

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM222178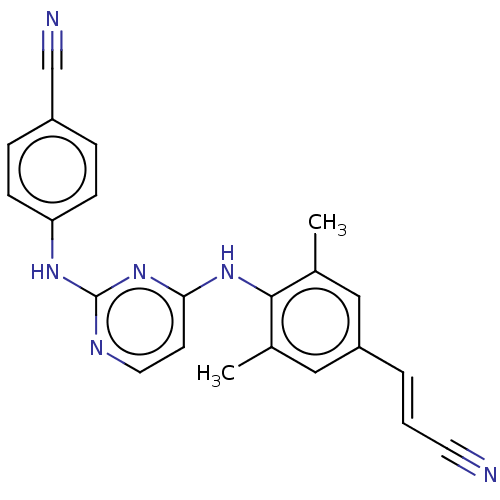 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 Y181C mutant expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed ... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25826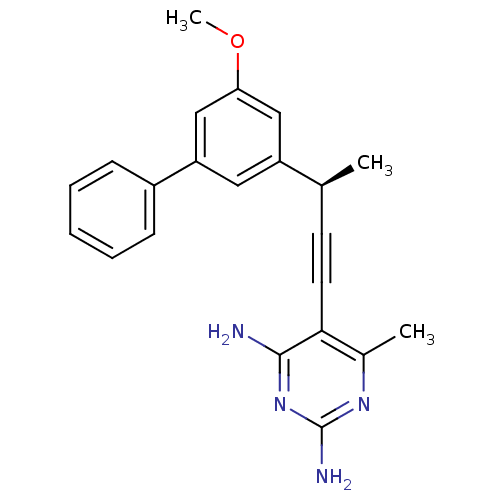 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134209 (CHEMBL3342974) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25818 (5-[3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]-6-me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25818 (5-[3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]-6-me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25820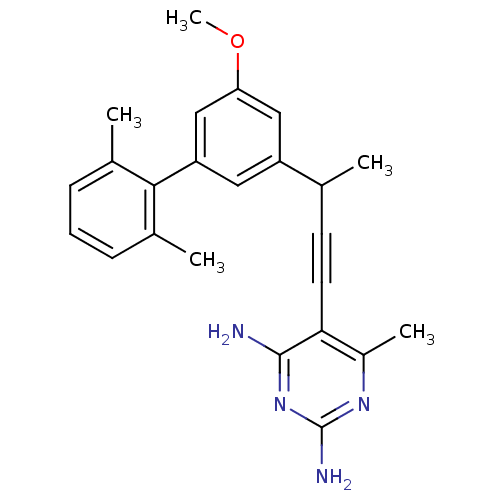 (5-{3-[3-(2,6-dimethylphenyl)-5-methoxyphenyl]but-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25819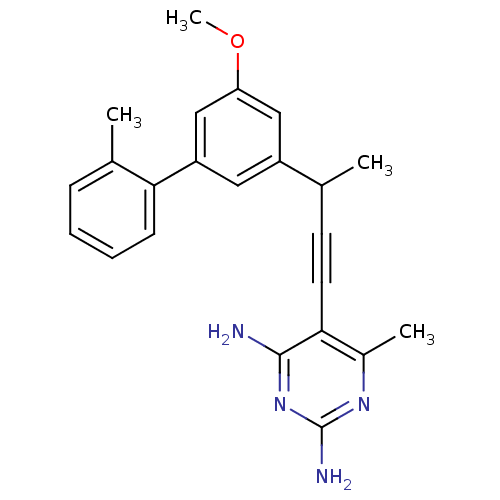 (5-{3-[3-methoxy-5-(2-methylphenyl)phenyl]but-1-yn-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25820 (5-{3-[3-(2,6-dimethylphenyl)-5-methoxyphenyl]but-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25819 (5-{3-[3-methoxy-5-(2-methylphenyl)phenyl]but-1-yn-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329610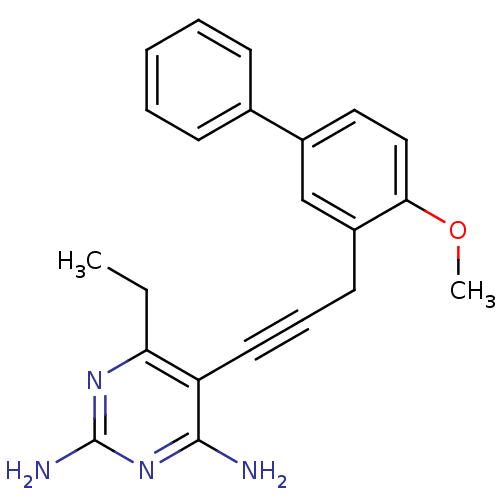 (6-ethyl-5-(3-(4-methoxybiphenyl-3-yl)prop-1-ynyl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134211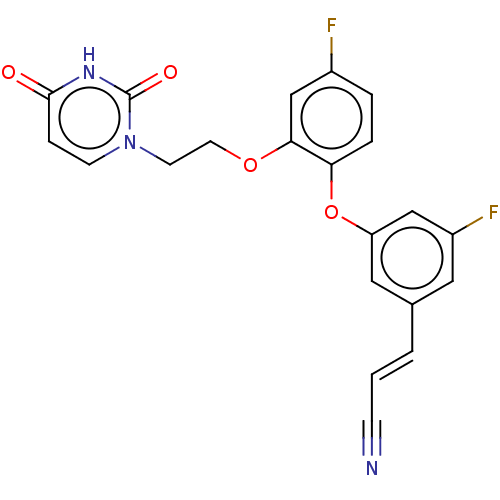 (CHEMBL1923492) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134210 (CHEMBL3342966) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50494905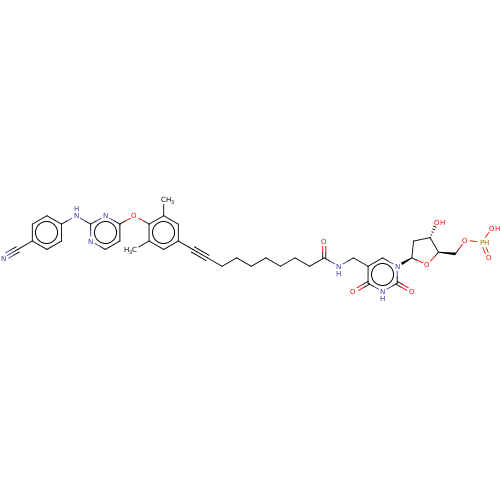 (CHEMBL3099595) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV-1 6His-tagged reverse transcriptase p66/p51-mediated TTP incorporation into D23/D36 primer/template preincubated for 15 mins follow... | ACS Med Chem Lett 4: 1183-8 (2013) Article DOI: 10.1021/ml4002979 BindingDB Entry DOI: 10.7270/Q2GF0XGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50501292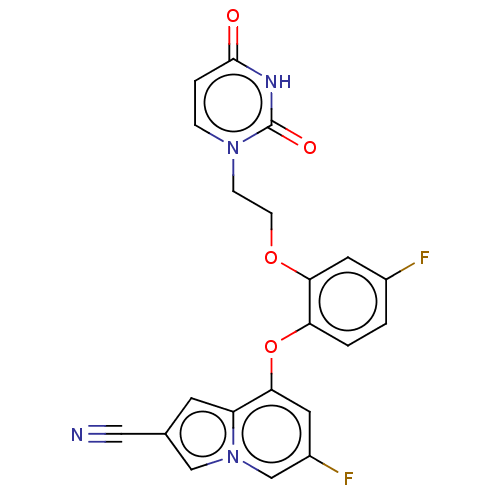 (CHEMBL3342978) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed by primer/tem... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50501292 (CHEMBL3342978) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 Y181C mutant expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed ... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50329610 (6-ethyl-5-(3-(4-methoxybiphenyl-3-yl)prop-1-ynyl)p...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50494904 (CHEMBL3099596) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV-1 6His-tagged reverse transcriptase p66/p51-mediated TTP incorporation into D23/D36 primer/template preincubated for 15 mins follow... | ACS Med Chem Lett 4: 1183-8 (2013) Article DOI: 10.1021/ml4002979 BindingDB Entry DOI: 10.7270/Q2GF0XGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25824 (5-{3-[4-(2,6-dimethylphenyl)-3-methoxyphenyl]but-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM134283 (US8853228, F26M) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25821 (5-(3-{3-[2,6-bis(propan-2-yl)phenyl]-5-methoxyphen...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25821 (5-(3-{3-[2,6-bis(propan-2-yl)phenyl]-5-methoxyphen...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134287 (US8853228, 150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM222178 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM134284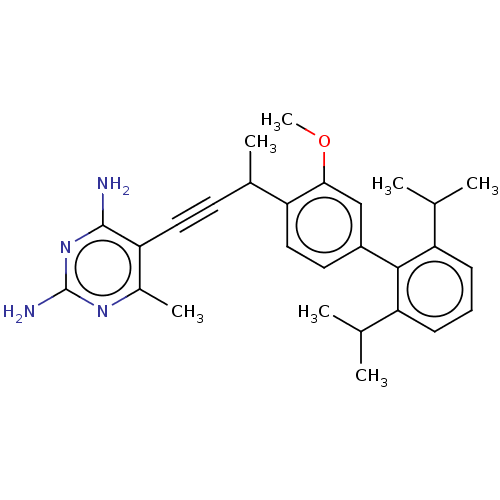 (US8853228, F26I) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25825 (5-(3-{4-[2,6-bis(propan-2-yl)phenyl]-3-methoxyphen...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429697 (CHEMBL2335419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25822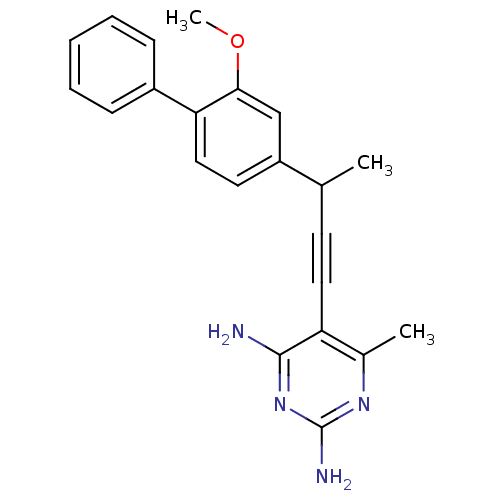 (5-[3-(3-methoxy-4-phenylphenyl)but-1-yn-1-yl]-6-me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM50329607 (5-(3-(3-methoxybiphenyl-4-yl)but-1-ynyl)-6-methylp...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134286 (US8853228, 149) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134288 (US8853228, 151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134286 (US8853228, 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50007817 (CHEMBL3234115) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134290 (US8853228, 155) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50329609 (5-(3-(biphenyl-3-yl)prop-1-ynyl)-6-ethylpyrimidine...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50429700 (CHEMBL2335416) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429698 (CHEMBL2335418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134285 (US8853228, 146) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329609 (5-(3-(biphenyl-3-yl)prop-1-ynyl)-6-ethylpyrimidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134287 (US8853228, 150) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429700 (CHEMBL2335416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134288 (US8853228, 151) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25827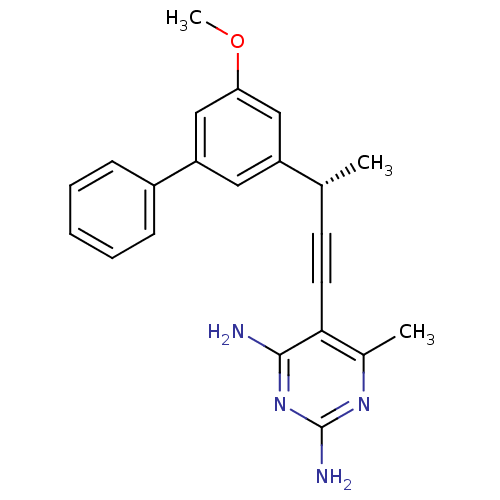 (5-[(3S)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25827 (5-[(3S)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134289 (US8853228, 154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134290 (US8853228, 155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25823 (5-{3-[3-methoxy-4-(2-methylphenyl)phenyl]but-1-yn-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM134282 (US8853228, F2M) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM18497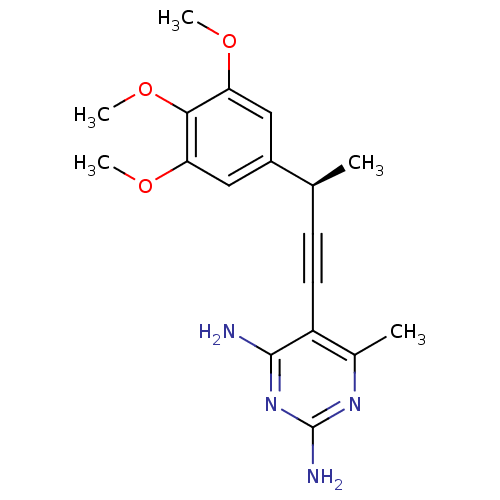 (6-methyl-5-[(3R)-3-(3,4,5-trimethoxyphenyl)but-1-y...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50298801 ((+/-)-5-(3-(5-methoxy-3',5'-dimethylbiphenyl-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |