 Found 12594 hits with Last Name = 'gan' and Initial = 'm'
Found 12594 hits with Last Name = 'gan' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402775
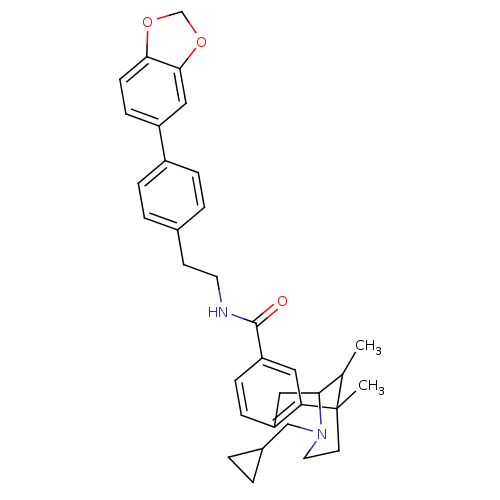
(CHEMBL2208351)Show SMILES CC1C2Cc3ccc(cc3C1(C)CCN2CC1CC1)C(=O)NCCc1ccc(cc1)-c1ccc2OCOc2c1 |TLB:15:14:1:4.9.3,8:9:1:14.13.12| Show InChI InChI=1S/C34H38N2O3/c1-22-30-18-27-9-10-28(17-29(27)34(22,2)14-16-36(30)20-24-3-4-24)33(37)35-15-13-23-5-7-25(8-6-23)26-11-12-31-32(19-26)39-21-38-31/h5-12,17,19,22,24,30H,3-4,13-16,18,20-21H2,1-2H3,(H,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402777
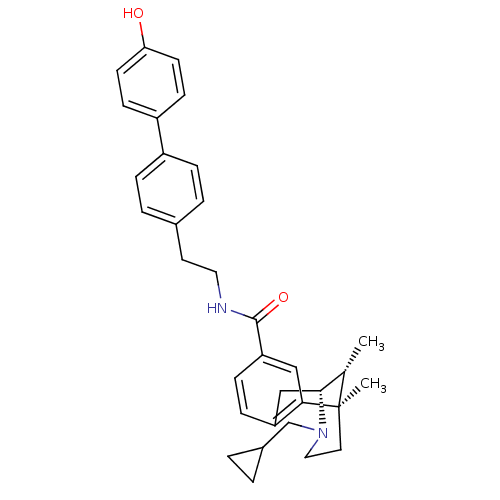
(CHEMBL2208347)Show SMILES C[C@H]1[C@H]2Cc3ccc(cc3[C@]1(C)CCN2CC1CC1)C(=O)NCCc1ccc(cc1)-c1ccc(O)cc1 |r,TLB:15:14:1:4.9.3| Show InChI InChI=1S/C33H38N2O2/c1-22-31-20-27-9-10-28(19-30(27)33(22,2)16-18-35(31)21-24-3-4-24)32(37)34-17-15-23-5-7-25(8-6-23)26-11-13-29(36)14-12-26/h5-14,19,22,24,31,36H,3-4,15-18,20-21H2,1-2H3,(H,34,37)/t22-,31+,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402777

(CHEMBL2208347)Show SMILES C[C@H]1[C@H]2Cc3ccc(cc3[C@]1(C)CCN2CC1CC1)C(=O)NCCc1ccc(cc1)-c1ccc(O)cc1 |r,TLB:15:14:1:4.9.3| Show InChI InChI=1S/C33H38N2O2/c1-22-31-20-27-9-10-28(19-30(27)33(22,2)16-18-35(31)21-24-3-4-24)32(37)34-17-15-23-5-7-25(8-6-23)26-11-13-29(36)14-12-26/h5-14,19,22,24,31,36H,3-4,15-18,20-21H2,1-2H3,(H,34,37)/t22-,31+,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402776
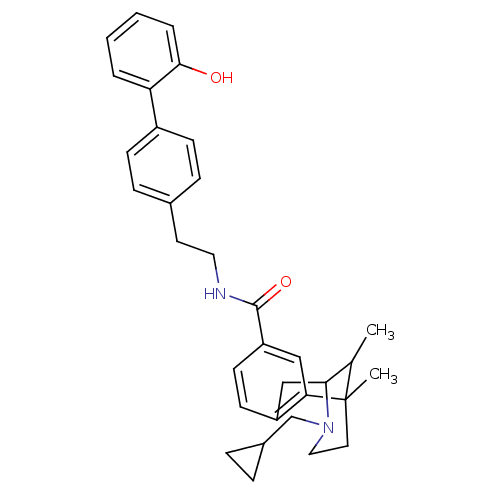
(CHEMBL2208349)Show SMILES CC1C2Cc3ccc(cc3C1(C)CCN2CC1CC1)C(=O)NCCc1ccc(cc1)-c1ccccc1O |TLB:15:14:1:4.9.3,8:9:1:14.13.12| Show InChI InChI=1S/C33H38N2O2/c1-22-30-20-26-13-14-27(19-29(26)33(22,2)16-18-35(30)21-24-7-8-24)32(37)34-17-15-23-9-11-25(12-10-23)28-5-3-4-6-31(28)36/h3-6,9-14,19,22,24,30,36H,7-8,15-18,20-21H2,1-2H3,(H,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402772
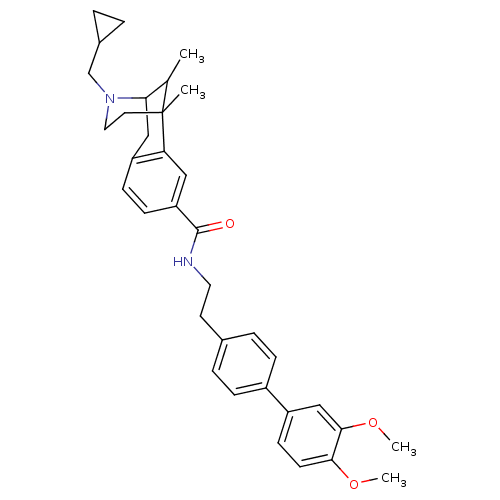
(CHEMBL2208350)Show SMILES COc1ccc(cc1OC)-c1ccc(CCNC(=O)c2ccc3CC4C(C)C(C)(CCN4CC4CC4)c3c2)cc1 |TLB:32:31:25:22.36.23,37:36:25:31.30.29| Show InChI InChI=1S/C35H42N2O3/c1-23-31-20-28-11-12-29(19-30(28)35(23,2)16-18-37(31)22-25-5-6-25)34(38)36-17-15-24-7-9-26(10-8-24)27-13-14-32(39-3)33(21-27)40-4/h7-14,19,21,23,25,31H,5-6,15-18,20,22H2,1-4H3,(H,36,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402773
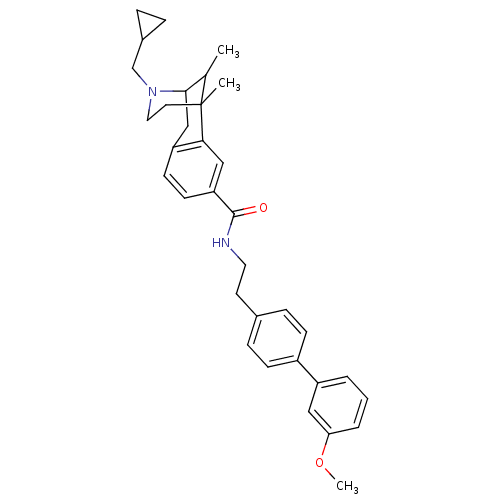
(CHEMBL2208358)Show SMILES COc1cccc(c1)-c1ccc(CCNC(=O)c2ccc3CC4C(C)C(C)(CCN4CC4CC4)c3c2)cc1 |TLB:30:29:23:20.34.21,35:34:23:29.28.27| Show InChI InChI=1S/C34H40N2O2/c1-23-32-21-28-13-14-29(20-31(28)34(23,2)16-18-36(32)22-25-7-8-25)33(37)35-17-15-24-9-11-26(12-10-24)27-5-4-6-30(19-27)38-3/h4-6,9-14,19-20,23,25,32H,7-8,15-18,21-22H2,1-3H3,(H,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402780
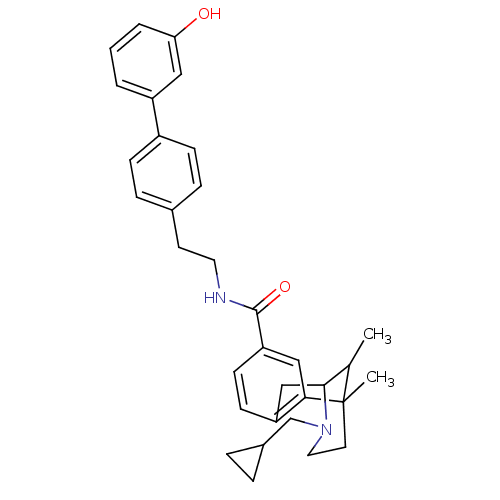
(CHEMBL2208348)Show SMILES CC1C2Cc3ccc(cc3C1(C)CCN2CC1CC1)C(=O)NCCc1ccc(cc1)-c1cccc(O)c1 |TLB:15:14:1:4.9.3,8:9:1:14.13.12| Show InChI InChI=1S/C33H38N2O2/c1-22-31-20-27-12-13-28(19-30(27)33(22,2)15-17-35(31)21-24-6-7-24)32(37)34-16-14-23-8-10-25(11-9-23)26-4-3-5-29(36)18-26/h3-5,8-13,18-19,22,24,31,36H,6-7,14-17,20-21H2,1-2H3,(H,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035179
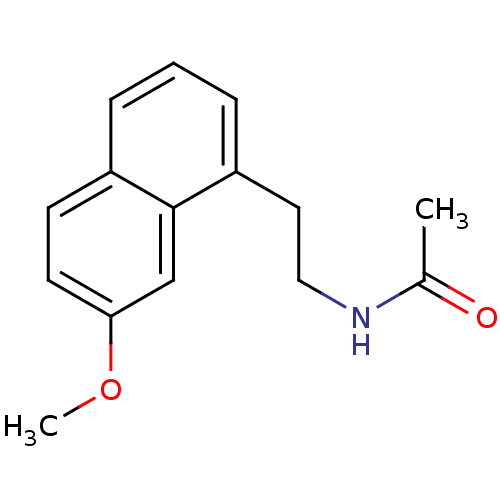
(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402774
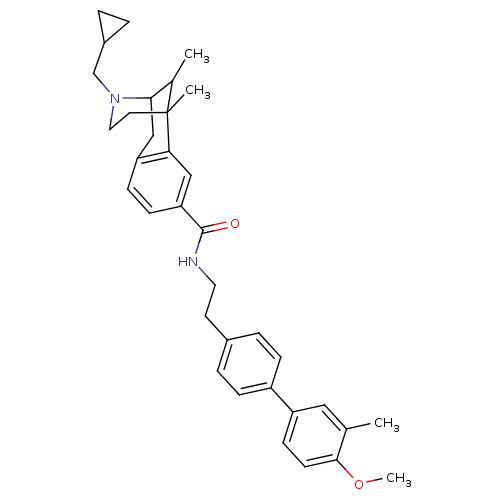
(CHEMBL2208352)Show SMILES COc1ccc(cc1C)-c1ccc(CCNC(=O)c2ccc3CC4C(C)C(C)(CCN4CC4CC4)c3c2)cc1 |TLB:31:30:24:21.35.22,36:35:24:30.29.28| Show InChI InChI=1S/C35H42N2O2/c1-23-19-28(13-14-33(23)39-4)27-9-7-25(8-10-27)15-17-36-34(38)30-12-11-29-21-32-24(2)35(3,31(29)20-30)16-18-37(32)22-26-5-6-26/h7-14,19-20,24,26,32H,5-6,15-18,21-22H2,1-4H3,(H,36,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50101160
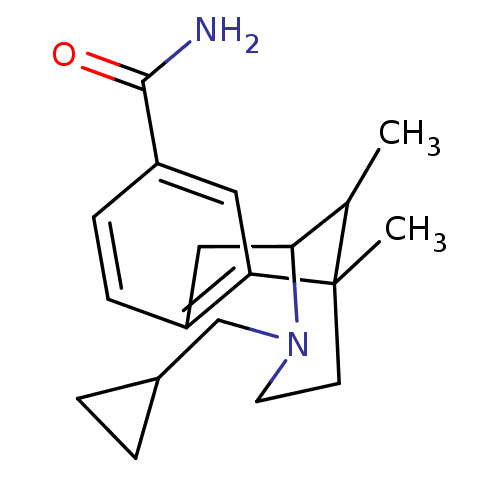
(3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hexa...)Show SMILES CC1C2Cc3ccc(cc3C1(C)CCN2CC1CC1)C(N)=O |TLB:8:9:1:14.13.12,15:14:1:4.9.3| Show InChI InChI=1S/C19H26N2O/c1-12-17-10-14-5-6-15(18(20)22)9-16(14)19(12,2)7-8-21(17)11-13-3-4-13/h5-6,9,12-13,17H,3-4,7-8,10-11H2,1-2H3,(H2,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402771
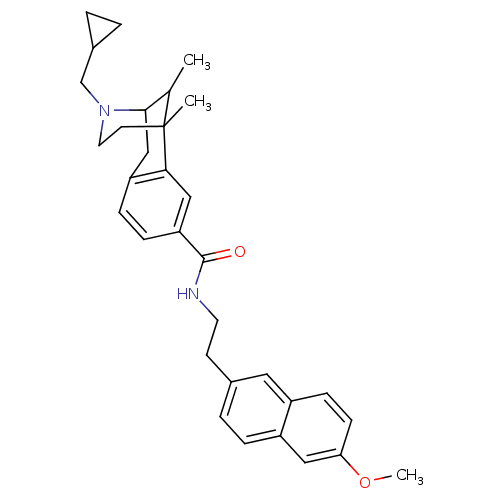
(CHEMBL2208354)Show SMILES COc1ccc2cc(CCNC(=O)c3ccc4CC5C(C)C(C)(CCN5CC5CC5)c4c3)ccc2c1 |TLB:26:25:19:16.30.17,31:30:19:25.24.23| Show InChI InChI=1S/C32H38N2O2/c1-21-30-19-26-8-9-27(18-29(26)32(21,2)13-15-34(30)20-23-4-5-23)31(35)33-14-12-22-6-7-25-17-28(36-3)11-10-24(25)16-22/h6-11,16-18,21,23,30H,4-5,12-15,19-20H2,1-3H3,(H,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320375
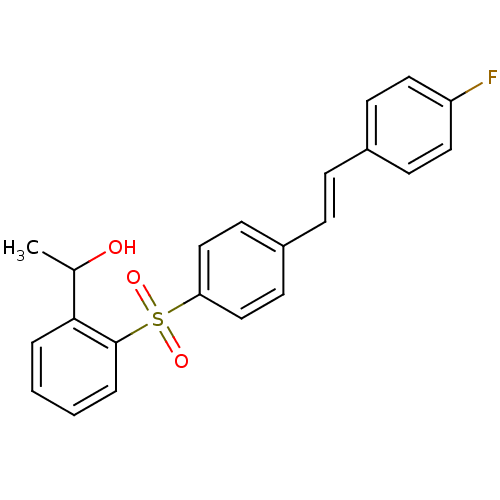
((E)-1-(2-(4-(4-fluorostyryl)phenylsulfonyl)phenyl)...)Show SMILES CC(O)c1ccccc1S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2)cc1 Show InChI InChI=1S/C22H19FO3S/c1-16(24)21-4-2-3-5-22(21)27(25,26)20-14-10-18(11-15-20)7-6-17-8-12-19(23)13-9-17/h2-16,24H,1H3/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50018731
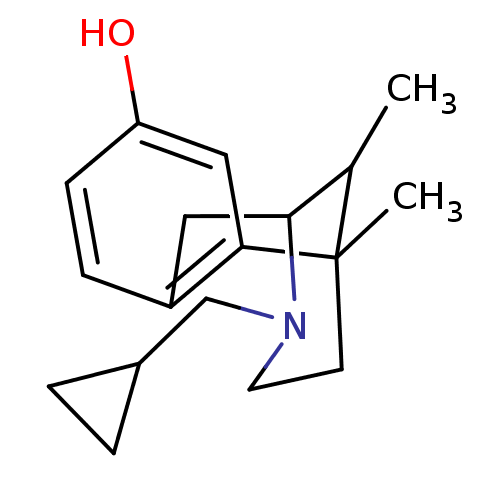
((Cyclazocine) 3-Cyclopropylmethyl-6,11-dimethyl-1,...)Show SMILES CC1C2Cc3ccc(O)cc3C1(C)CCN2CC1CC1 |TLB:16:15:1:3.4.10| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247744
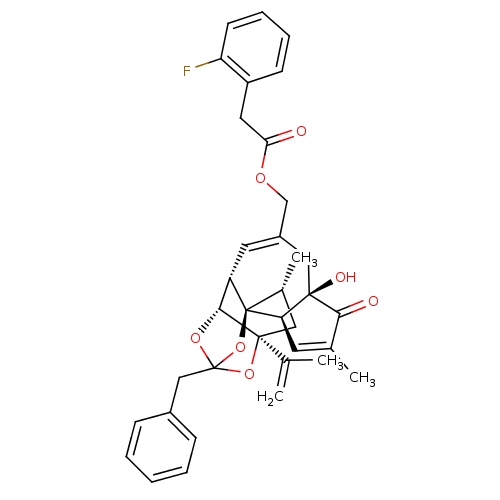
(CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-27(32(34)42-35(43-34,44-36)19-24-10-6-5-7-11-24)15-25(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-26-12-8-9-13-28(26)37/h5-15,23,27,29,32,40H,1,16-20H2,2-4H3/t23-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50224873
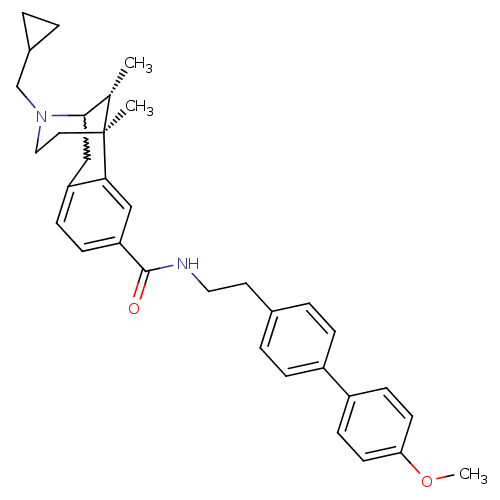
((6S,11R)-3-cyclopropylmethyl-6,11-dimethyl-1,2,3,4...)Show SMILES COc1ccc(cc1)-c1ccc(CCNC(=O)c2ccc3CC4[C@H](C)[C@](C)(CCN4CC4CC4)c3c2)cc1 |w:22.22,TLB:35:34:23:29.28.27,30:29:23:20.34.21| Show InChI InChI=1S/C34H40N2O2/c1-23-32-21-28-10-11-29(20-31(28)34(23,2)17-19-36(32)22-25-4-5-25)33(37)35-18-16-24-6-8-26(9-7-24)27-12-14-30(38-3)15-13-27/h6-15,20,23,25,32H,4-5,16-19,21-22H2,1-3H3,(H,35,37)/t23-,32?,34-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118528
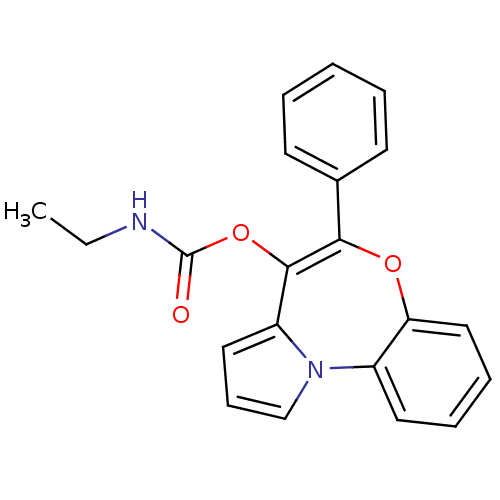
(CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...)Show SMILES CCNC(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H18N2O3/c1-2-22-21(24)26-20-17-12-8-14-23(17)16-11-6-7-13-18(16)25-19(20)15-9-4-3-5-10-15/h3-14H,2H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro-5-4864 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118528

(CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...)Show SMILES CCNC(=O)OC1=C(Oc2ccccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H18N2O3/c1-2-22-21(24)26-20-17-12-8-14-23(17)16-11-6-7-13-18(16)25-19(20)15-9-4-3-5-10-15/h3-14H,2H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
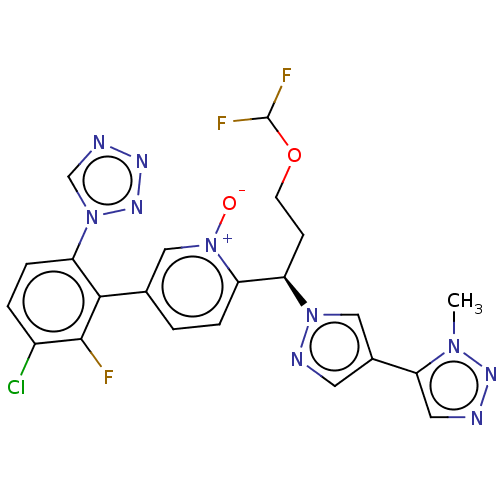
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402786
(CHEMBL2208359)Show SMILES COc1ccccc1-c1ccc(CCNC(=O)c2ccc3CC4C(C)C(C)(CCN4CC4CC4)c3c2)cc1 |TLB:30:29:23:20.34.21,35:34:23:29.28.27| Show InChI InChI=1S/C34H40N2O2/c1-23-31-21-27-14-15-28(20-30(27)34(23,2)17-19-36(31)22-25-8-9-25)33(37)35-18-16-24-10-12-26(13-11-24)29-6-4-5-7-32(29)38-3/h4-7,10-15,20,23,25,31H,8-9,16-19,21-22H2,1-3H3,(H,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50402783
(CHEMBL2208363)Show SMILES CC(C)Oc1ccccc1-c1ccc(CCNC(=O)c2ccc3CC4C(C)C(C)(CCN4CC4CC4)c3c2)cc1 |TLB:32:31:25:22.36.23,37:36:25:31.30.29| Show InChI InChI=1S/C36H44N2O2/c1-24(2)40-34-8-6-5-7-31(34)28-13-11-26(12-14-28)17-19-37-35(39)30-16-15-29-22-33-25(3)36(4,32(29)21-30)18-20-38(33)23-27-9-10-27/h5-8,11-16,21,24-25,27,33H,9-10,17-20,22-23H2,1-4H3,(H,37,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50043289
(CHEMBL34730 | N-[2-(6-Chloro-5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H15ClN2O2/c1-8(17)15-4-3-9-7-16-12-6-11(14)13(18-2)5-10(9)12/h5-7,16H,3-4H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007568
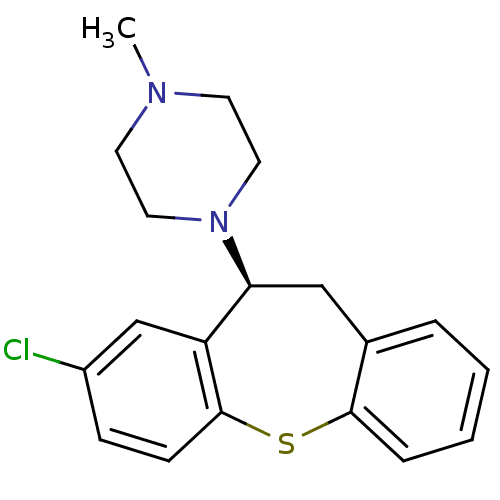
(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex homogenate |
J Med Chem 45: 344-59 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3G26 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM85064
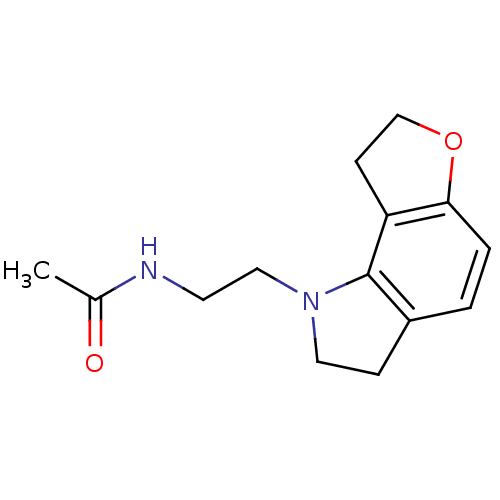
(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50056378
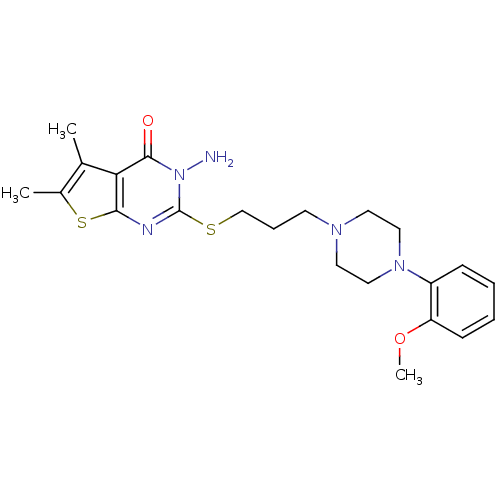
(3-Amino-2-{3-[4-(2-methoxy-phenyl)-piperazin-1-yl]...)Show SMILES COc1ccccc1N1CCN(CCCSc2nc3sc(C)c(C)c3c(=O)n2N)CC1 Show InChI InChI=1S/C22H29N5O2S2/c1-15-16(2)31-20-19(15)21(28)27(23)22(24-20)30-14-6-9-25-10-12-26(13-11-25)17-7-4-5-8-18(17)29-3/h4-5,7-8H,6,9-14,23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampus membranes |
Bioorg Med Chem Lett 10: 1089-92 (2000)
BindingDB Entry DOI: 10.7270/Q25D8R2B |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50018731

((Cyclazocine) 3-Cyclopropylmethyl-6,11-dimethyl-1,...)Show SMILES CC1C2Cc3ccc(O)cc3C1(C)CCN2CC1CC1 |TLB:16:15:1:3.4.10| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM85064

(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50224873

((6S,11R)-3-cyclopropylmethyl-6,11-dimethyl-1,2,3,4...)Show SMILES COc1ccc(cc1)-c1ccc(CCNC(=O)c2ccc3CC4[C@H](C)[C@](C)(CCN4CC4CC4)c3c2)cc1 |w:22.22,TLB:35:34:23:29.28.27,30:29:23:20.34.21| Show InChI InChI=1S/C34H40N2O2/c1-23-32-21-28-10-11-29(20-31(28)34(23,2)17-19-36(32)22-25-4-5-25)33(37)35-18-16-24-6-8-26(9-7-24)27-12-14-30(38-3)15-13-27/h6-15,20,23,25,32H,4-5,16-19,21-22H2,1-3H3,(H,35,37)/t23-,32?,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50224879
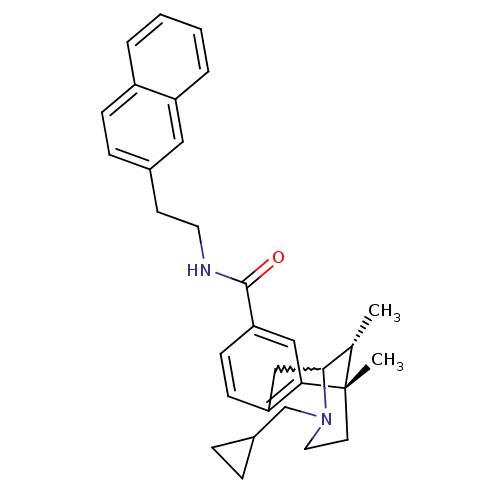
((6S,11R)-3-cyclopropylmethyl-6,11-dimethyl-1,2,3,4...)Show SMILES C[C@H]1C2Cc3ccc(cc3[C@@]1(C)CCN2CC1CC1)C(=O)NCCc1ccc2ccccc2c1 |w:2.2,TLB:8:9:1:14.13.12,15:14:1:4.9.3| Show InChI InChI=1S/C31H36N2O/c1-21-29-19-26-11-12-27(18-28(26)31(21,2)14-16-33(29)20-23-7-8-23)30(34)32-15-13-22-9-10-24-5-3-4-6-25(24)17-22/h3-6,9-12,17-18,21,23,29H,7-8,13-16,19-20H2,1-2H3,(H,32,34)/t21-,29?,31-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 7340-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.081
BindingDB Entry DOI: 10.7270/Q2N017Q6 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50118537

(CHEMBL135391 | Ethyl-carbamic acid 7-chloro-5-phen...)Show SMILES CCNC(=O)OC1=C(Oc2c(Cl)cccc2-n2cccc12)c1ccccc1 |t:6| Show InChI InChI=1S/C21H17ClN2O3/c1-2-23-21(25)27-20-17-12-7-13-24(17)16-11-6-10-15(22)19(16)26-18(20)14-8-4-3-5-9-14/h3-13H,2H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% |
J Med Chem 45: 4276-81 (2002)
BindingDB Entry DOI: 10.7270/Q20Z72MT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690
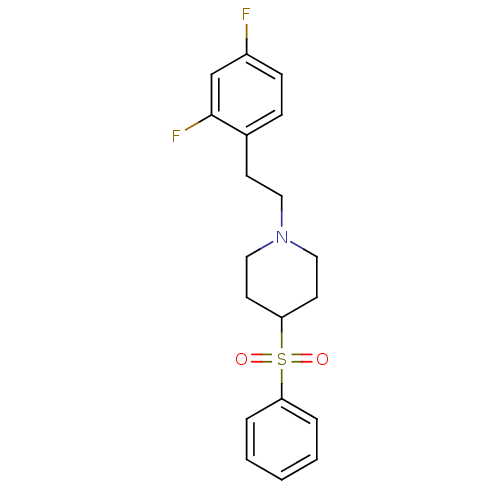
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50088696

(3-Amino-6-ethyl-2-{3-[4-(2-methoxy-phenyl)-piperaz...)Show SMILES CCc1cc2c(nc(SCCCN3CCN(CC3)c3ccccc3OC)n(N)c2=O)s1 Show InChI InChI=1S/C22H29N5O2S2/c1-3-16-15-17-20(31-16)24-22(27(23)21(17)28)30-14-6-9-25-10-12-26(13-11-25)18-7-4-5-8-19(18)29-2/h4-5,7-8,15H,3,6,9-14,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampus membranes |
Bioorg Med Chem Lett 10: 1089-92 (2000)
BindingDB Entry DOI: 10.7270/Q25D8R2B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50061649
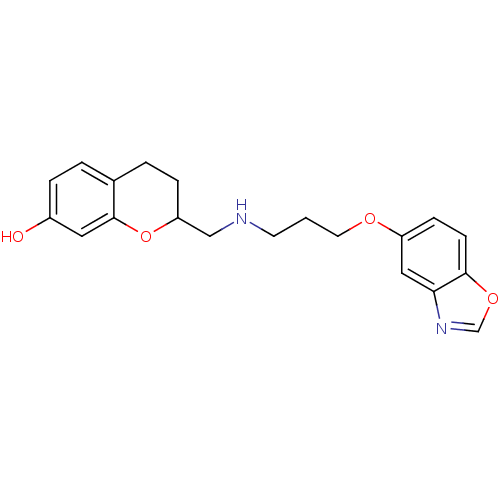
(2-{[3-(Benzooxazol-5-yloxy)-propylamino]-methyl}-c...)Show InChI InChI=1S/C20H22N2O4/c23-15-4-2-14-3-5-17(26-20(14)10-15)12-21-8-1-9-24-16-6-7-19-18(11-16)22-13-25-19/h2,4,6-7,10-11,13,17,21,23H,1,3,5,8-9,12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity to rat hippocampal 5-hydroxytryptamine 1A receptor was determined using [3H]8-OH-DPAT as radioligand |
J Med Chem 40: 4235-56 (1998)
Article DOI: 10.1021/jm9703653
BindingDB Entry DOI: 10.7270/Q2KP82TD |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50061682
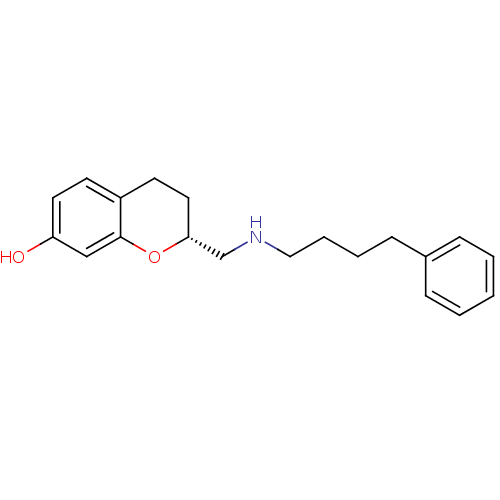
((R)-2-[(4-Phenyl-butylamino)-methyl]-chroman-7-ol;...)Show InChI InChI=1S/C20H25NO2/c22-18-11-9-17-10-12-19(23-20(17)14-18)15-21-13-5-4-8-16-6-2-1-3-7-16/h1-3,6-7,9,11,14,19,21-22H,4-5,8,10,12-13,15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... |
J Med Chem 40: 4235-56 (1998)
Article DOI: 10.1021/jm9703653
BindingDB Entry DOI: 10.7270/Q2KP82TD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50061684
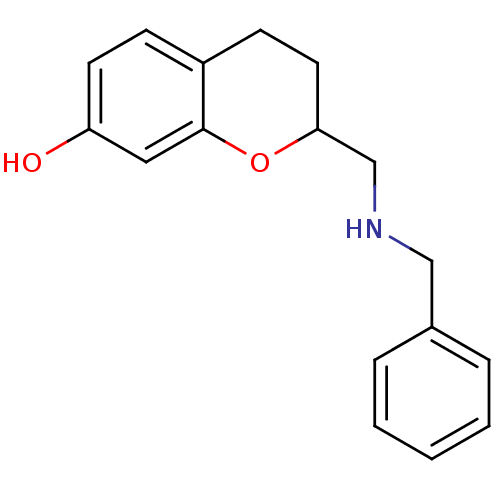
(2-(Benzylamino-methyl)-chroman-7-ol; oxalic acid |...)Show InChI InChI=1S/C17H19NO2/c19-15-8-6-14-7-9-16(20-17(14)10-15)12-18-11-13-4-2-1-3-5-13/h1-6,8,10,16,18-19H,7,9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... |
J Med Chem 40: 4235-56 (1998)
Article DOI: 10.1021/jm9703653
BindingDB Entry DOI: 10.7270/Q2KP82TD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50061669

((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...)Show InChI InChI=1S/C17H19NO2/c19-15-8-6-14-7-9-16(20-17(14)10-15)12-18-11-13-4-2-1-3-5-13/h1-6,8,10,16,18-19H,7,9,11-12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... |
J Med Chem 40: 4235-56 (1998)
Article DOI: 10.1021/jm9703653
BindingDB Entry DOI: 10.7270/Q2KP82TD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50061681
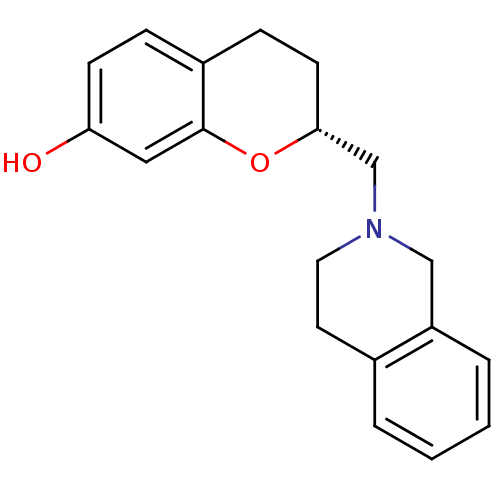
((R)-2-(3,4-Dihydro-1H-isoquinolin-2-ylmethyl)-chro...)Show InChI InChI=1S/C19H21NO2/c21-17-7-5-15-6-8-18(22-19(15)11-17)13-20-10-9-14-3-1-2-4-16(14)12-20/h1-5,7,11,18,21H,6,8-10,12-13H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human Dopamine receptor D3 (hD3) using [3H]spiperone radioligand. |
J Med Chem 40: 4235-56 (1998)
Article DOI: 10.1021/jm9703653
BindingDB Entry DOI: 10.7270/Q2KP82TD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data