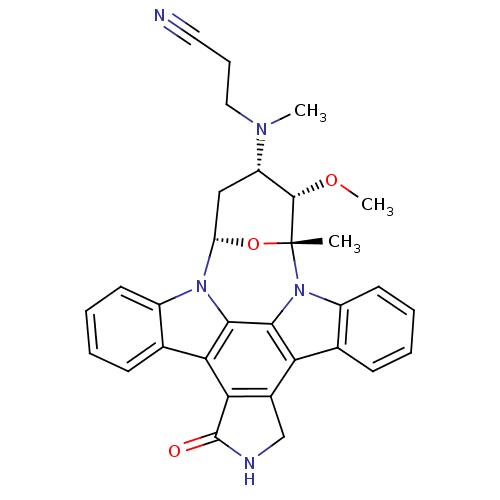
(2-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)CCC#N Show InChI InChI=1S/C31H29N5O3/c1-31-29(38-3)22(34(2)14-8-13-32)15-23(39-31)35-20-11-6-4-9-17(20)25-26-19(16-33-30(26)37)24-18-10-5-7-12-21(18)36(31)28(24)27(25)35/h4-7,9-12,22-23,29H,8,14-16H2,1-3H3,(H,33,37)/t22-,23-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50103602

(CHEMBL3526396)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(N)=O)cc1 |r| Show InChI InChI=1S/C21H23N3O2/c1-20-21(26,17-4-2-3-5-18(17)23-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(22)25/h2-11,23,26H,12-14H2,1H3,(H2,22,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283887
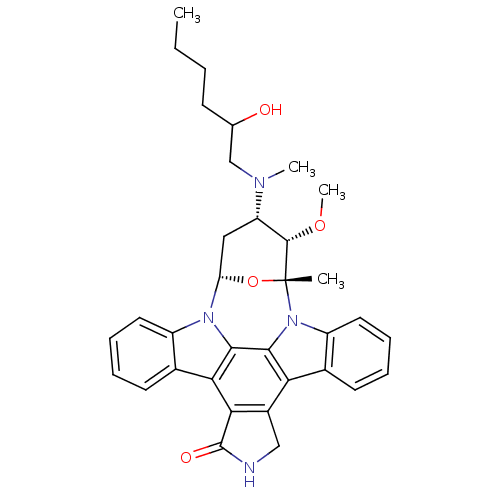
(4-[2-hydroxyhexyl(methyl)amino]-3-methoxy-2-methyl...)Show SMILES CCCCC(O)CN(C)[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C34H38N4O4/c1-5-6-11-19(39)18-36(3)25-16-26-37-23-14-9-7-12-20(23)28-29-22(17-35-33(29)40)27-21-13-8-10-15-24(21)38(31(27)30(28)37)34(2,42-26)32(25)41-4/h7-10,12-15,19,25-26,32,39H,5-6,11,16-18H2,1-4H3,(H,35,40)/t19?,25-,26-,32-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283883
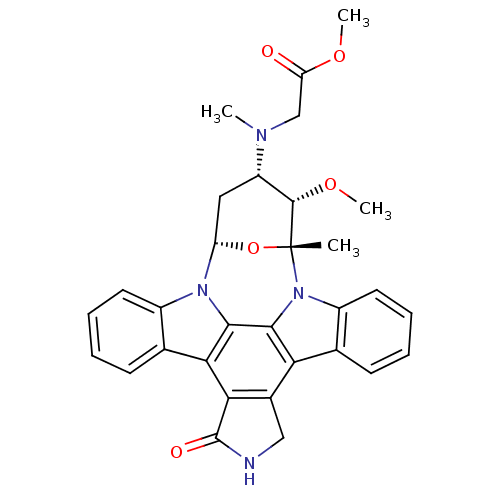
(CHEMBL444337 | methyl 2-[3-methoxy-2-methyl-16-oxo...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)CC(=O)OC Show InChI InChI=1S/C31H30N4O5/c1-31-29(39-4)21(33(2)15-23(36)38-3)13-22(40-31)34-19-11-7-5-9-16(19)25-26-18(14-32-30(26)37)24-17-10-6-8-12-20(17)35(31)28(24)27(25)34/h5-12,21-22,29H,13-15H2,1-4H3,(H,32,37)/t21-,22-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50103604

(CHEMBL3526395)Show SMILES C[C@@]12Nc3ccccc3[C@]1(O)CCN2Cc1ccc(\C=C\C(=O)NO)cc1 |r| Show InChI InChI=1S/C21H23N3O3/c1-20-21(26,17-4-2-3-5-18(17)22-20)12-13-24(20)14-16-8-6-15(7-9-16)10-11-19(25)23-27/h2-11,22,26-27H,12-14H2,1H3,(H,23,25)/b11-10+/t20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC2 (unknown origin) expressed in SF21 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation ... |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50283903

(18-hydroxy-5-methoxy-6-methyl-4-methylamino-(2S,4S...)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4C(=O)N[C@@H](O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25-26,29,33H,12H2,1-3H3,(H,30,34)/t15-,18-,25-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM31094

(PKC-412 | cid_24202429)Show SMILES [H][C@@]12C[C@@H]([C@H](OC)[C@@](C)(O1)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |TLB:11:10:9:4.3.2,17:33:9:4.3.2,THB:24:32:9:4.3.2,5:4:9:10.31.33.32,30:31:9:4.3.2| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283889

(CHEMBL130049 | Staurosporine derivative)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)OC Show InChI InChI=1S/C30H28N4O5/c1-30-27(37-3)20(32(2)29(36)38-4)13-21(39-30)33-18-11-7-5-9-15(18)23-24-17(14-31-28(24)35)22-16-10-6-8-12-19(16)34(30)26(22)25(23)33/h5-12,20-21,27H,13-14H2,1-4H3,(H,31,35)/t20-,21-,27-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283893
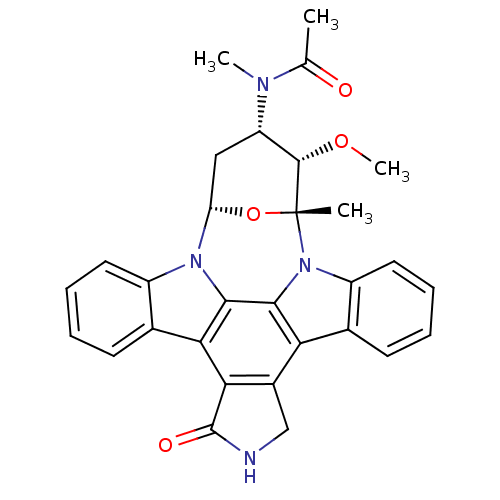
(1N-[3-methoxy-2-methyl-16-oxo-(2R,3S,4S,6S)-29-oxa...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(C)=O Show InChI InChI=1S/C30H28N4O4/c1-15(35)32(3)21-13-22-33-19-11-7-5-9-16(19)24-25-18(14-31-29(25)36)23-17-10-6-8-12-20(17)34(27(23)26(24)33)30(2,38-22)28(21)37-4/h5-12,21-22,28H,13-14H2,1-4H3,(H,31,36)/t21-,22-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50103603
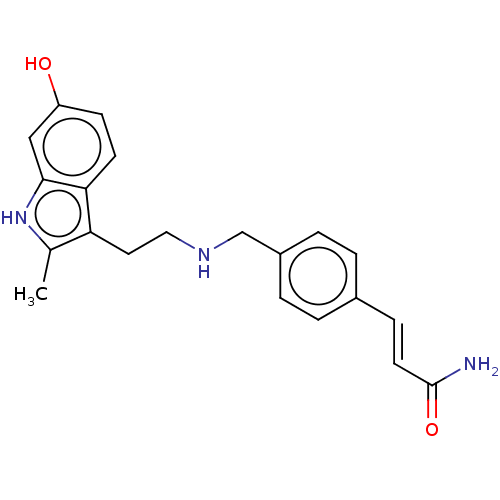
(CHEMBL3526942)Show SMILES Cc1[nH]c2cc(O)ccc2c1CCNCc1ccc(\C=C\C(N)=O)cc1 Show InChI InChI=1S/C21H23N3O2/c1-14-18(19-8-7-17(25)12-20(19)24-14)10-11-23-13-16-4-2-15(3-5-16)6-9-21(22)26/h2-9,12,23-25H,10-11,13H2,1H3,(H2,22,26)/b9-6+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283901

(CHEMBL335931 | Staurosporine derivative)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)CNC(=O)OC(C)(C)C Show InChI InChI=1S/C35H37N5O6/c1-34(2,3)46-33(43)37-17-24(41)38(5)23-15-25-39-21-13-9-7-11-18(21)27-28-20(16-36-32(28)42)26-19-12-8-10-14-22(19)40(30(26)29(27)39)35(4,45-25)31(23)44-6/h7-14,23,25,31H,15-17H2,1-6H3,(H,36,42)(H,37,43)/t23-,25-,31-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283882
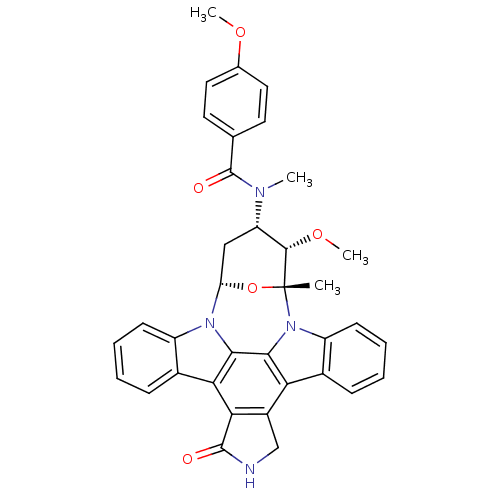
(3-methoxy-4-[4-methoxyphenyl(methyl)carboxamido]-2...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C36H32N4O5/c1-36-33(44-4)26(38(2)35(42)19-13-15-20(43-3)16-14-19)17-27(45-36)39-24-11-7-5-9-21(24)29-30-23(18-37-34(30)41)28-22-10-6-8-12-25(22)40(36)32(28)31(29)39/h5-16,26-27,33H,17-18H2,1-4H3,(H,37,41)/t26-,27-,33-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50103603

(CHEMBL3526942)Show SMILES Cc1[nH]c2cc(O)ccc2c1CCNCc1ccc(\C=C\C(N)=O)cc1 Show InChI InChI=1S/C21H23N3O2/c1-14-18(19-8-7-17(25)12-20(19)24-14)10-11-23-13-16-4-2-15(3-5-16)6-9-21(22)26/h2-9,12,23-25H,10-11,13H2,1H3,(H2,22,26)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283898
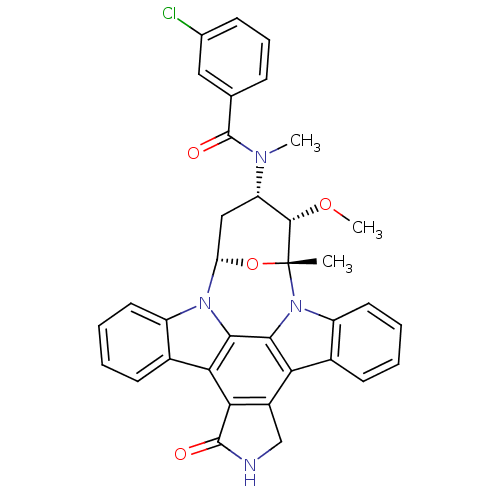
(4-[3-chlorophenyl(methyl)carboxamido]-3-methoxy-2-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C35H29ClN4O4/c1-35-32(43-3)25(38(2)34(42)18-9-8-10-19(36)15-18)16-26(44-35)39-23-13-6-4-11-20(23)28-29-22(17-37-33(29)41)27-21-12-5-7-14-24(21)40(35)31(27)30(28)39/h4-15,25-26,32H,16-17H2,1-3H3,(H,37,41)/t25-,26-,32-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50103603

(CHEMBL3526942)Show SMILES Cc1[nH]c2cc(O)ccc2c1CCNCc1ccc(\C=C\C(N)=O)cc1 Show InChI InChI=1S/C21H23N3O2/c1-14-18(19-8-7-17(25)12-20(19)24-14)10-11-23-13-16-4-2-15(3-5-16)6-9-21(22)26/h2-9,12,23-25H,10-11,13H2,1H3,(H2,22,26)/b9-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50283897
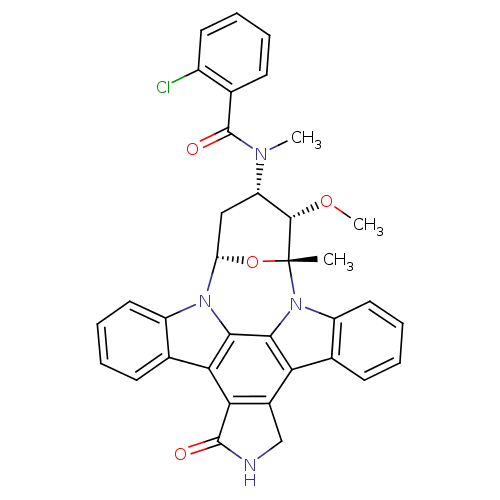
(4-[2-chlorophenyl(methyl)carboxamido]-3-methoxy-2-...)Show SMILES CO[C@H]1[C@H](C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1Cl Show InChI InChI=1S/C35H29ClN4O4/c1-35-32(43-3)25(38(2)34(42)18-10-4-7-13-22(18)36)16-26(44-35)39-23-14-8-5-11-19(23)28-29-21(17-37-33(29)41)27-20-12-6-9-15-24(20)40(35)31(27)30(28)39/h4-15,25-26,32H,16-17H2,1-3H3,(H,37,41)/t25-,26-,32-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine kinase of Epidermal growth factor receptor was determined |
Bioorg Med Chem Lett 4: 399-404 (1994)
Article DOI: 10.1016/0960-894X(94)80004-9
BindingDB Entry DOI: 10.7270/Q2R211BD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data