

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021790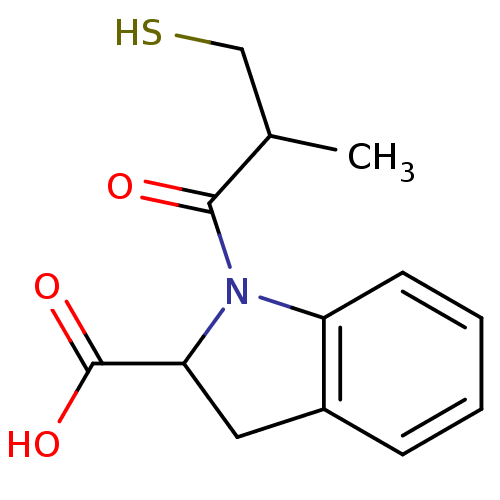 ((S,S)1-(3-Mercapto-2-methyl-propionyl)-2,3-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase in Escherichia coli | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021783 (1-(3-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004566 (9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367173 (CHEMBL1744333) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027697 (6-Fluoro-1-(3-mercapto-propionyl)-1,2,3,4-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367155 (CHEMBL1744329) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50023492 (5-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367149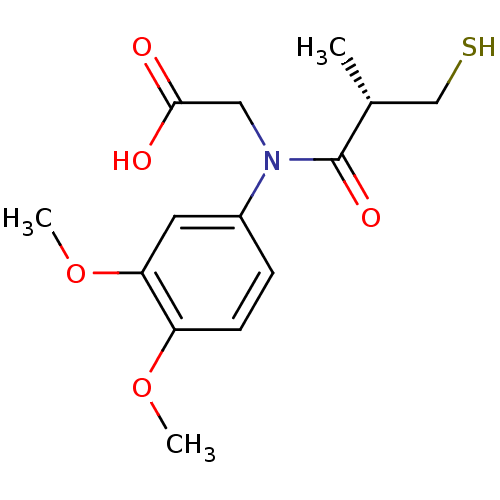 (CHEMBL1169540) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367146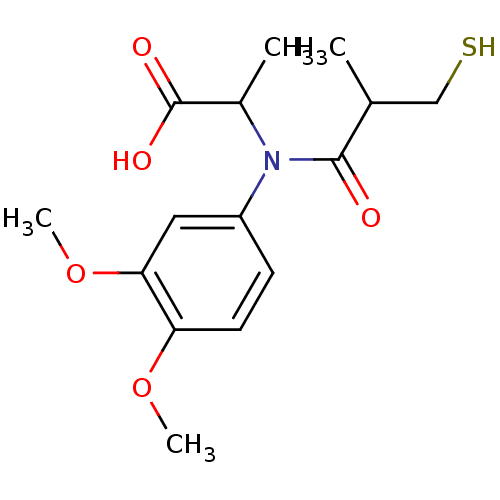 (CHEMBL1744332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10804 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020766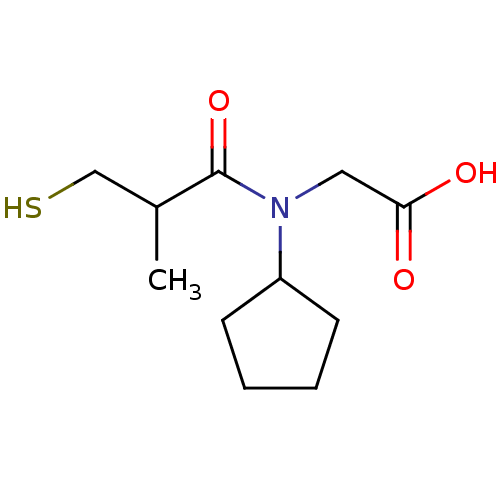 ((R+S)-[Cyclopentyl-(3-mercapto-2-methyl-propionyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020798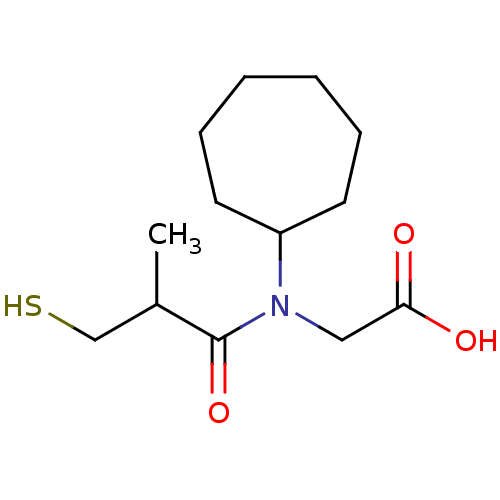 (CHEMBL1788148 | CHEMBL23841 | [Cycloheptyl-(3-merc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027736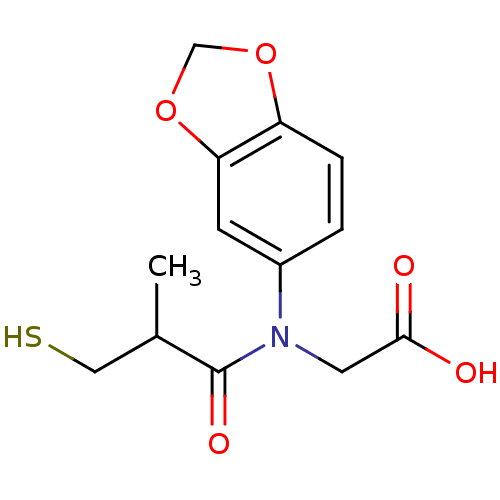 (CHEMBL22654 | [Benzo[1,3]dioxol-5-yl-(3-mercapto-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020768 (CHEMBL1788152 | CHEMBL23518 | [Cyclohexyl-(3-merca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367157 (CHEMBL1744325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367169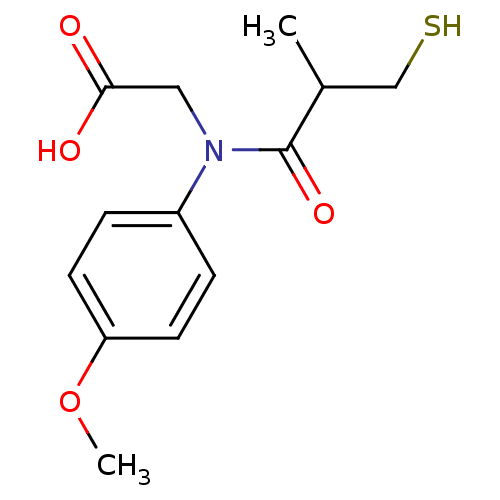 (CHEMBL1744334) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021783 (1-(3-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase in Lactobacillus casei | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10807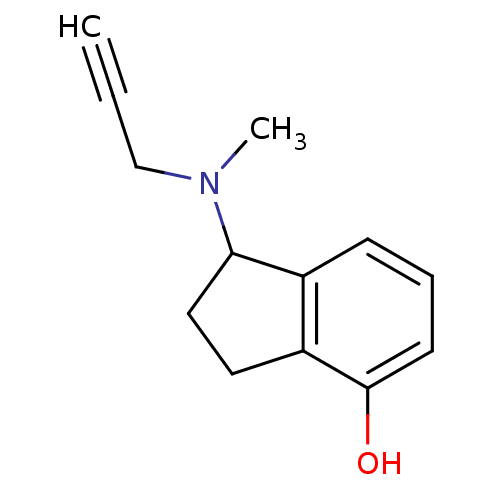 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027731 (1-(3-Mercapto-propionyl)-1,2,3,4-tetrahydro-quinol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase in Escherichia coli | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027717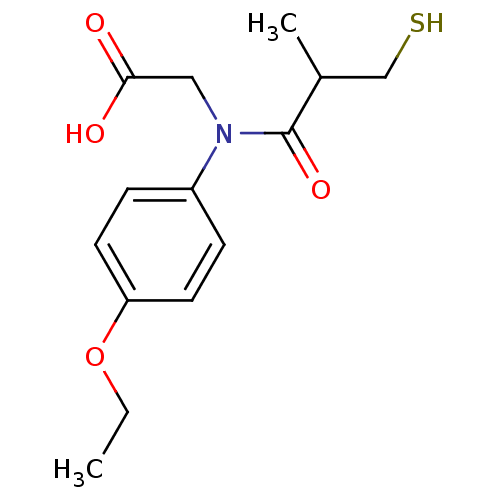 (CHEMBL282730 | [(4-Ethoxy-phenyl)-(3-mercapto-2-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020801 (CHEMBL23641 | [Indan-5-yl-(3-mercapto-2-methyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50023492 (5-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027716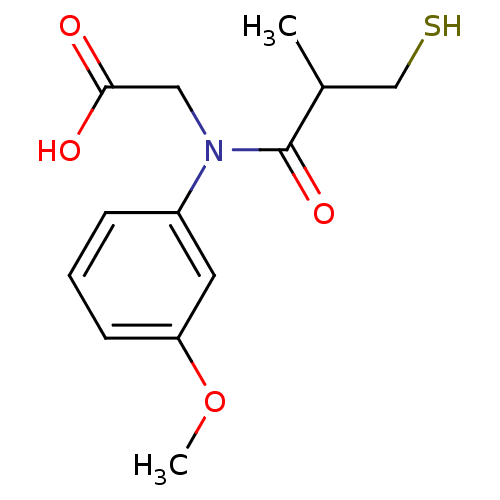 (CHEMBL23347 | [(3-Mercapto-2-methyl-propionyl)-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027696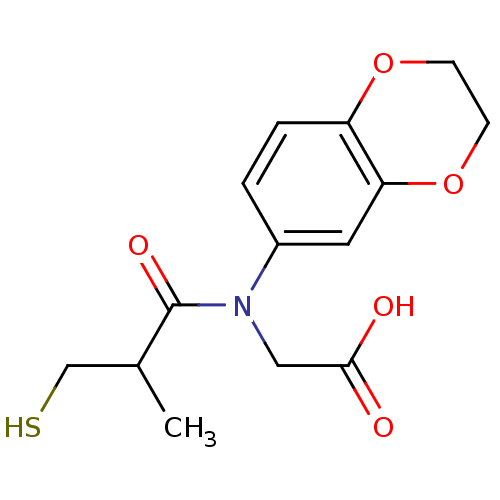 (CHEMBL23379 | [(2,3-Dihydro-benzo[1,4]dioxin-6-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020793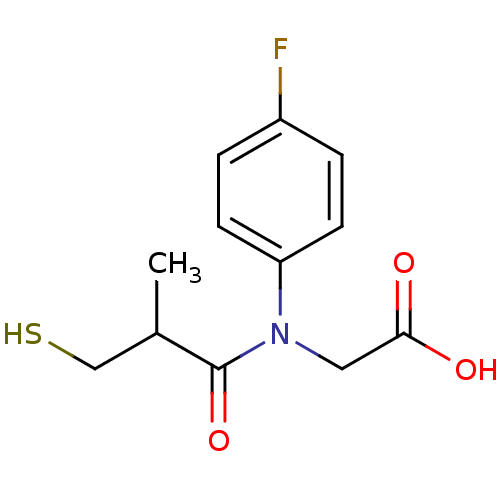 (CHEMBL23727 | [(4-Fluoro-phenyl)-(3-mercapto-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020808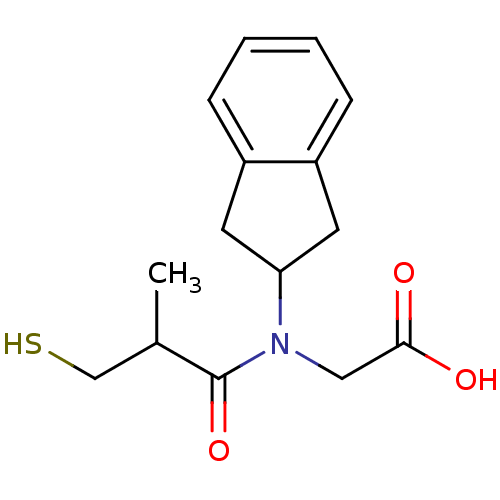 (CHEMBL1788147 | CHEMBL278348 | [Indan-2-yl-(3-merc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027721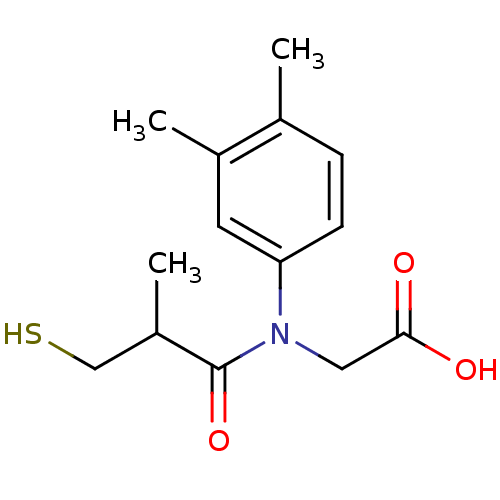 (CHEMBL279938 | [(3,4-Dimethyl-phenyl)-(3-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10796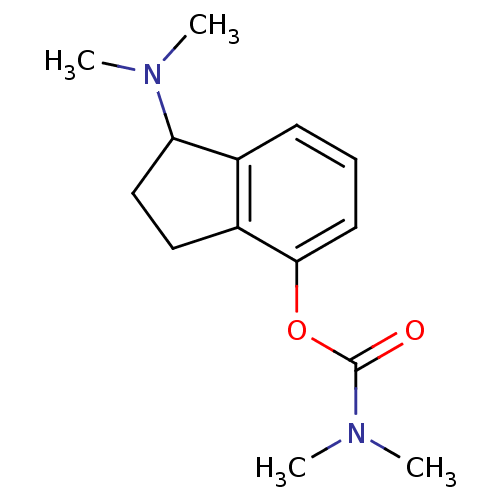 (1-(dimethylamino)-2,3-dihydro-1H-inden-4-yl N,N-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027731 (1-(3-Mercapto-propionyl)-1,2,3,4-tetrahydro-quinol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020800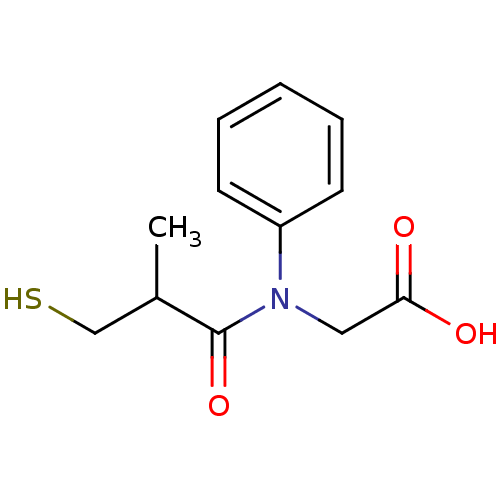 (CHEMBL26338 | [(3-Mercapto-2-methyl-propionyl)-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10803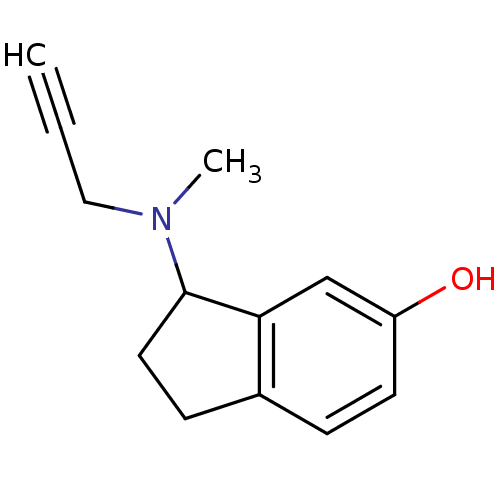 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027718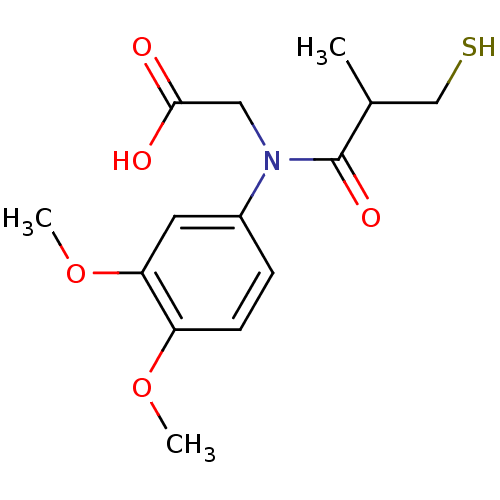 (CHEMBL280420 | [(3,4-Dimethoxy-phenyl)-(3-mercapto...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027722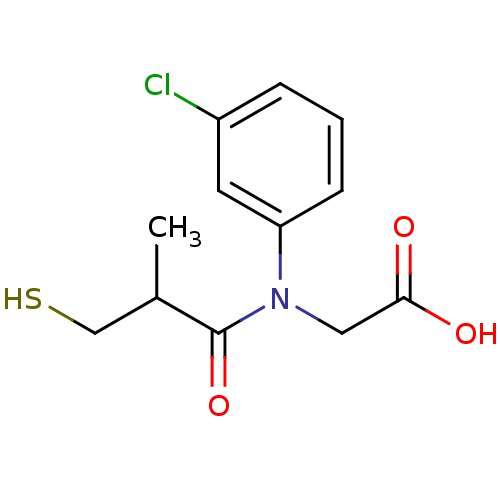 (CHEMBL24814 | [(3-Chloro-phenyl)-(3-mercapto-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367158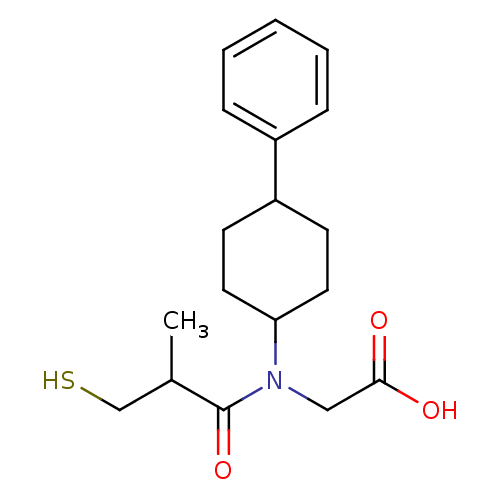 (CHEMBL1744320) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367156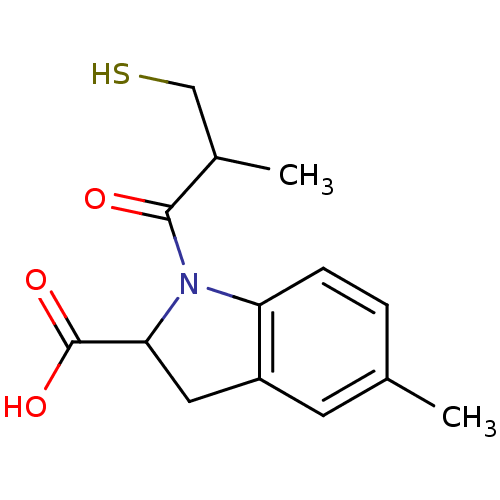 (CHEMBL1744327) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10784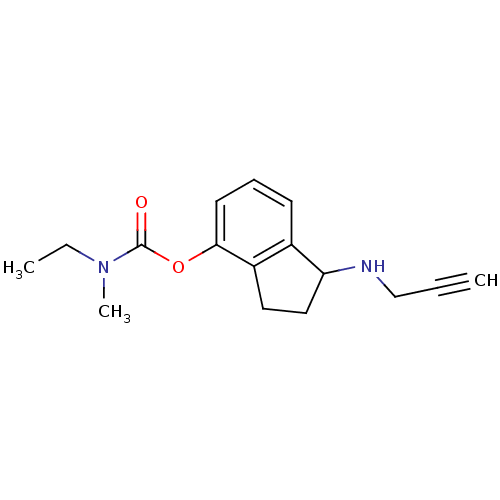 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367161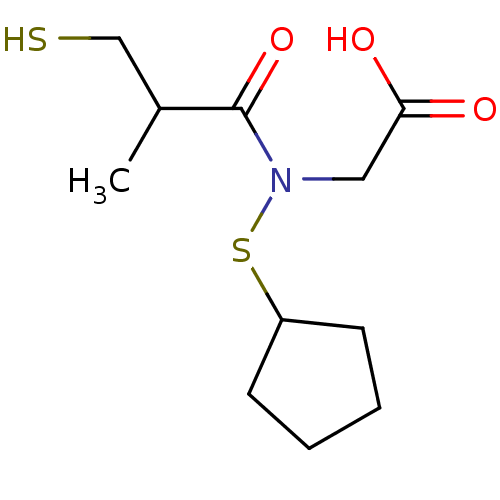 (CHEMBL1744326) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50023505 (2-Furan-2-yl-9-methoxy-[1,2,4]triazolo[1,5-c]quina...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10804 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021523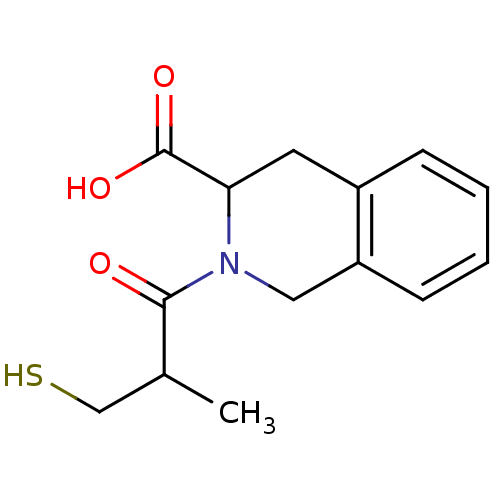 ((S,S) 2-(3-Mercapto-2-methyl-propionyl)-1,2,3,4-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase in Lactobacillus casei | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367148 (CHEMBL1744336) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50023495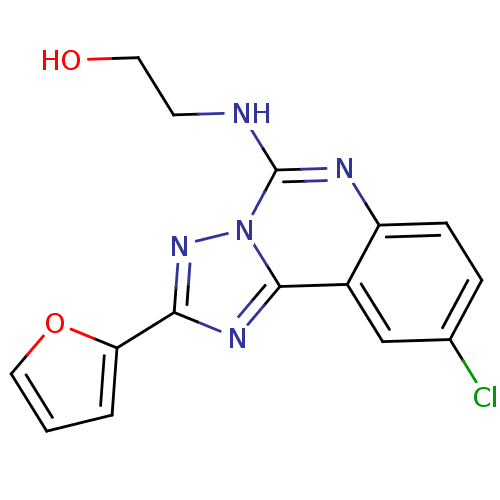 (2-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004566 (9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50023500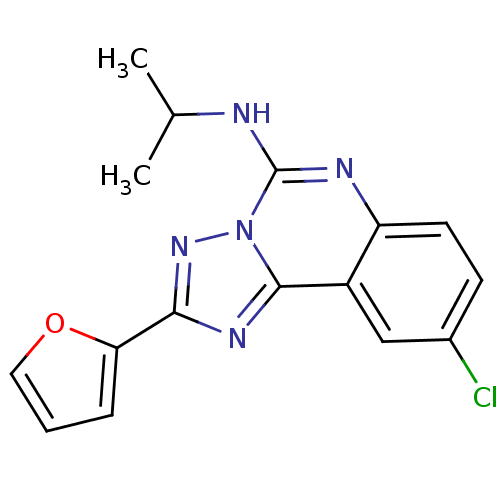 ((9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quina...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. | J Med Chem 31: 1014-20 (1988) BindingDB Entry DOI: 10.7270/Q2T43S4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027698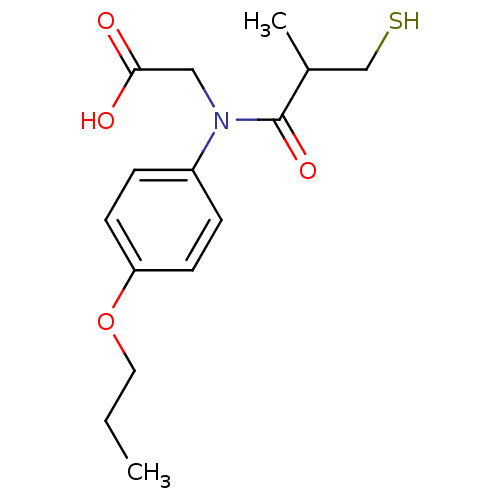 (CHEMBL23534 | [(3-Mercapto-2-methyl-propionyl)-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021790 ((S,S)1-(3-Mercapto-2-methyl-propionyl)-2,3-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367153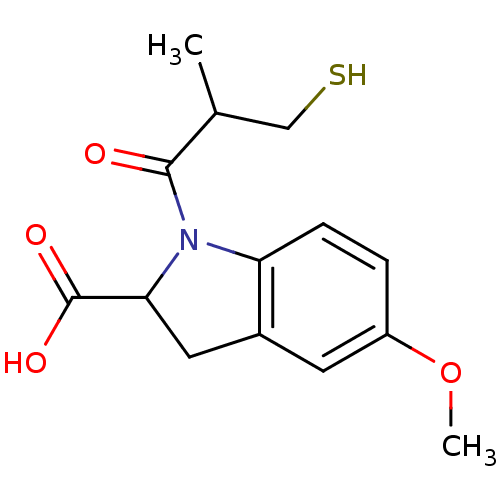 (CHEMBL1744324) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 395 total ) | Next | Last >> |