

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337114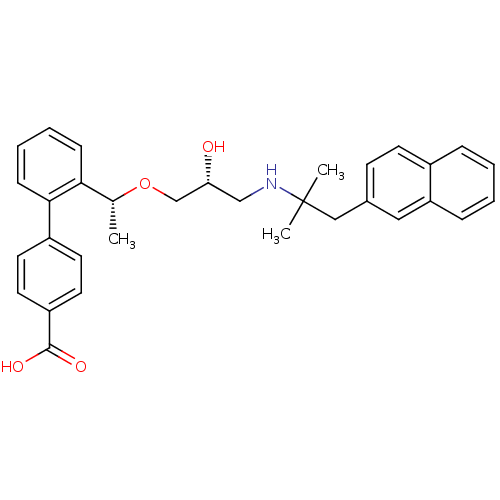 (2'-((1R)-1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337116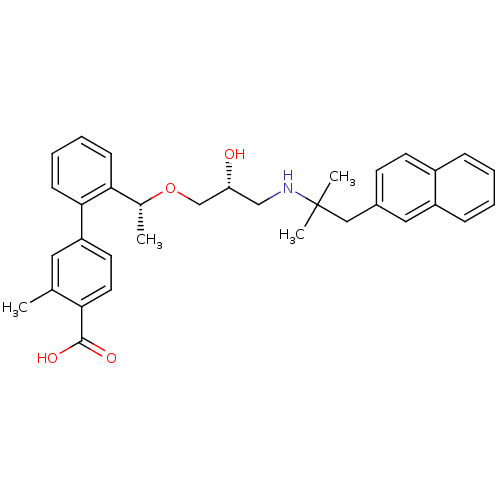 (2'-((1R)-1-{(2R)-3-[2-methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337113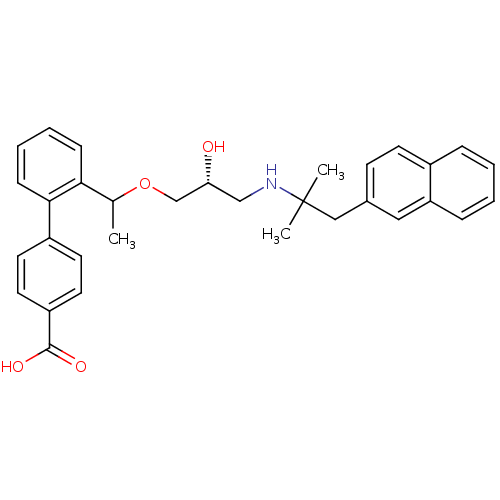 ((RS)-2'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337103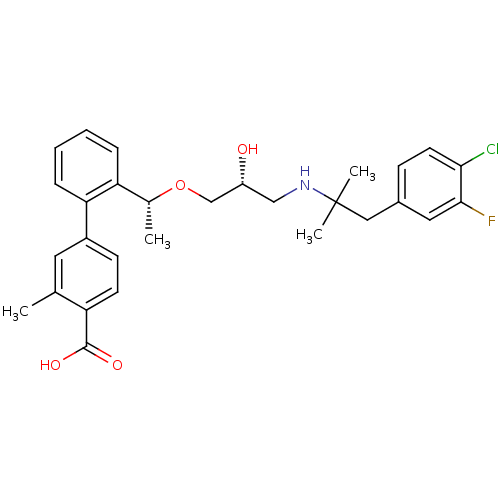 (2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337112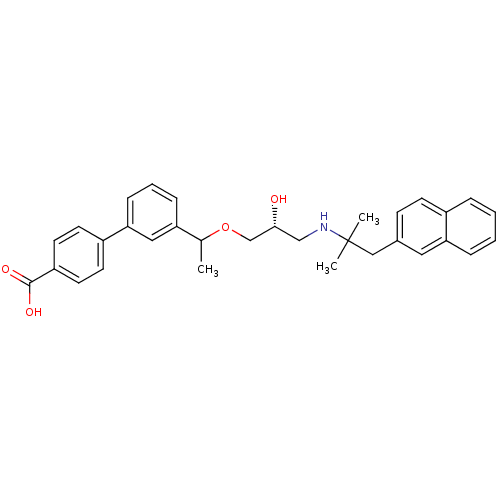 ((RS)-3'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337105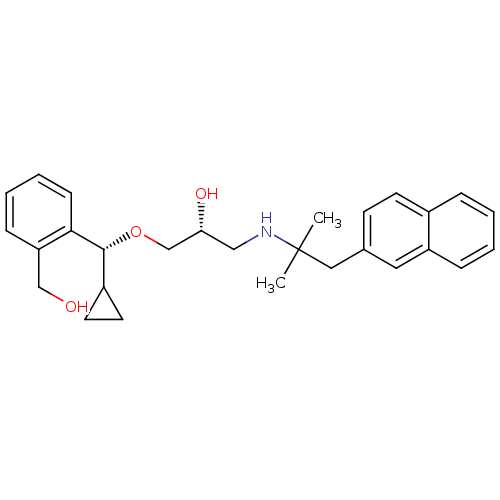 ((R)-1-((R)-cyclopropyl(2-(hydroxymethyl)phenyl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337115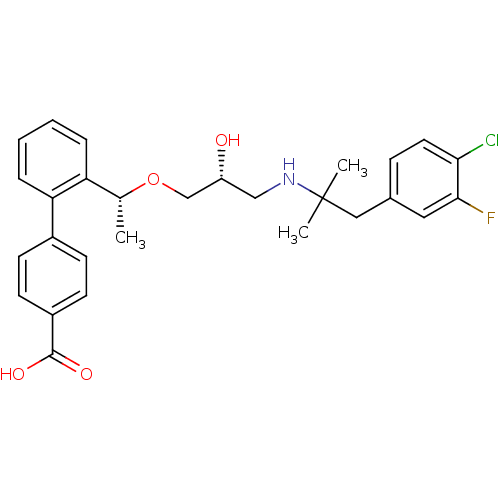 (2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320009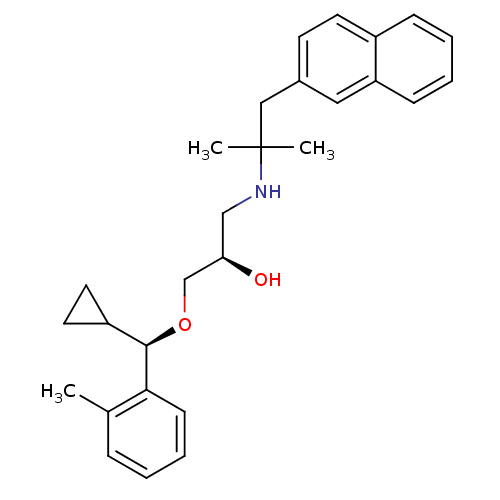 ((R)-1-((R)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337104 (3-[2-((1R)-1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283464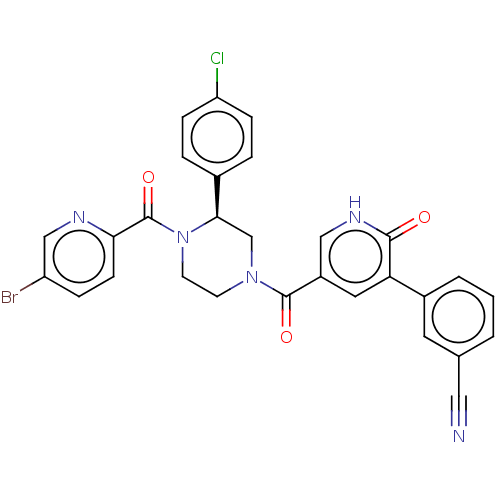 (CHEMBL4162764) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283443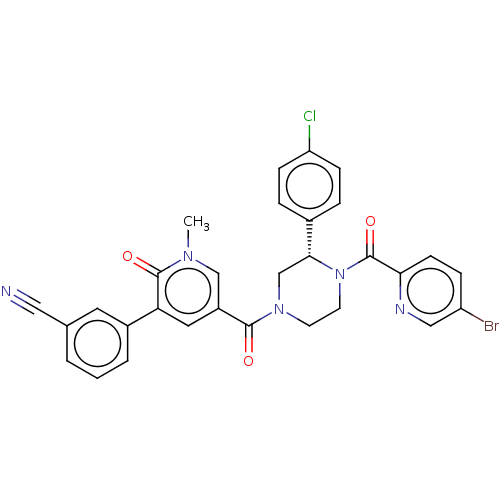 (CHEMBL4170030) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283445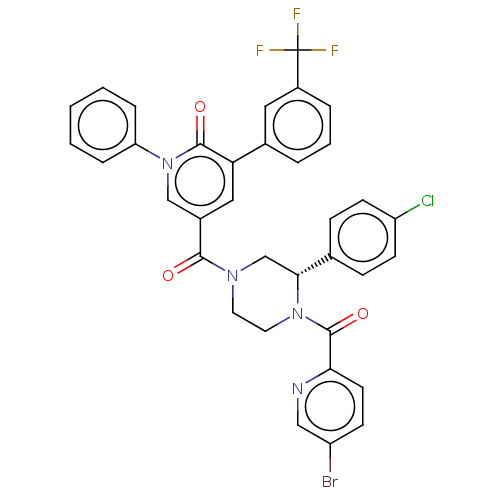 (CHEMBL4175313) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283462 (CHEMBL4176393) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283465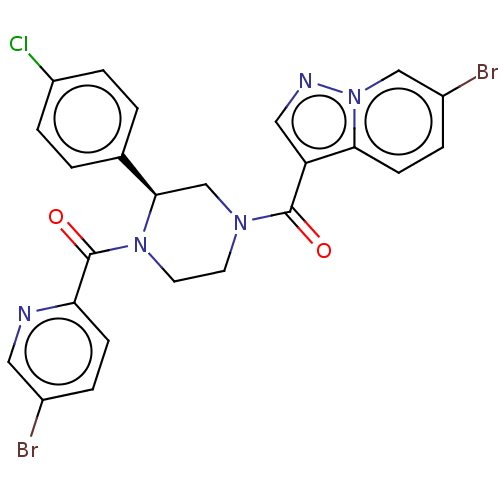 (CHEMBL4168783) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283444 (CHEMBL4173010) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283442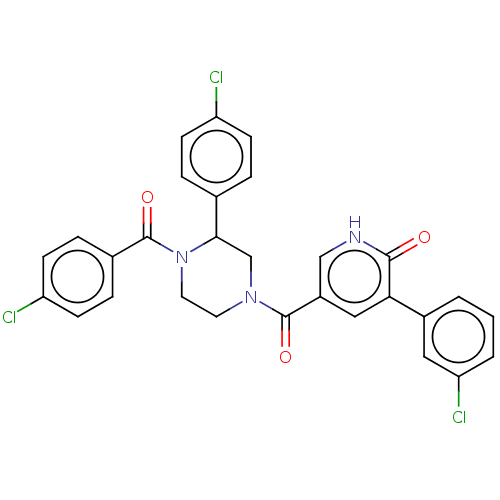 (CHEMBL4172770) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283446 (CHEMBL4169616) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283461 (CHEMBL4164548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283463 (CHEMBL4176581) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50320009 ((R)-1-((R)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283447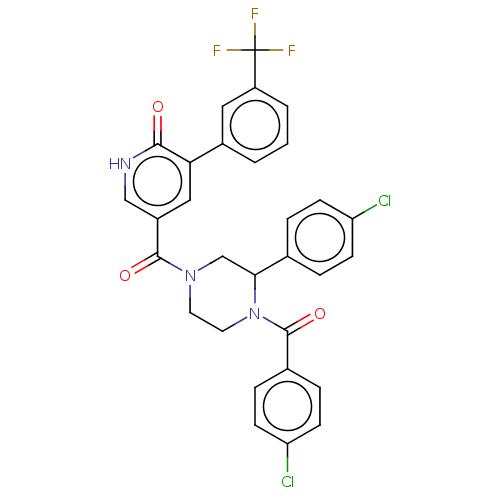 (CHEMBL4168400) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283459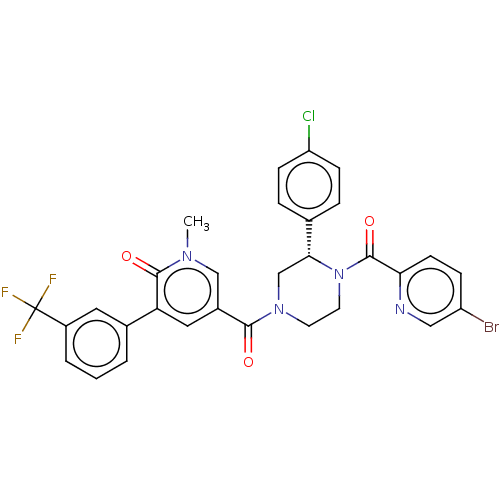 (CHEMBL4159400) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337107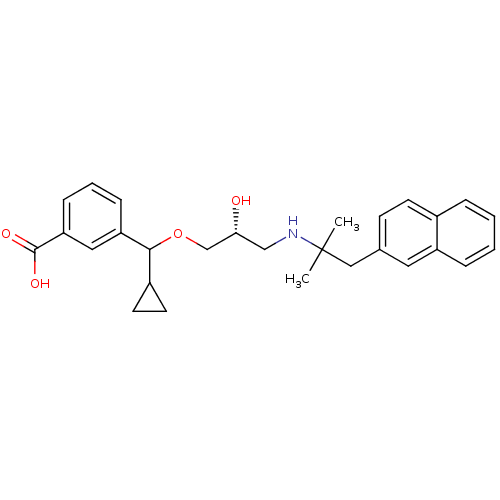 ((RS)-3-(Cyclopropyl-{(2R)-2-hydroxy-3-[2-methyl-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283468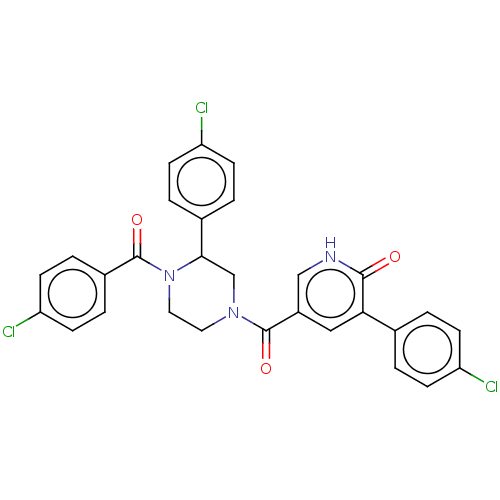 (CHEMBL4165785) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337105 ((R)-1-((R)-cyclopropyl(2-(hydroxymethyl)phenyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283460 (CHEMBL4177474) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337111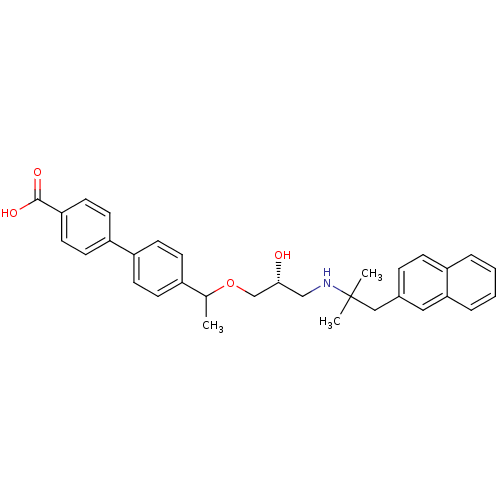 ((RS)-4'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283467 (CHEMBL4162337) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50337103 (2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of beta2 adrenergic receptor | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337110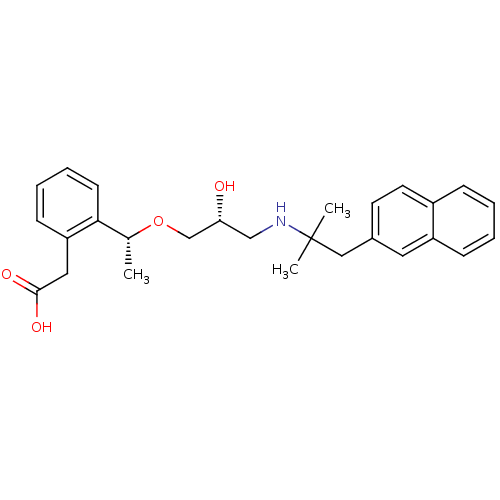 (2-[2-((1R)-1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283466 (CHEMBL4176640) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337106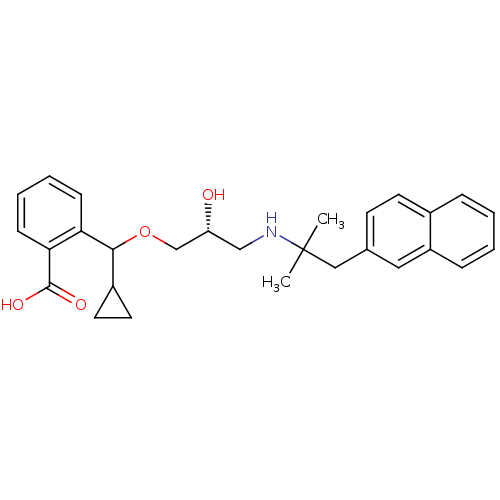 ((RS)-2-(Cyclopropyl-{(2R)-2-hydroxy-3-[2-methyl-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337108 ((RS)-4-(Cyclopropyl-{(2R)-2-hydroxy-3-[2-methyl-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337109 ((RS)-2-(1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphthale...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337104 (3-[2-((1R)-1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337104 (3-[2-((1R)-1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337107 ((RS)-3-(Cyclopropyl-{(2R)-2-hydroxy-3-[2-methyl-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337114 (2'-((1R)-1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337106 ((RS)-2-(Cyclopropyl-{(2R)-2-hydroxy-3-[2-methyl-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50337103 (2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-II (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of eIF4G-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A2 RNA dependent ATPase activity using single stranded poly... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase DHX29 (Homo sapiens (Human)) | BDBM50283445 (CHEMBL4175313) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal full length FLAG-His-tagged DHX29 RNA dependent ATPase activity expressed in baculovirus infected Sf9 inse... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase DHX29 (Homo sapiens (Human)) | BDBM50283443 (CHEMBL4170030) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal full length FLAG-His-tagged DHX29 RNA dependent ATPase activity expressed in baculovirus infected Sf9 inse... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase DHX29 (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal full length FLAG-His-tagged DHX29 RNA dependent ATPase activity expressed in baculovirus infected Sf9 inse... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| U5 small nuclear ribonucleoprotein 200 kDa helicase (Homo sapiens (Human)) | BDBM50283443 (CHEMBL4170030) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal full length His-tagged BRR2 RNA dependent ATPase activity expressed in baculovirus infected Sf9 insect cel... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| U5 small nuclear ribonucleoprotein 200 kDa helicase (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal full length His-tagged BRR2 RNA dependent ATPase activity expressed in baculovirus infected Sf9 insect cel... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-II (Homo sapiens (Human)) | BDBM50283443 (CHEMBL4170030) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of eIF4G-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A2 RNA dependent ATPase activity using single stranded poly... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of eIF4G-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A1 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| U5 small nuclear ribonucleoprotein 200 kDa helicase (Homo sapiens (Human)) | BDBM50283445 (CHEMBL4175313) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal full length His-tagged BRR2 RNA dependent ATPase activity expressed in baculovirus infected Sf9 insect cel... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 643 total ) | Next | Last >> |