 Found 335 hits with Last Name = 'fujii' and Initial = 'k'
Found 335 hits with Last Name = 'fujii' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142001
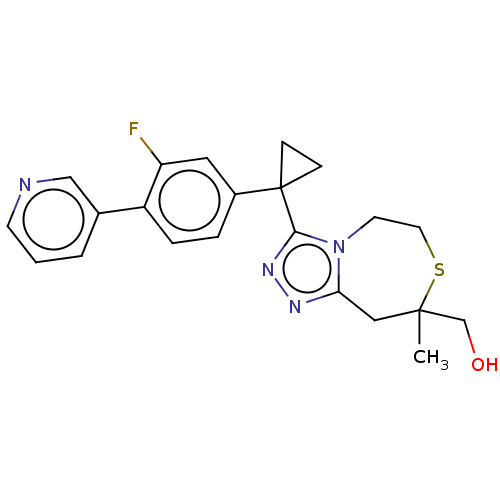
(US8927536, 26)Show SMILES CC1(CO)Cc2nnc(n2CCS1)C1(CC1)c1ccc(c(F)c1)-c1cccnc1 Show InChI InChI=1S/C22H23FN4OS/c1-21(14-28)12-19-25-26-20(27(19)9-10-29-21)22(6-7-22)16-4-5-17(18(23)11-16)15-3-2-8-24-13-15/h2-5,8,11,13,28H,6-7,9-10,12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142003
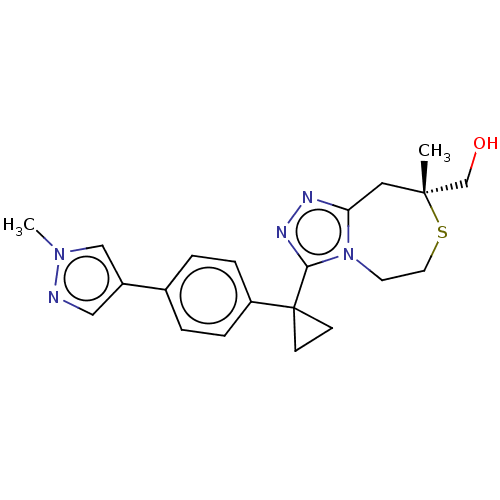
(US8927536, 34)Show SMILES Cn1cc(cn1)-c1ccc(cc1)C1(CC1)c1nnc2C[C@](C)(CO)SCCn12 |r| Show InChI InChI=1S/C21H25N5OS/c1-20(14-27)11-18-23-24-19(26(18)9-10-28-20)21(7-8-21)17-5-3-15(4-6-17)16-12-22-25(2)13-16/h3-6,12-13,27H,7-11,14H2,1-2H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM141998

(US8927536, 4)Show SMILES C[C@]1(CO)Cc2nnc(n2CCS1)C1(CC1)c1ccc(cc1)-c1cccnc1 |r| Show InChI InChI=1S/C22H24N4OS/c1-21(15-27)13-19-24-25-20(26(19)11-12-28-21)22(8-9-22)18-6-4-16(5-7-18)17-3-2-10-23-14-17/h2-7,10,14,27H,8-9,11-13,15H2,1H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142008

(US8927536, 89)Show SMILES Cc1ccc(nn1)-c1ccc(c(F)c1)C1(CC1)c1nnc2C[C@](C)(CO)SCCn12 |r| Show InChI InChI=1S/C22H24FN5OS/c1-14-3-6-18(25-24-14)15-4-5-16(17(23)11-15)22(7-8-22)20-27-26-19-12-21(2,13-29)30-10-9-28(19)20/h3-6,11,29H,7-10,12-13H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142007
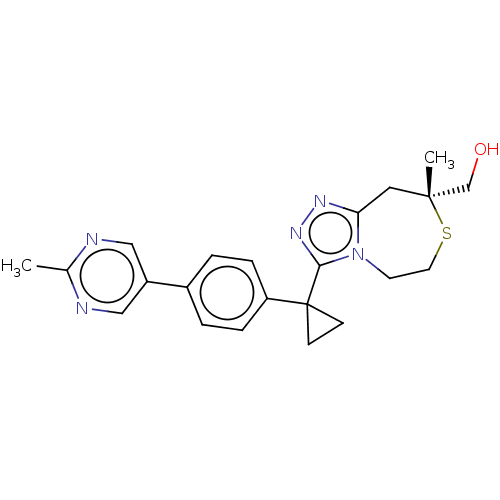
(US8927536, 88)Show SMILES Cc1ncc(cn1)-c1ccc(cc1)C1(CC1)c1nnc2C[C@](C)(CO)SCCn12 |r| Show InChI InChI=1S/C22H25N5OS/c1-15-23-12-17(13-24-15)16-3-5-18(6-4-16)22(7-8-22)20-26-25-19-11-21(2,14-28)29-10-9-27(19)20/h3-6,12-13,28H,7-11,14H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142000
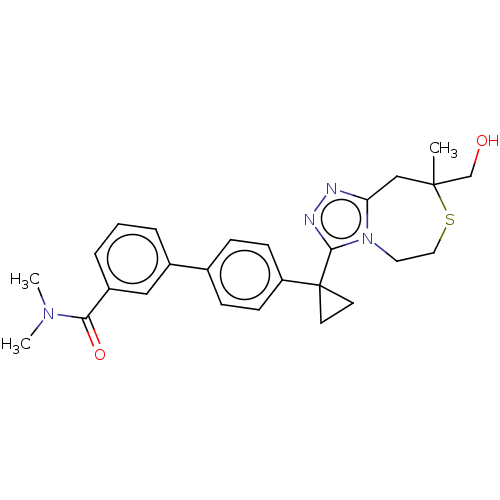
(US8927536, 9)Show SMILES CN(C)C(=O)c1cccc(c1)-c1ccc(cc1)C1(CC1)c1nnc2CC(C)(CO)SCCn12 Show InChI InChI=1S/C26H30N4O2S/c1-25(17-31)16-22-27-28-24(30(22)13-14-33-25)26(11-12-26)21-9-7-18(8-10-21)19-5-4-6-20(15-19)23(32)29(2)3/h4-10,15,31H,11-14,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142002
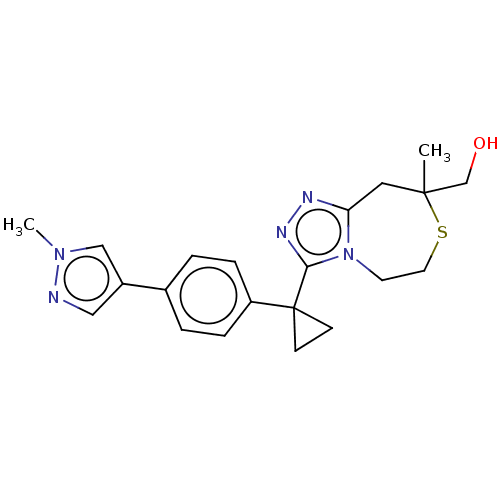
(US8927536, 32)Show SMILES Cn1cc(cn1)-c1ccc(cc1)C1(CC1)c1nnc2CC(C)(CO)SCCn12 Show InChI InChI=1S/C21H25N5OS/c1-20(14-27)11-18-23-24-19(26(18)9-10-28-20)21(7-8-21)17-5-3-15(4-6-17)16-12-22-25(2)13-16/h3-6,12-13,27H,7-11,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142006
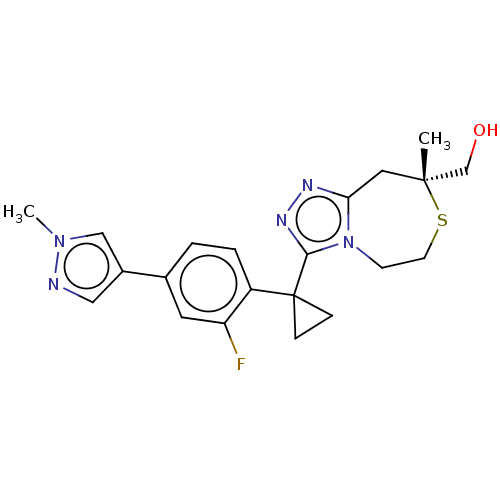
(US8927536, 81)Show SMILES Cn1cc(cn1)-c1ccc(c(F)c1)C1(CC1)c1nnc2C[C@](C)(CO)SCCn12 |r| Show InChI InChI=1S/C21H24FN5OS/c1-20(13-28)10-18-24-25-19(27(18)7-8-29-20)21(5-6-21)16-4-3-14(9-17(16)22)15-11-23-26(2)12-15/h3-4,9,11-12,28H,5-8,10,13H2,1-2H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM141999
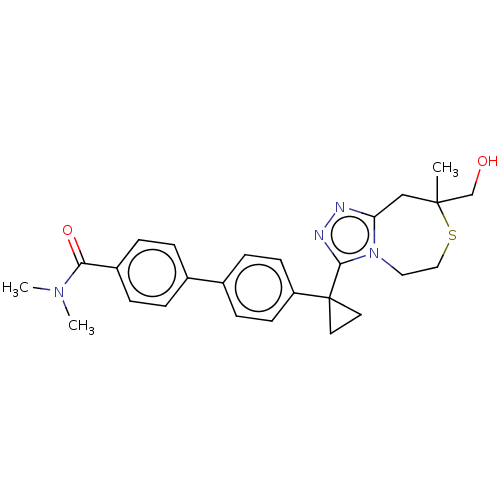
(US8927536, 6)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc(cc1)C1(CC1)c1nnc2CC(C)(CO)SCCn12 Show InChI InChI=1S/C26H30N4O2S/c1-25(17-31)16-22-27-28-24(30(22)14-15-33-25)26(12-13-26)21-10-8-19(9-11-21)18-4-6-20(7-5-18)23(32)29(2)3/h4-11,31H,12-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM141996

(US8927536, 2)Show SMILES CC1(CO)Cc2nnc(n2CCS1)C1(CC1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C22H24N4OS/c1-21(15-27)13-19-24-25-20(26(19)11-12-28-21)22(8-9-22)18-6-4-16(5-7-18)17-3-2-10-23-14-17/h2-7,10,14,27H,8-9,11-13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM141995
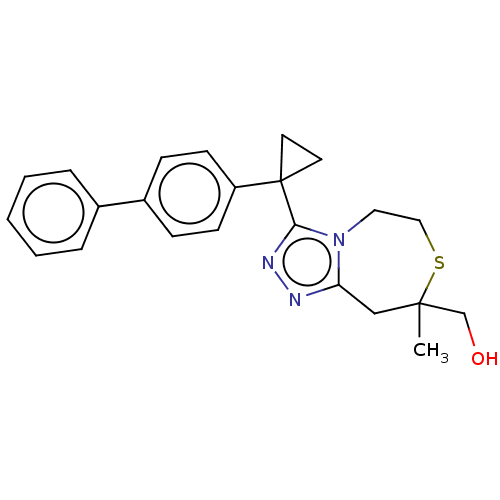
(US8927536, 1)Show SMILES CC1(CO)Cc2nnc(n2CCS1)C1(CC1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H25N3OS/c1-22(16-27)15-20-24-25-21(26(20)13-14-28-22)23(11-12-23)19-9-7-18(8-10-19)17-5-3-2-4-6-17/h2-10,27H,11-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142004
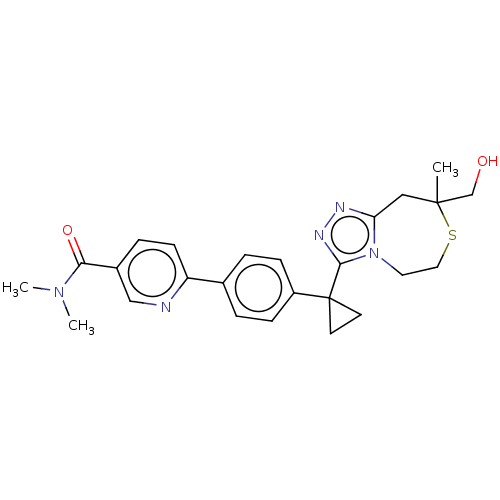
(US8927536, 67)Show SMILES CN(C)C(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)c1nnc2CC(C)(CO)SCCn12 Show InChI InChI=1S/C25H29N5O2S/c1-24(16-31)14-21-27-28-23(30(21)12-13-33-24)25(10-11-25)19-7-4-17(5-8-19)20-9-6-18(15-26-20)22(32)29(2)3/h4-9,15,31H,10-14,16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM141997
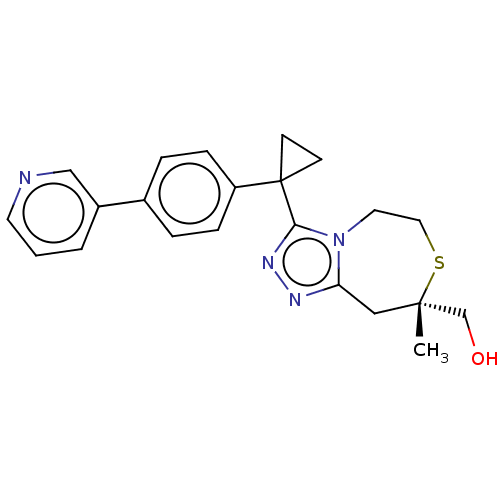
(US8927536, 3)Show SMILES C[C@@]1(CO)Cc2nnc(n2CCS1)C1(CC1)c1ccc(cc1)-c1cccnc1 |r| Show InChI InChI=1S/C22H24N4OS/c1-21(15-27)13-19-24-25-20(26(19)11-12-28-21)22(8-9-22)18-6-4-16(5-7-18)17-3-2-10-23-14-17/h2-7,10,14,27H,8-9,11-13,15H2,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM142005
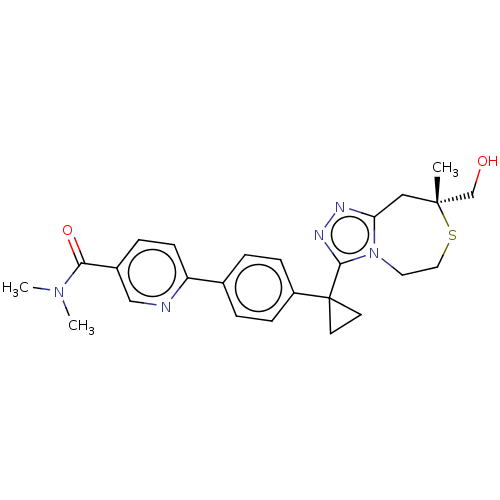
(US8927536, 68)Show SMILES CN(C)C(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)c1nnc2C[C@](C)(CO)SCCn12 |r| Show InChI InChI=1S/C25H29N5O2S/c1-24(16-31)14-21-27-28-23(30(21)12-13-33-24)25(10-11-25)19-7-4-17(5-8-19)20-9-6-18(15-26-20)22(32)29(2)3/h4-9,15,31H,10-14,16H2,1-3H3/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 無, and all the samples were diluted with an assay buffe... |
US Patent US8927536 (2015)
BindingDB Entry DOI: 10.7270/Q2Q23XZP |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550011
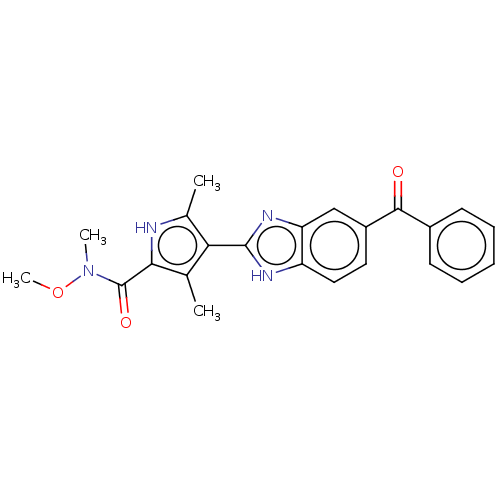
(CHEMBL4747168)Show SMILES Cl.CON(C)C(=O)c1[nH]c(C)c(c1C)-c1nc2cc(ccc2[nH]1)C(=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
N-formyl peptide receptor 2
(Homo sapiens (Human)) | BDBM50559829
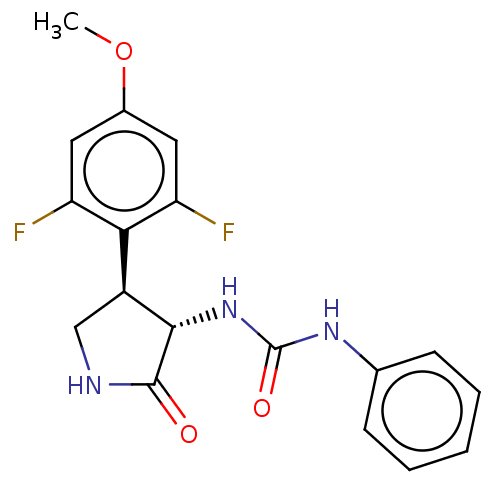
(CHEMBL4784510)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccccc2)c(F)c1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at FPR2 in human HL-60 cells assessed as reduction in chemoattractant induced chemotaxis by luminescence cell viability assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722
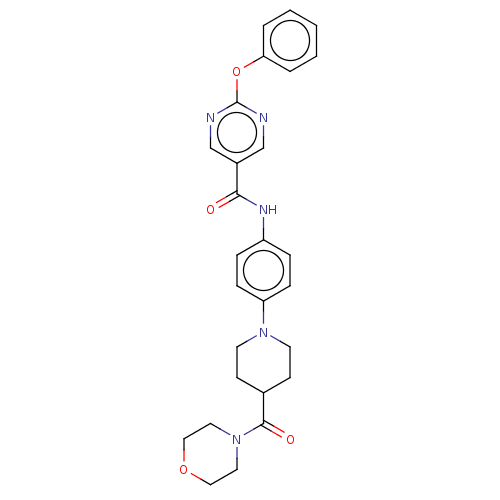
(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 3 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722

(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS using [14C]-PGH2 as substrate preincubated for 1 min in presence of MgCl2 followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50587271

(CHEMBL5075403)Show SMILES CN1C(=O)CCC(N2C(=O)c3cccc(N4CCN(CC4)C(=O)C4CCN(CC4)c4ccc(NC(=O)c5cnc(Oc6ccccc6)nc5)cc4)c3C2=O)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of H-PGDS (unknown origin) using fluorescent probe by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01206
BindingDB Entry DOI: 10.7270/Q2T157KT |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722

(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of H-PGDS (unknown origin) using fluorescent probe by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01206
BindingDB Entry DOI: 10.7270/Q2T157KT |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50587270
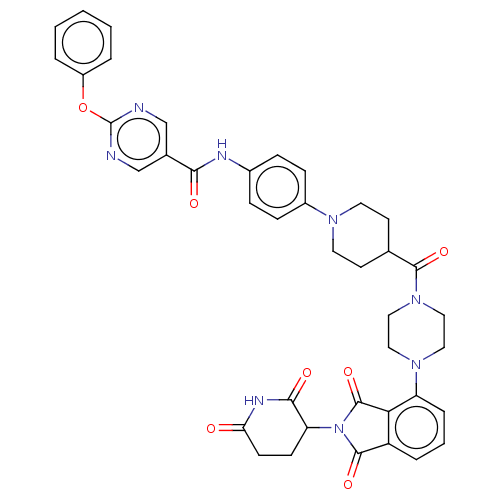
(CHEMBL5076100)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCN(CC1)c1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)c1cnc(Oc2ccccc2)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of H-PGDS (unknown origin) using fluorescent probe by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01206
BindingDB Entry DOI: 10.7270/Q2T157KT |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550010

(CHEMBL4762935)Show SMILES O=C(CCOCCOCCOCCOCCOCCNc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)N1CCN(CC1)C(=O)C1CCN(CC1)c1ccc(NC(=O)c2cnc(Oc3ccccc3)nc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 3 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550009

(CHEMBL4758395)Show SMILES CN1C(=O)CCC(N2C(=O)c3cccc(NCCOCCOCCOCCOCCOCCC(=O)N4CCN(CC4)C(=O)C4CCN(CC4)c4ccc(NC(=O)c5cnc(Oc6ccccc6)nc5)cc4)c3C2=O)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 1 hr by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550009

(CHEMBL4758395)Show SMILES CN1C(=O)CCC(N2C(=O)c3cccc(NCCOCCOCCOCCOCCOCCC(=O)N4CCN(CC4)C(=O)C4CCN(CC4)c4ccc(NC(=O)c5cnc(Oc6ccccc6)nc5)cc4)c3C2=O)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 3 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550010

(CHEMBL4762935)Show SMILES O=C(CCOCCOCCOCCOCCOCCNc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)N1CCN(CC1)C(=O)C1CCN(CC1)c1ccc(NC(=O)c2cnc(Oc3ccccc3)nc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 1 hr by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722

(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 1 hr by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50559840
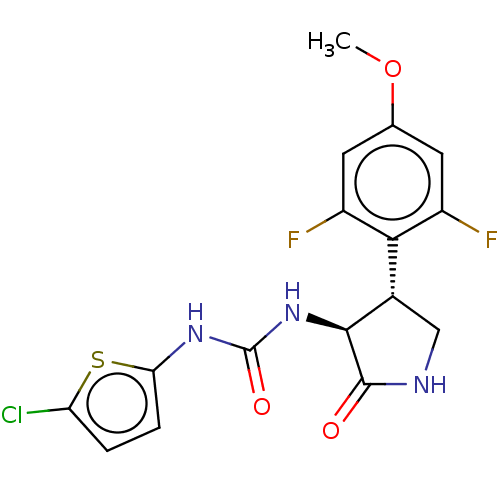
(CHEMBL4760897)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccc(Cl)s2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50559838
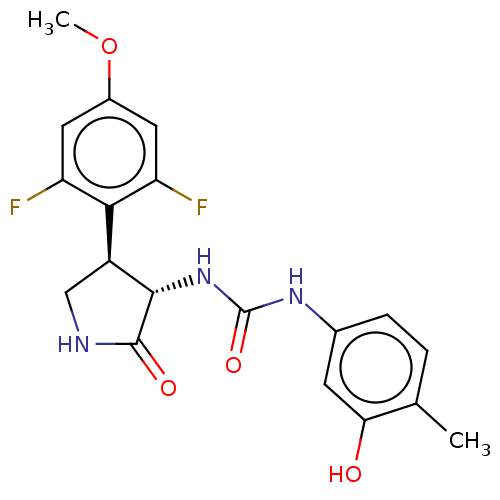
(CHEMBL4790836)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccc(C)c(O)c2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50559835
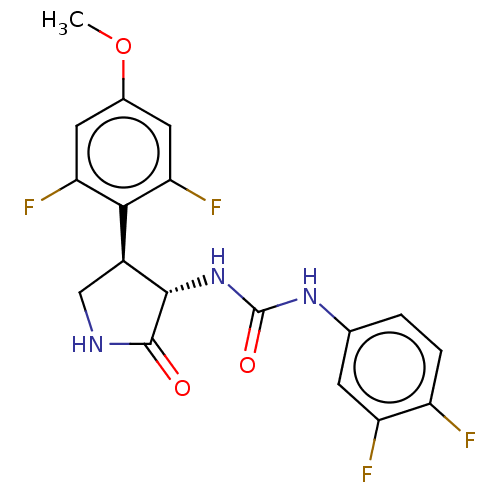
(CHEMBL4762030)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccc(F)c(F)c2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50559829

(CHEMBL4784510)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccccc2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50559821
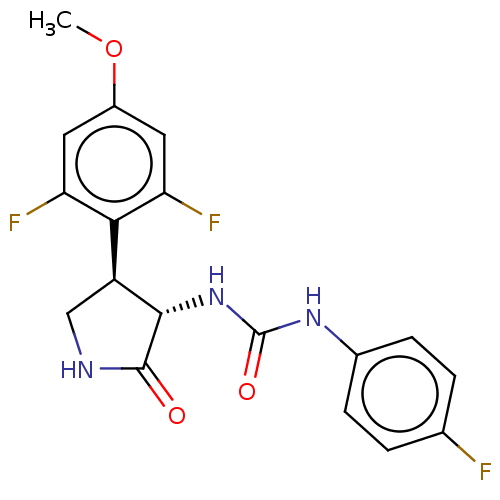
(CHEMBL4781596)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccc(F)cc2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50450105

(CHEMBL4161810)Show SMILES Cc1cccc(Oc2ccc3nc(COc4cccc(c4)C(O)=O)n(C)c3c2)n1 Show InChI InChI=1S/C22H19N3O4/c1-14-5-3-8-21(23-14)29-17-9-10-18-19(12-17)25(2)20(24-18)13-28-16-7-4-6-15(11-16)22(26)27/h3-12H,13H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARalpha LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50450106

(CHEMBL4162863)Show SMILES Cc1cccnc1Oc1ccc2nc(COc3cccc(c3)C(O)=O)n(C)c2c1 Show InChI InChI=1S/C22H19N3O4/c1-14-5-4-10-23-21(14)29-17-8-9-18-19(12-17)25(2)20(24-18)13-28-16-7-3-6-15(11-16)22(26)27/h3-12H,13H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARalpha LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50450107
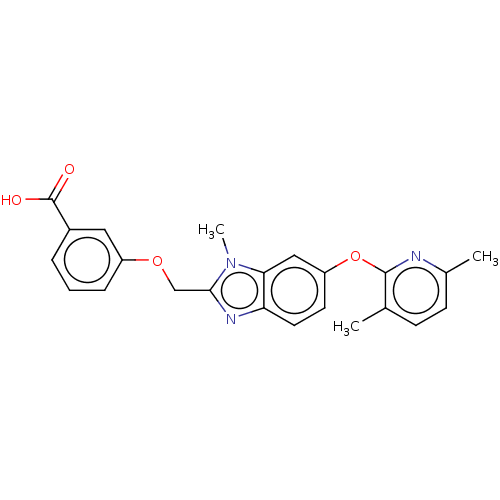
(CHEMBL4164828)Show SMILES Cc1ccc(C)c(Oc2ccc3nc(COc4cccc(c4)C(O)=O)n(C)c3c2)n1 Show InChI InChI=1S/C23H21N3O4/c1-14-7-8-15(2)24-22(14)30-18-9-10-19-20(12-18)26(3)21(25-19)13-29-17-6-4-5-16(11-17)23(27)28/h4-12H,13H2,1-3H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARalpha LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450108
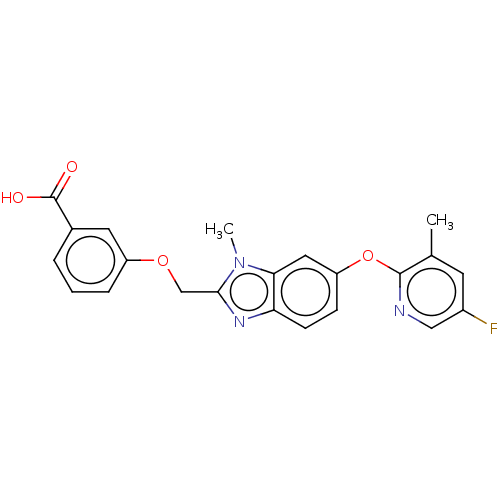
(CHEMBL4176941)Show SMILES Cc1cc(F)cnc1Oc1ccc2nc(COc3cccc(c3)C(O)=O)n(C)c2c1 Show InChI InChI=1S/C22H18FN3O4/c1-13-8-15(23)11-24-21(13)30-17-6-7-18-19(10-17)26(2)20(25-18)12-29-16-5-3-4-14(9-16)22(27)28/h3-11H,12H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50030474
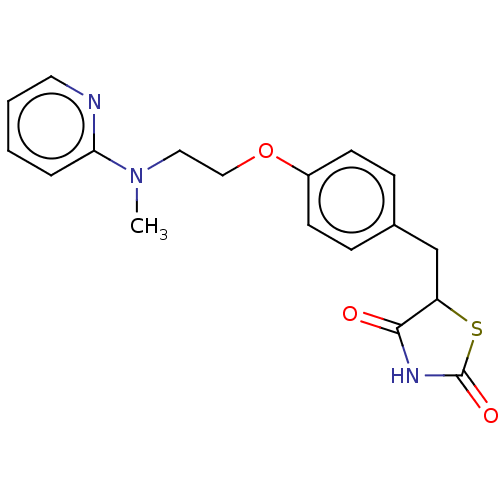
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 396 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450109

(CHEMBL4161905)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3ncccc3F)cc12 Show InChI InChI=1S/C21H16FN3O4/c1-25-18-11-15(29-20-16(22)6-3-9-23-20)7-8-17(18)24-19(25)12-28-14-5-2-4-13(10-14)21(26)27/h2-11H,12H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450110
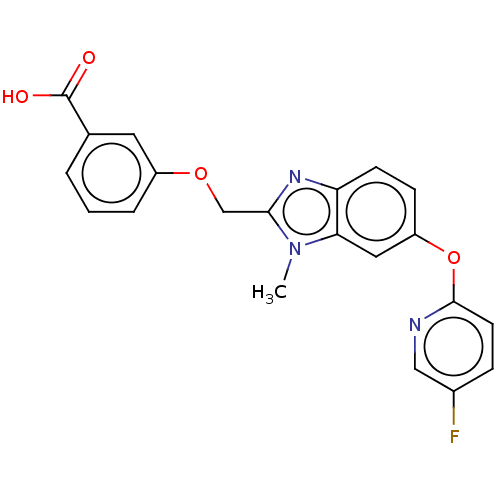
(CHEMBL4172034)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3ccc(F)cn3)cc12 Show InChI InChI=1S/C21H16FN3O4/c1-25-18-10-16(29-20-8-5-14(22)11-23-20)6-7-17(18)24-19(25)12-28-15-4-2-3-13(9-15)21(26)27/h2-11H,12H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 808 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450106

(CHEMBL4162863)Show SMILES Cc1cccnc1Oc1ccc2nc(COc3cccc(c3)C(O)=O)n(C)c2c1 Show InChI InChI=1S/C22H19N3O4/c1-14-5-4-10-23-21(14)29-17-8-9-18-19(12-17)25(2)20(24-18)13-28-16-7-3-6-15(11-16)22(26)27/h3-12H,13H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 351 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450111

(CHEMBL4176938)Show SMILES Cc1ccnc(Oc2ccc3nc(COc4cccc(c4)C(O)=O)n(C)c3c2)c1 Show InChI InChI=1S/C22H19N3O4/c1-14-8-9-23-21(10-14)29-17-6-7-18-19(12-17)25(2)20(24-18)13-28-16-5-3-4-15(11-16)22(26)27/h3-12H,13H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 458 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450112
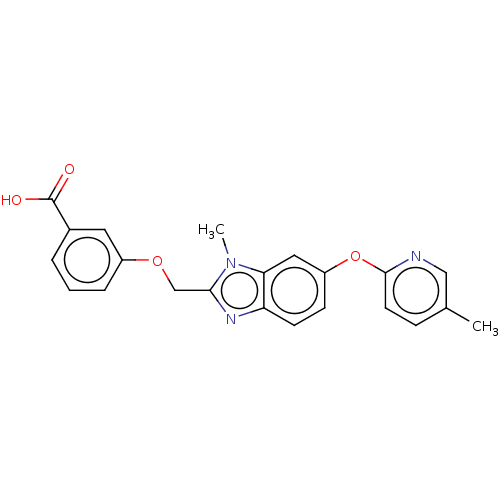
(CHEMBL4165766)Show SMILES Cc1ccc(Oc2ccc3nc(COc4cccc(c4)C(O)=O)n(C)c3c2)nc1 Show InChI InChI=1S/C22H19N3O4/c1-14-6-9-21(23-12-14)29-17-7-8-18-19(11-17)25(2)20(24-18)13-28-16-5-3-4-15(10-16)22(26)27/h3-12H,13H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 129 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450113

(CHEMBL4167706)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3ncccc3Cl)cc12 Show InChI InChI=1S/C21H16ClN3O4/c1-25-18-11-15(29-20-16(22)6-3-9-23-20)7-8-17(18)24-19(25)12-28-14-5-2-4-13(10-14)21(26)27/h2-11H,12H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450114
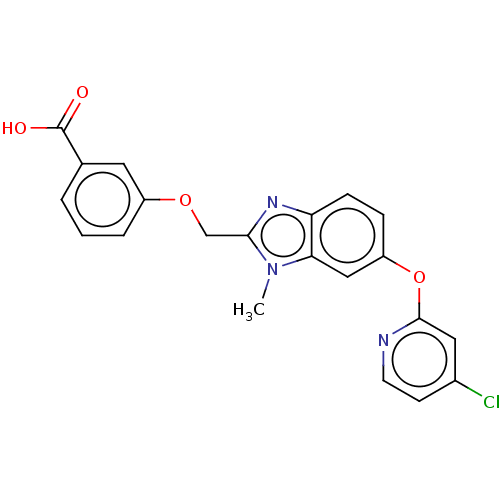
(CHEMBL4168900)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3cc(Cl)ccn3)cc12 Show InChI InChI=1S/C21H16ClN3O4/c1-25-18-11-16(29-20-10-14(22)7-8-23-20)5-6-17(18)24-19(25)12-28-15-4-2-3-13(9-15)21(26)27/h2-11H,12H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 615 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450115
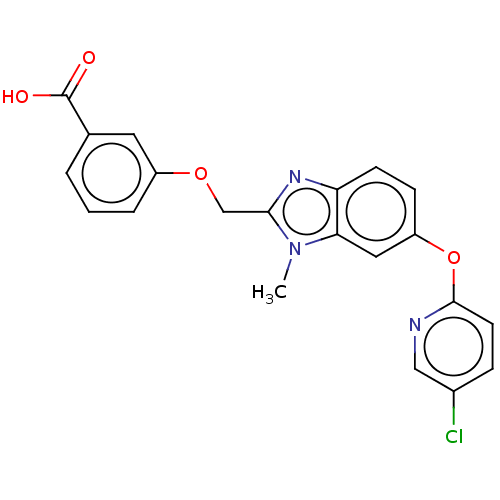
(CHEMBL4176762)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3ccc(Cl)cn3)cc12 Show InChI InChI=1S/C21H16ClN3O4/c1-25-18-10-16(29-20-8-5-14(22)11-23-20)6-7-17(18)24-19(25)12-28-15-4-2-3-13(9-15)21(26)27/h2-11H,12H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450116
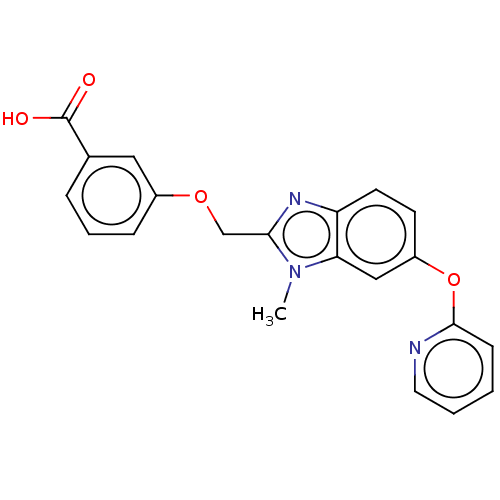
(CHEMBL4159720)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3ccccn3)cc12 Show InChI InChI=1S/C21H17N3O4/c1-24-18-12-16(28-20-7-2-3-10-22-20)8-9-17(18)23-19(24)13-27-15-6-4-5-14(11-15)21(25)26/h2-12H,13H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450117
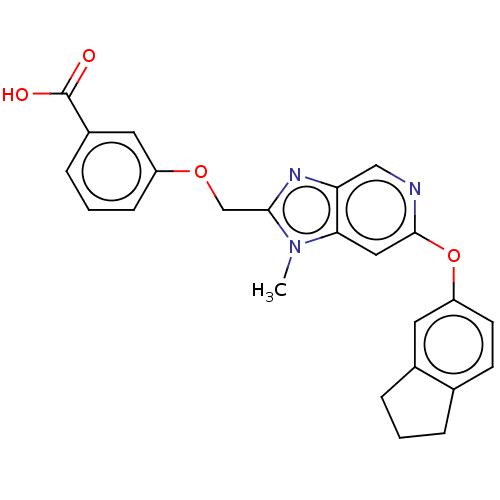
(CHEMBL4166946)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2cnc(Oc3ccc4CCCc4c3)cc12 Show InChI InChI=1S/C24H21N3O4/c1-27-21-12-23(31-19-9-8-15-4-2-5-16(15)10-19)25-13-20(21)26-22(27)14-30-18-7-3-6-17(11-18)24(28)29/h3,6-13H,2,4-5,14H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450118

(CHEMBL4177154)Show SMILES Cc1ccc(Oc2cc3n(C)c(COc4cccc(c4)C(O)=O)nc3cn2)cc1 Show InChI InChI=1S/C22H19N3O4/c1-14-6-8-16(9-7-14)29-21-11-19-18(12-23-21)24-20(25(19)2)13-28-17-5-3-4-15(10-17)22(26)27/h3-12H,13H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 746 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450119

(CHEMBL4167180)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2cnc(Oc3ccc(Cl)c(F)c3)cc12 Show InChI InChI=1S/C21H15ClFN3O4/c1-26-18-9-20(30-14-5-6-15(22)16(23)8-14)24-10-17(18)25-19(26)11-29-13-4-2-3-12(7-13)21(27)28/h2-10H,11H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450120
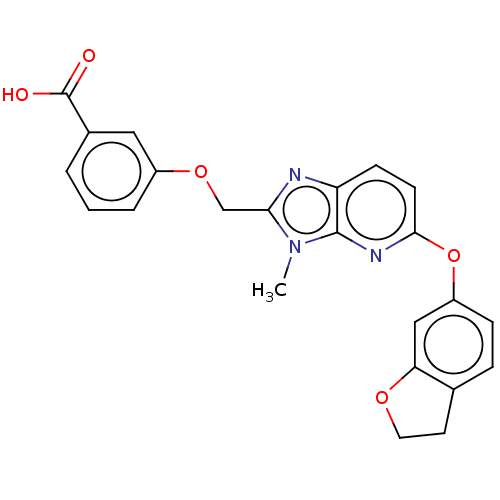
(CHEMBL4173802)Show SMILES Cn1c(COc2cccc(c2)C(O)=O)nc2ccc(Oc3ccc4CCOc4c3)nc12 Show InChI InChI=1S/C23H19N3O5/c1-26-20(13-30-16-4-2-3-15(11-16)23(27)28)24-18-7-8-21(25-22(18)26)31-17-6-5-14-9-10-29-19(14)12-17/h2-8,11-12H,9-10,13H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450121

(CHEMBL4167767)Show SMILES Cc1ccc(Oc2ccc3nc(COc4cccc(c4)C(O)=O)n(C)c3n2)cc1C Show InChI InChI=1S/C23H21N3O4/c1-14-7-8-18(11-15(14)2)30-21-10-9-19-22(25-21)26(3)20(24-19)13-29-17-6-4-5-16(12-17)23(27)28/h4-12H,13H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5099-5117 (2018)
Article DOI: 10.1016/j.bmc.2018.09.005
BindingDB Entry DOI: 10.7270/Q2XK8J3V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data