 Found 108 hits with Last Name = 'fur' and Initial = 'gl'
Found 108 hits with Last Name = 'fur' and Initial = 'gl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281769
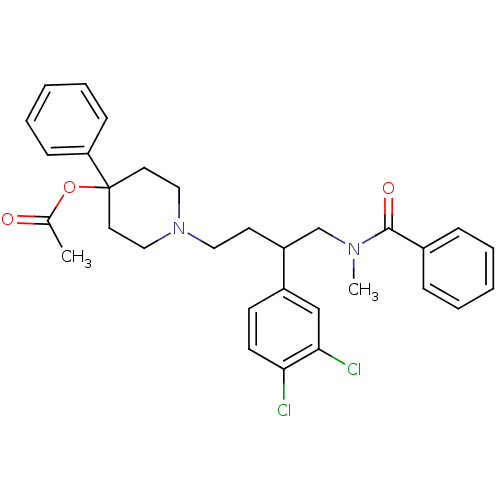
(Acetic acid 1-[4-(benzoyl-methyl-amino)-3-(3,4-dic...)Show SMILES CN(CC(CCN1CCC(CC1)(OC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H34Cl2N2O3/c1-23(36)38-31(27-11-7-4-8-12-27)16-19-35(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-34(2)30(37)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281767

(Acetic acid 1-{3-(3,4-dichloro-phenyl)-4-[ethyl-(t...)Show SMILES CCN(CC(CCN1CCC(CC1)(OC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1cccs1 Show InChI InChI=1S/C30H34Cl2N2O3S/c1-3-34(29(36)28-10-7-19-38-28)21-24(23-11-12-26(31)27(32)20-23)13-16-33-17-14-30(15-18-33,37-22(2)35)25-8-5-4-6-9-25/h4-12,19-20,24H,3,13-18,21H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50071484
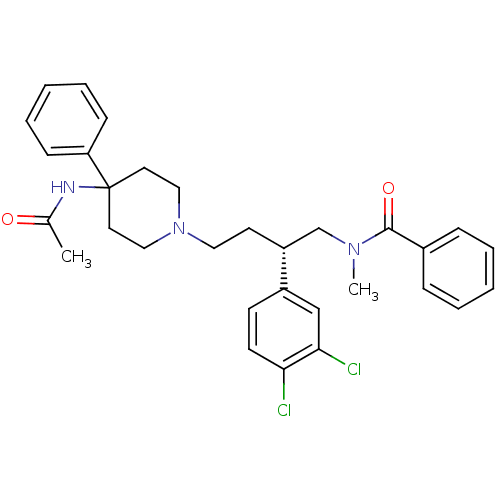
(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281771
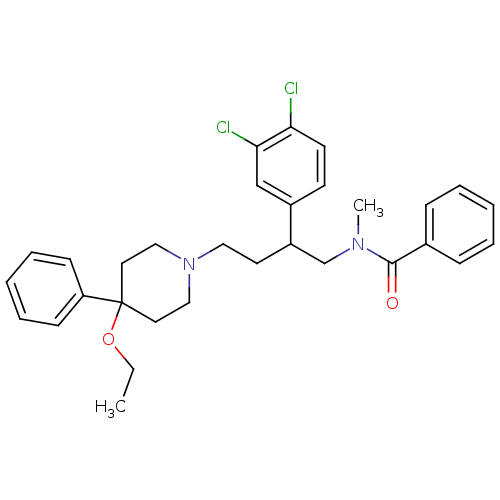
(CHEMBL173841 | N-[2-(3,4-Dichloro-phenyl)-4-(4-eth...)Show SMILES CCOC1(CCN(CCC(CN(C)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C31H36Cl2N2O2/c1-3-37-31(27-12-8-5-9-13-27)17-20-35(21-18-31)19-16-26(25-14-15-28(32)29(33)22-25)23-34(2)30(36)24-10-6-4-7-11-24/h4-15,22,26H,3,16-21,23H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50129513
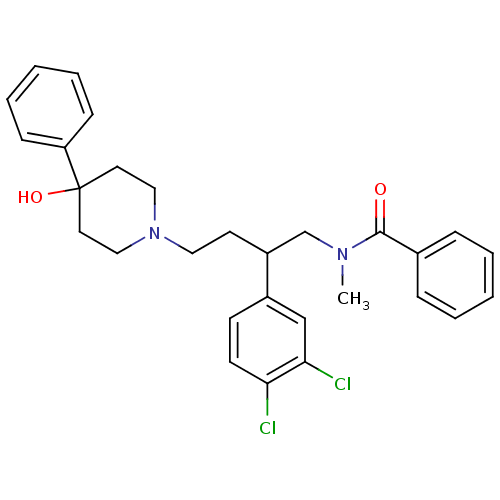
(CHEMBL71397 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hydr...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C29H32Cl2N2O2/c1-32(28(34)22-8-4-2-5-9-22)21-24(23-12-13-26(30)27(31)20-23)14-17-33-18-15-29(35,16-19-33)25-10-6-3-7-11-25/h2-13,20,24,35H,14-19,21H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281768

(CHEMBL176588 | Thiophene-3-carboxylic acid [2-(3,4...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccsc1 Show InChI InChI=1S/C27H30Cl2N2O2S/c1-30(26(32)22-10-16-34-19-22)18-21(20-7-8-24(28)25(29)17-20)9-13-31-14-11-27(33,12-15-31)23-5-3-2-4-6-23/h2-8,10,16-17,19,21,33H,9,11-15,18H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(HAMSTER) | BDBM50071484

(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-NKA binding to neurokinin NK2 receptor from hamster urinary bladder membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281766

(CHEMBL173008 | Thiophene-2-carboxylic acid [2-(3,4...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1cccs1 Show InChI InChI=1S/C27H30Cl2N2O2S/c1-30(26(32)25-8-5-17-34-25)19-21(20-9-10-23(28)24(29)18-20)11-14-31-15-12-27(33,13-16-31)22-6-3-2-4-7-22/h2-10,17-18,21,33H,11-16,19H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281773

(CHEMBL176853 | Naphthalene-1-carboxylic acid [2-(3...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C33H34Cl2N2O2/c1-36(32(38)29-13-7-9-24-8-5-6-12-28(24)29)23-26(25-14-15-30(34)31(35)22-25)16-19-37-20-17-33(39,18-21-37)27-10-3-2-4-11-27/h2-15,22,26,39H,16-21,23H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281770
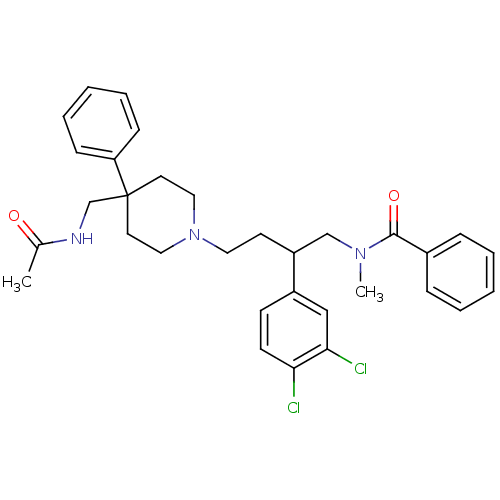
(CHEMBL367542 | N-[4-[4-(Acetylamino-methyl)-4-phen...)Show SMILES CN(CC(CCN1CCC(CNC(C)=O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C32H37Cl2N3O2/c1-24(38)35-23-32(28-11-7-4-8-12-28)16-19-37(20-17-32)18-15-27(26-13-14-29(33)30(34)21-26)22-36(2)31(39)25-9-5-3-6-10-25/h3-14,21,27H,15-20,22-23H2,1-2H3,(H,35,38) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281772

(CHEMBL368372 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hyd...)Show SMILES CN(CC(CCN1CCC(CO)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C30H34Cl2N2O2/c1-33(29(36)23-8-4-2-5-9-23)21-25(24-12-13-27(31)28(32)20-24)14-17-34-18-15-30(22-35,16-19-34)26-10-6-3-7-11-26/h2-13,20,25,35H,14-19,21-22H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50129520
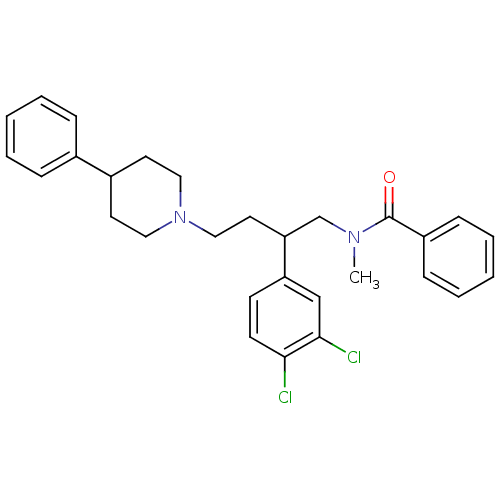
(CHEMBL69335 | N-[2-(3,4-Dichloro-phenyl)-4-(4-phen...)Show SMILES CN(CC(CCN1CCC(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C29H32Cl2N2O/c1-32(29(34)24-10-6-3-7-11-24)21-26(25-12-13-27(30)28(31)20-25)16-19-33-17-14-23(15-18-33)22-8-4-2-5-9-22/h2-13,20,23,26H,14-19,21H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281774
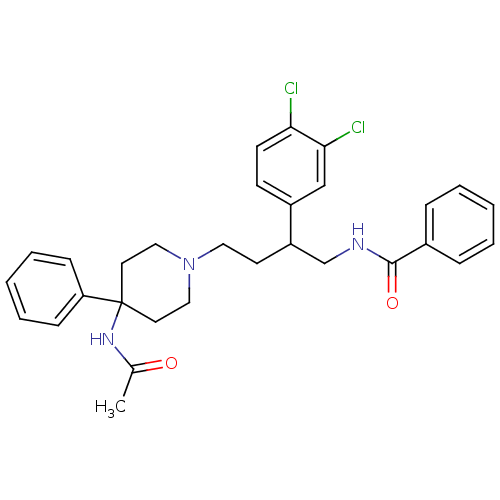
(CHEMBL366382 | N-[4-(4-Acetylamino-4-phenyl-piperi...)Show SMILES CC(=O)NC1(CCN(CCC(CNC(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C30H33Cl2N3O2/c1-22(36)34-30(26-10-6-3-7-11-26)15-18-35(19-16-30)17-14-25(24-12-13-27(31)28(32)20-24)21-33-29(37)23-8-4-2-5-9-23/h2-13,20,25H,14-19,21H2,1H3,(H,33,37)(H,34,36) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50071484

(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 945 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-NKA binding to Tachykinin receptor 2 from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Peripheral-type benzodiazepine receptor-associated protein 1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid type B receptor subunit 1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Galanin peptides
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM21280

(5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2[C@@]3(C)CCC(C3)C2(C)C)cc1 |r,TLB:21:22:28:26.25| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22?,27?,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 284: 644-50 (1998)
BindingDB Entry DOI: 10.7270/Q2D50KHT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data