 Found 50 hits with Last Name = 'garapaty-rao' and Initial = 's'
Found 50 hits with Last Name = 'garapaty-rao' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010824
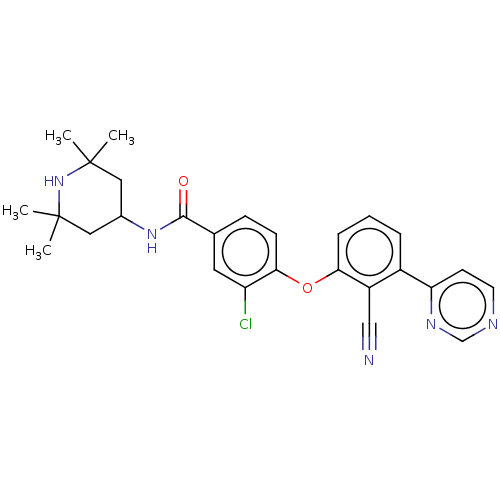
(CHEMBL3264788)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccncn3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-18(14-27(3,4)33-26)32-25(34)17-8-9-24(21(28)12-17)35-23-7-5-6-19(20(23)15-29)22-10-11-30-16-31-22/h5-12,16,18,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823
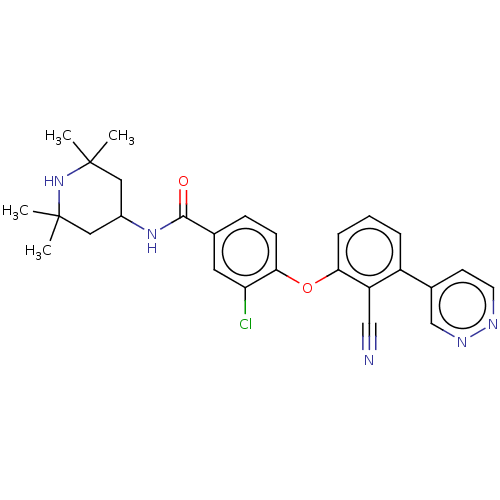
(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010822
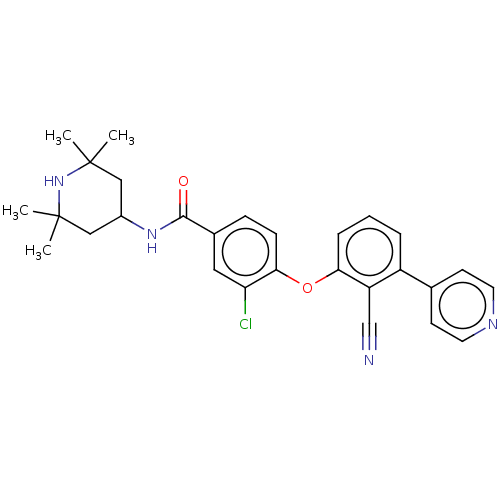
(CHEMBL3264786)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccncc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C28H29ClN4O2/c1-27(2)15-20(16-28(3,4)33-27)32-26(34)19-8-9-25(23(29)14-19)35-24-7-5-6-21(22(24)17-30)18-10-12-31-13-11-18/h5-14,20,33H,15-16H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010820
(CHEMBL3264784)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(Cl)c2C#N)c(Cl)c1 Show InChI InChI=1S/C23H25Cl2N3O2/c1-22(2)11-15(12-23(3,4)28-22)27-21(29)14-8-9-20(18(25)10-14)30-19-7-5-6-17(24)16(19)13-26/h5-10,15,28H,11-12H2,1-4H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010819
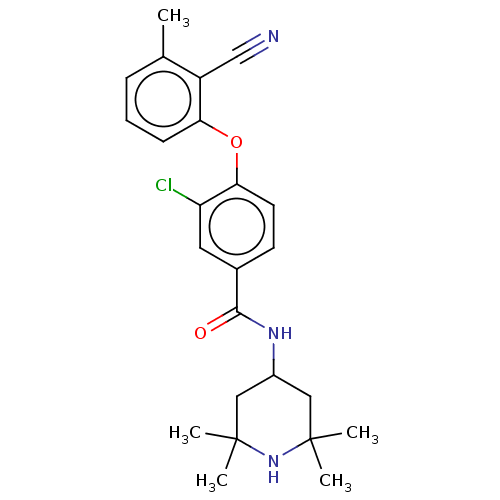
(CHEMBL3264783)Show SMILES Cc1cccc(Oc2ccc(cc2Cl)C(=O)NC2CC(C)(C)NC(C)(C)C2)c1C#N Show InChI InChI=1S/C24H28ClN3O2/c1-15-7-6-8-20(18(15)14-26)30-21-10-9-16(11-19(21)25)22(29)27-17-12-23(2,3)28-24(4,5)13-17/h6-11,17,28H,12-13H2,1-5H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010835
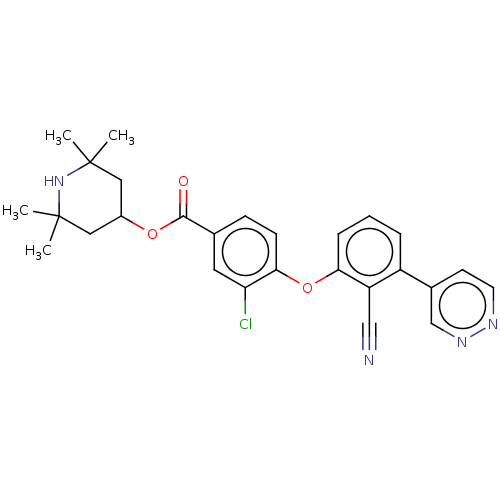
(CHEMBL3264794)Show SMILES CC1(C)CC(CC(C)(C)N1)OC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H27ClN4O3/c1-26(2)13-19(14-27(3,4)32-26)34-25(33)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,32H,13-14H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010836
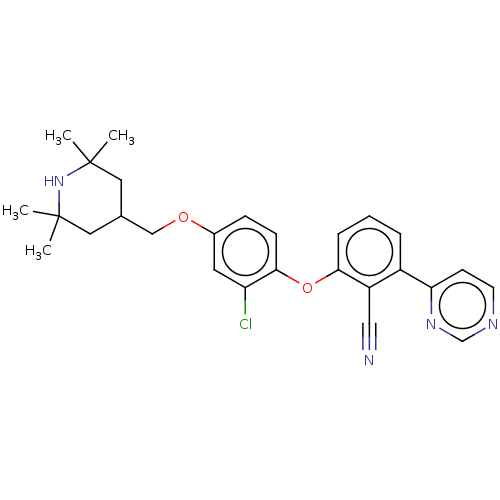
(CHEMBL3264795)Show SMILES CC1(C)CC(COc2ccc(Oc3cccc(-c4ccncn4)c3C#N)c(Cl)c2)CC(C)(C)N1 Show InChI InChI=1S/C27H29ClN4O2/c1-26(2)13-18(14-27(3,4)32-26)16-33-19-8-9-25(22(28)12-19)34-24-7-5-6-20(21(24)15-29)23-10-11-30-17-31-23/h5-12,17-18,32H,13-14,16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010821

(CHEMBL3264785)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3cn[nH]c3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C26H28ClN5O2/c1-25(2)11-18(12-26(3,4)32-25)31-24(33)16-8-9-23(21(27)10-16)34-22-7-5-6-19(20(22)13-28)17-14-29-30-15-17/h5-10,14-15,18,32H,11-12H2,1-4H3,(H,29,30)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010816
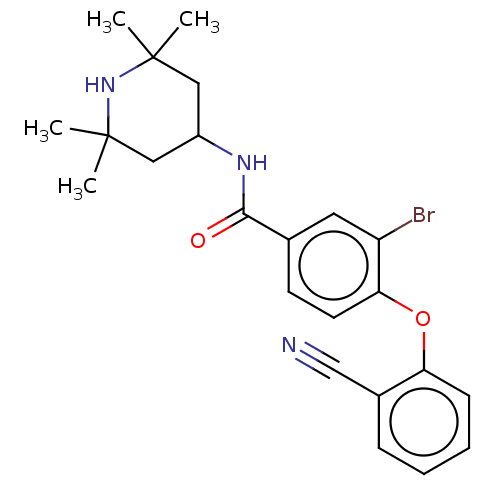
(CHEMBL3264780)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)c(Br)c1 Show InChI InChI=1S/C23H26BrN3O2/c1-22(2)12-17(13-23(3,4)27-22)26-21(28)15-9-10-20(18(24)11-15)29-19-8-6-5-7-16(19)14-25/h5-11,17,27H,12-13H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010815
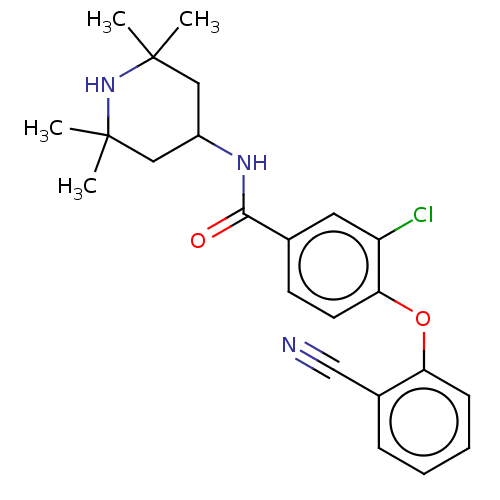
(CHEMBL3264779)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)c(Cl)c1 Show InChI InChI=1S/C23H26ClN3O2/c1-22(2)12-17(13-23(3,4)27-22)26-21(28)15-9-10-20(18(24)11-15)29-19-8-6-5-7-16(19)14-25/h5-11,17,27H,12-13H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010814
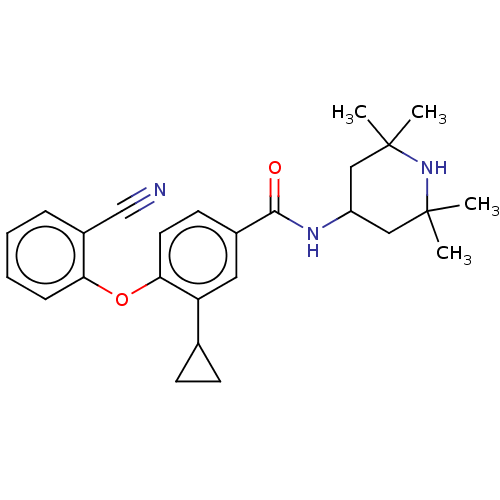
(CHEMBL3264778)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)c(c1)C1CC1 Show InChI InChI=1S/C26H31N3O2/c1-25(2)14-20(15-26(3,4)29-25)28-24(30)18-11-12-23(21(13-18)17-9-10-17)31-22-8-6-5-7-19(22)16-27/h5-8,11-13,17,20,29H,9-10,14-15H2,1-4H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010722
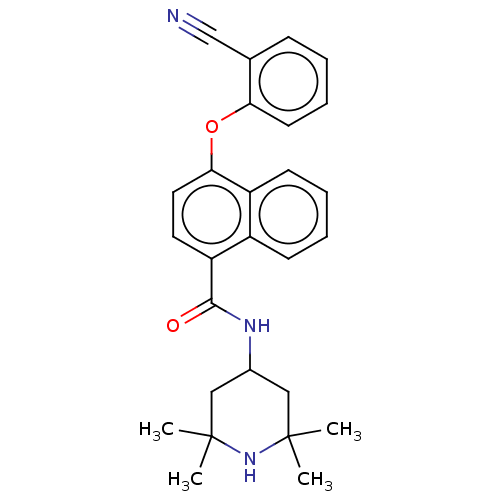
(CHEMBL3264774)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-26(2)15-19(16-27(3,4)30-26)29-25(31)22-13-14-24(21-11-7-6-10-20(21)22)32-23-12-8-5-9-18(23)17-28/h5-14,19,30H,15-16H2,1-4H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010831
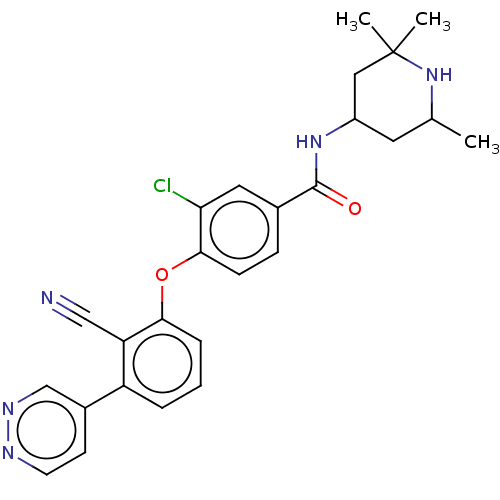
(CHEMBL3264792)Show SMILES CC1CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C26H26ClN5O2/c1-16-11-19(13-26(2,3)32-16)31-25(33)17-7-8-24(22(27)12-17)34-23-6-4-5-20(21(23)14-28)18-9-10-29-30-15-18/h4-10,12,15-16,19,32H,11,13H2,1-3H3,(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 871 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010812

(CHEMBL3264776)Show SMILES Cc1cc(ccc1Oc1ccccc1C#N)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C24H29N3O2/c1-16-12-17(10-11-20(16)29-21-9-7-6-8-18(21)15-25)22(28)26-19-13-23(2,3)27-24(4,5)14-19/h6-12,19,27H,13-14H2,1-5H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010813
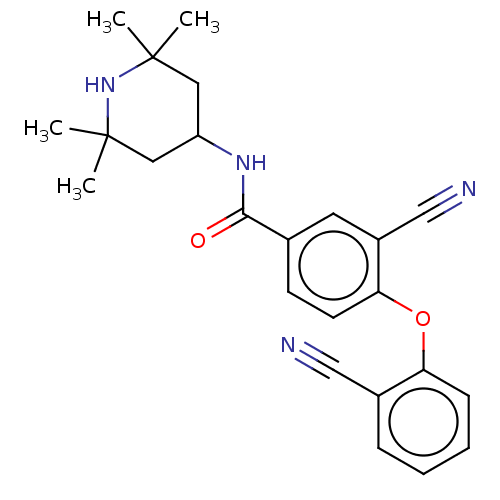
(CHEMBL3264777)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)c(c1)C#N Show InChI InChI=1S/C24H26N4O2/c1-23(2)12-19(13-24(3,4)28-23)27-22(29)16-9-10-21(18(11-16)15-26)30-20-8-6-5-7-17(20)14-25/h5-11,19,28H,12-13H2,1-4H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010817
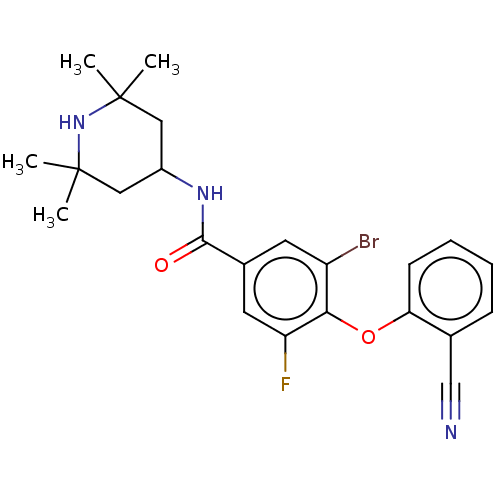
(CHEMBL3264781)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1cc(F)c(Oc2ccccc2C#N)c(Br)c1 Show InChI InChI=1S/C23H25BrFN3O2/c1-22(2)11-16(12-23(3,4)28-22)27-21(29)15-9-17(24)20(18(25)10-15)30-19-8-6-5-7-14(19)13-26/h5-10,16,28H,11-12H2,1-4H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010811
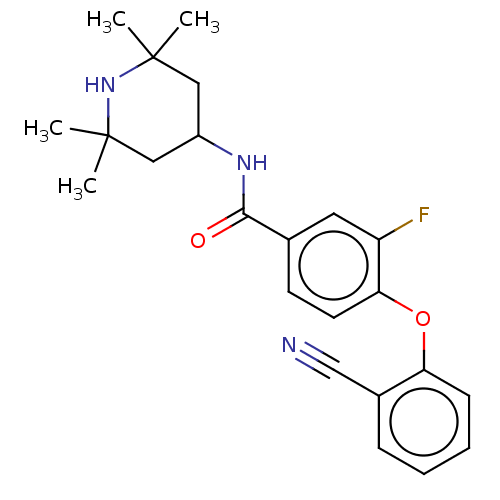
(CHEMBL3264775)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)c(F)c1 Show InChI InChI=1S/C23H26FN3O2/c1-22(2)12-17(13-23(3,4)27-22)26-21(28)15-9-10-20(18(24)11-15)29-19-8-6-5-7-16(19)14-25/h5-11,17,27H,12-13H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010827
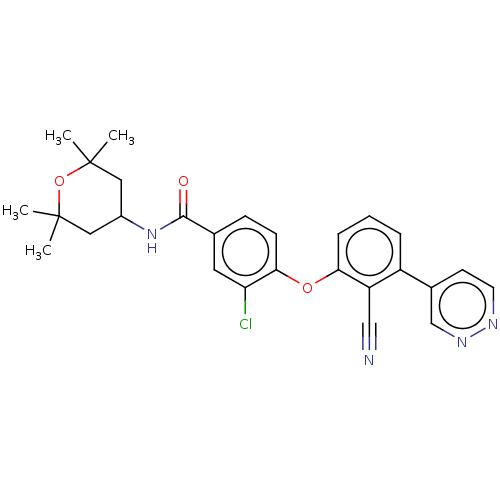
(CHEMBL3264791)Show SMILES CC1(C)CC(CC(C)(C)O1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H27ClN4O3/c1-26(2)13-19(14-27(3,4)35-26)32-25(33)17-8-9-24(22(28)12-17)34-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19H,13-14H2,1-4H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010832
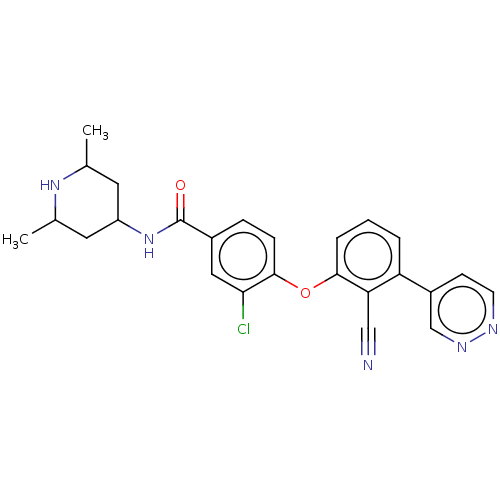
(CHEMBL3264793)Show SMILES CC1CC(CC(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C25H24ClN5O2/c1-15-10-19(11-16(2)30-15)31-25(32)17-6-7-24(22(26)12-17)33-23-5-3-4-20(21(23)13-27)18-8-9-28-29-14-18/h3-9,12,14-16,19,30H,10-11H2,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010681
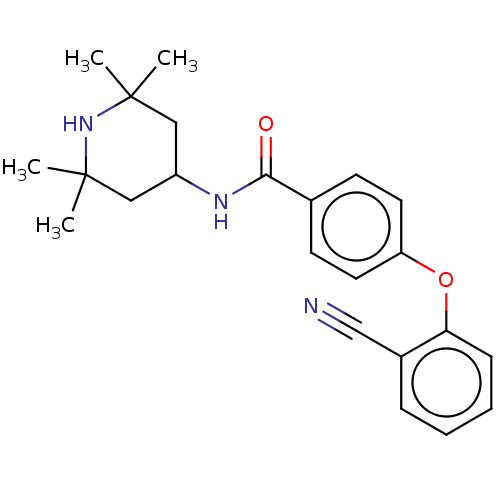
(CHEMBL3264770)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)cc1 Show InChI InChI=1S/C23H27N3O2/c1-22(2)13-18(14-23(3,4)26-22)25-21(27)16-9-11-19(12-10-16)28-20-8-6-5-7-17(20)15-24/h5-12,18,26H,13-14H2,1-4H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010678
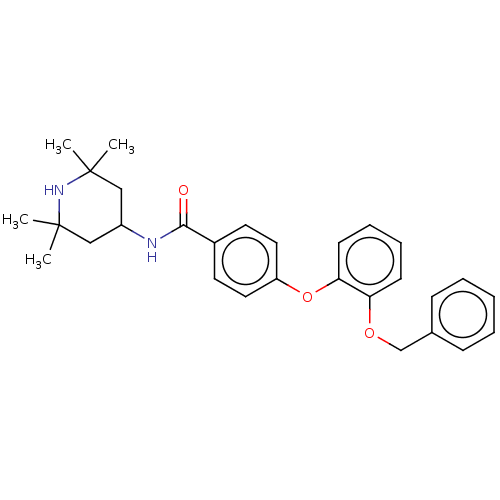
(CHEMBL3264767)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2OCc2ccccc2)cc1 Show InChI InChI=1S/C29H34N2O3/c1-28(2)18-23(19-29(3,4)31-28)30-27(32)22-14-16-24(17-15-22)34-26-13-9-8-12-25(26)33-20-21-10-6-5-7-11-21/h5-17,23,31H,18-20H2,1-4H3,(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010825
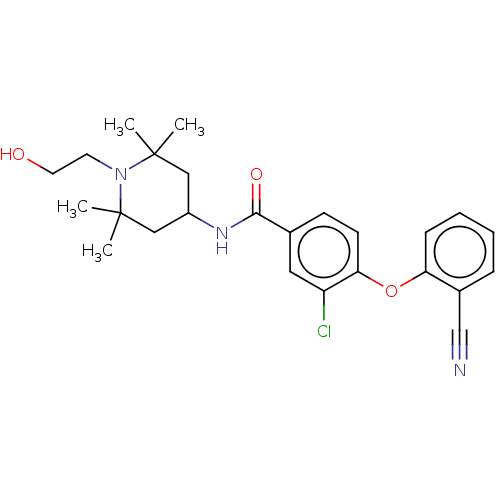
(CHEMBL3264789)Show SMILES CC1(C)CC(CC(C)(C)N1CCO)NC(=O)c1ccc(Oc2ccccc2C#N)c(Cl)c1 Show InChI InChI=1S/C25H30ClN3O3/c1-24(2)14-19(15-25(3,4)29(24)11-12-30)28-23(31)17-9-10-22(20(26)13-17)32-21-8-6-5-7-18(21)16-27/h5-10,13,19,30H,11-12,14-15H2,1-4H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010679
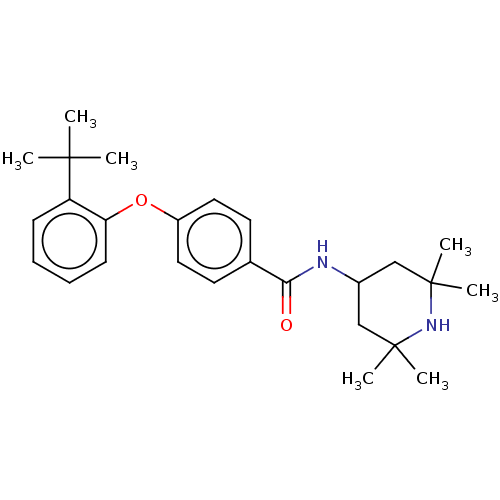
(CHEMBL3264768)Show SMILES CC(C)(C)c1ccccc1Oc1ccc(cc1)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C26H36N2O2/c1-24(2,3)21-10-8-9-11-22(21)30-20-14-12-18(13-15-20)23(29)27-19-16-25(4,5)28-26(6,7)17-19/h8-15,19,28H,16-17H2,1-7H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as global reduction in H3K27me3 level after 10 days by ELISA-based assay |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as global reduction in H3K27me3 level after 7 days by ELISA-based assay |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010682
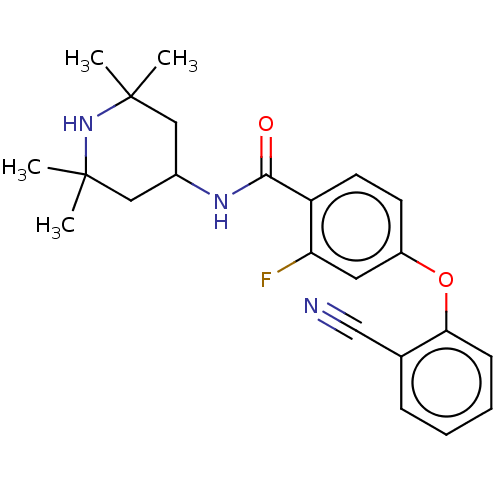
(CHEMBL3264771)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)cc1F Show InChI InChI=1S/C23H26FN3O2/c1-22(2)12-16(13-23(3,4)27-22)26-21(28)18-10-9-17(11-19(18)24)29-20-8-6-5-7-15(20)14-25/h5-11,16,27H,12-13H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010680
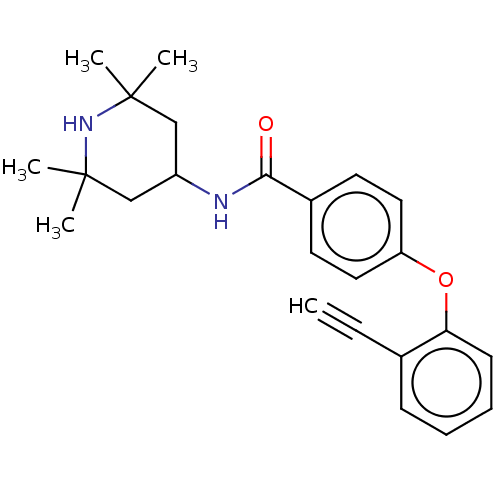
(CHEMBL3264769)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#C)cc1 Show InChI InChI=1S/C24H28N2O2/c1-6-17-9-7-8-10-21(17)28-20-13-11-18(12-14-20)22(27)25-19-15-23(2,3)26-24(4,5)16-19/h1,7-14,19,26H,15-16H2,2-5H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010676
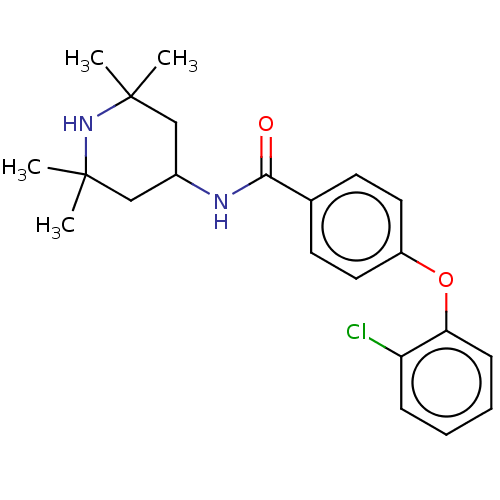
(CHEMBL3264765)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2Cl)cc1 Show InChI InChI=1S/C22H27ClN2O2/c1-21(2)13-16(14-22(3,4)25-21)24-20(26)15-9-11-17(12-10-15)27-19-8-6-5-7-18(19)23/h5-12,16,25H,13-14H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010663

(CHEMBL3264752)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C22H28N2OS/c1-21(2)14-17(15-22(3,4)24-21)23-20(25)16-10-12-19(13-11-16)26-18-8-6-5-7-9-18/h5-13,17,24H,14-15H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010683
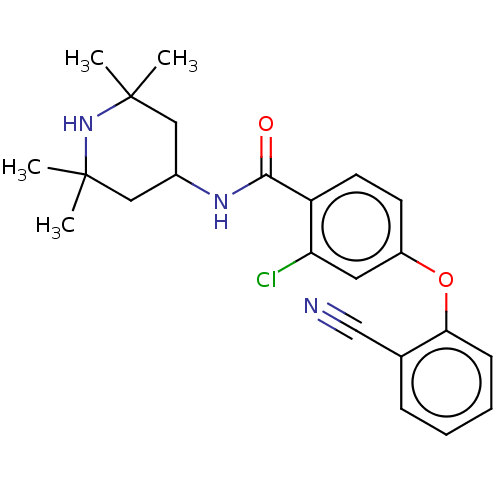
(CHEMBL3264772)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2C#N)cc1Cl Show InChI InChI=1S/C23H26ClN3O2/c1-22(2)12-16(13-23(3,4)27-22)26-21(28)18-10-9-17(11-19(18)24)29-20-8-6-5-7-15(20)14-25/h5-11,16,27H,12-13H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010673
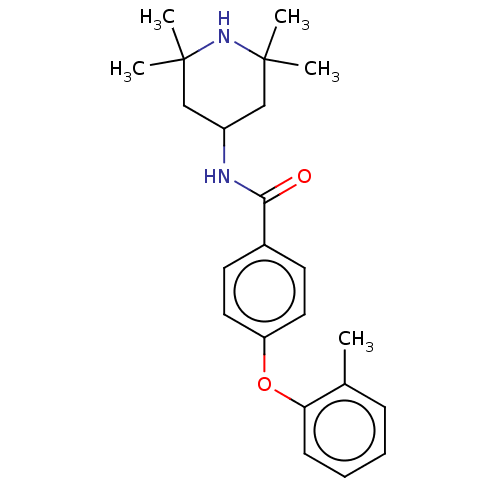
(CHEMBL3264762)Show SMILES Cc1ccccc1Oc1ccc(cc1)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C23H30N2O2/c1-16-8-6-7-9-20(16)27-19-12-10-17(11-13-19)21(26)24-18-14-22(2,3)25-23(4,5)15-18/h6-13,18,25H,14-15H2,1-5H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010661

(CHEMBL3264750)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(COc2ccccc2)cc1 Show InChI InChI=1S/C23H30N2O2/c1-22(2)14-19(15-23(3,4)25-22)24-21(26)18-12-10-17(11-13-18)16-27-20-8-6-5-7-9-20/h5-13,19,25H,14-16H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010818
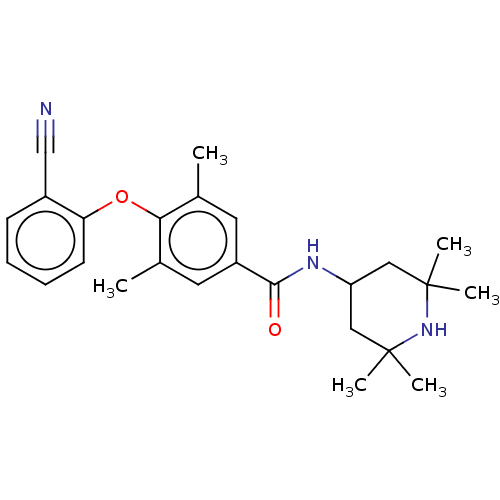
(CHEMBL3264782)Show SMILES Cc1cc(cc(C)c1Oc1ccccc1C#N)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C25H31N3O2/c1-16-11-19(23(29)27-20-13-24(3,4)28-25(5,6)14-20)12-17(2)22(16)30-21-10-8-7-9-18(21)15-26/h7-12,20,28H,13-14H2,1-6H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010660
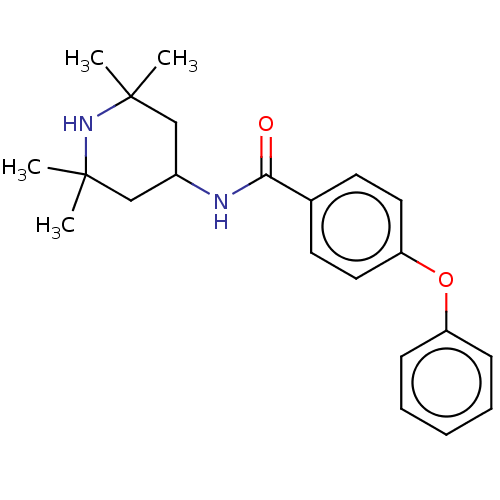
(CHEMBL3264749)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C22H28N2O2/c1-21(2)14-17(15-22(3,4)24-21)23-20(25)16-10-12-19(13-11-16)26-18-8-6-5-7-9-18/h5-13,17,24H,14-15H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010666
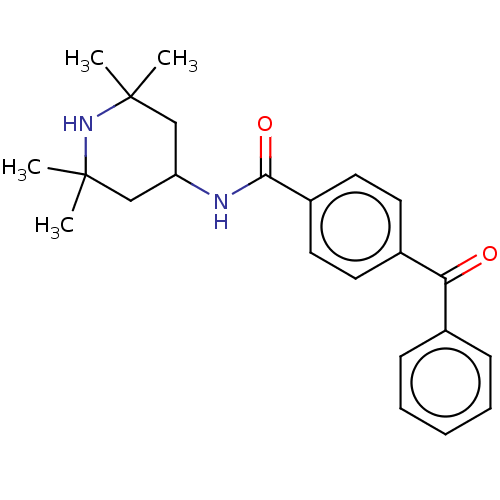
(CHEMBL3264755)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C23H28N2O2/c1-22(2)14-19(15-23(3,4)25-22)24-21(27)18-12-10-17(11-13-18)20(26)16-8-6-5-7-9-16/h5-13,19,25H,14-15H2,1-4H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as global reduction in H3K27me3 level after 4 days by ELISA-based assay |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010677
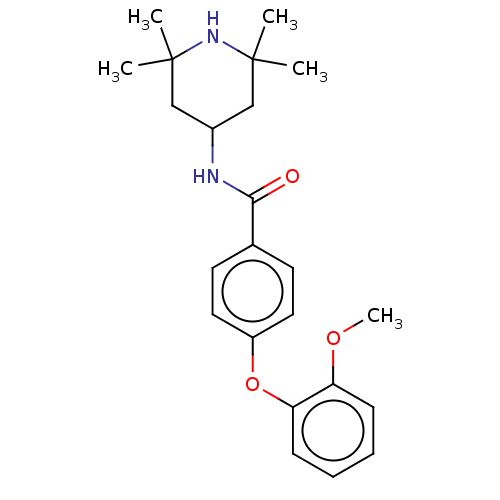
(CHEMBL3264766)Show SMILES COc1ccccc1Oc1ccc(cc1)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C23H30N2O3/c1-22(2)14-17(15-23(3,4)25-22)24-21(26)16-10-12-18(13-11-16)28-20-9-7-6-8-19(20)27-5/h6-13,17,25H,14-15H2,1-5H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010674
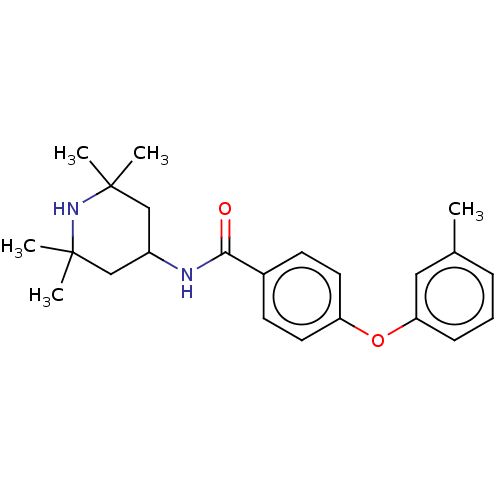
(CHEMBL3264763)Show SMILES Cc1cccc(Oc2ccc(cc2)C(=O)NC2CC(C)(C)NC(C)(C)C2)c1 Show InChI InChI=1S/C23H30N2O2/c1-16-7-6-8-20(13-16)27-19-11-9-17(10-12-19)21(26)24-18-14-22(2,3)25-23(4,5)15-18/h6-13,18,25H,14-15H2,1-5H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010669
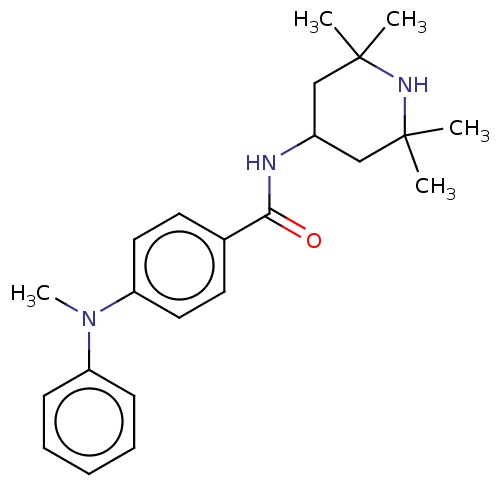
(CHEMBL3264758)Show SMILES CN(c1ccccc1)c1ccc(cc1)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C23H31N3O/c1-22(2)15-18(16-23(3,4)25-22)24-21(27)17-11-13-20(14-12-17)26(5)19-9-7-6-8-10-19/h6-14,18,25H,15-16H2,1-5H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010668
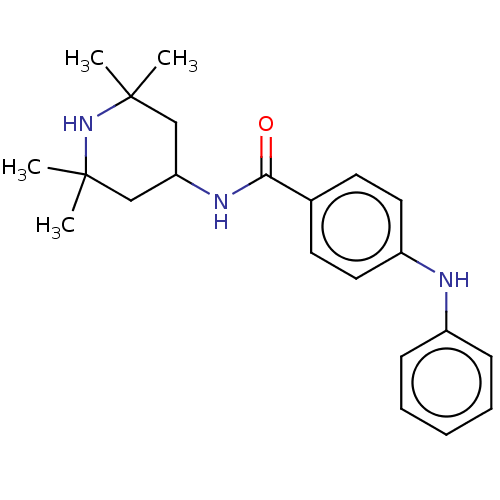
(CHEMBL3264757)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Nc2ccccc2)cc1 Show InChI InChI=1S/C22H29N3O/c1-21(2)14-19(15-22(3,4)25-21)24-20(26)16-10-12-18(13-11-16)23-17-8-6-5-7-9-17/h5-13,19,23,25H,14-15H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010667

(CHEMBL3264756)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(cc1)C(O)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-22(2)14-19(15-23(3,4)25-22)24-21(27)18-12-10-17(11-13-18)20(26)16-8-6-5-7-9-16/h5-13,19-20,25-26H,14-15H2,1-4H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010665
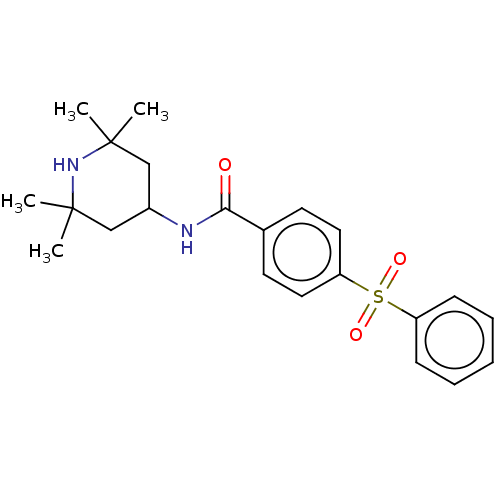
(CHEMBL3264754)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(cc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C22H28N2O3S/c1-21(2)14-17(15-22(3,4)24-21)23-20(25)16-10-12-19(13-11-16)28(26,27)18-8-6-5-7-9-18/h5-13,17,24H,14-15H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
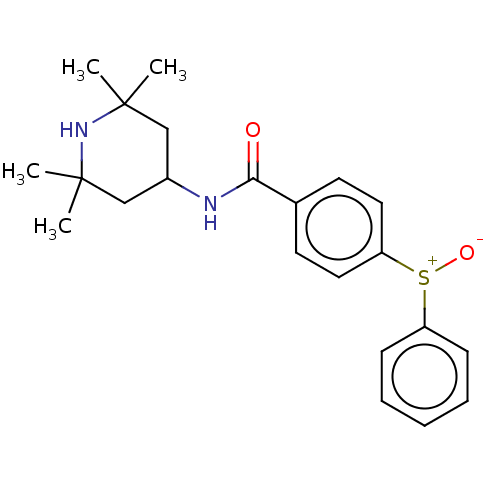
(Homo sapiens (Human)) | BDBM50010664
(CHEMBL3264753)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(cc1)[S+]([O-])c1ccccc1 Show InChI InChI=1S/C22H28N2O2S/c1-21(2)14-17(15-22(3,4)24-21)23-20(25)16-10-12-19(13-11-16)27(26)18-8-6-5-7-9-18/h5-13,17,24H,14-15H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
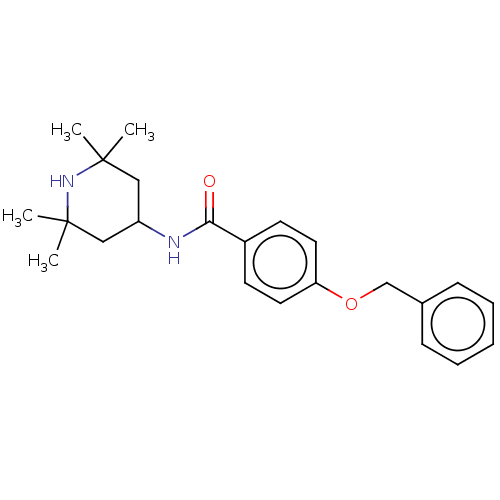
(Homo sapiens (Human)) | BDBM50010662
(CHEMBL3264751)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C23H30N2O2/c1-22(2)14-19(15-23(3,4)25-22)24-21(26)18-10-12-20(13-11-18)27-16-17-8-6-5-7-9-17/h5-13,19,25H,14-16H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010713
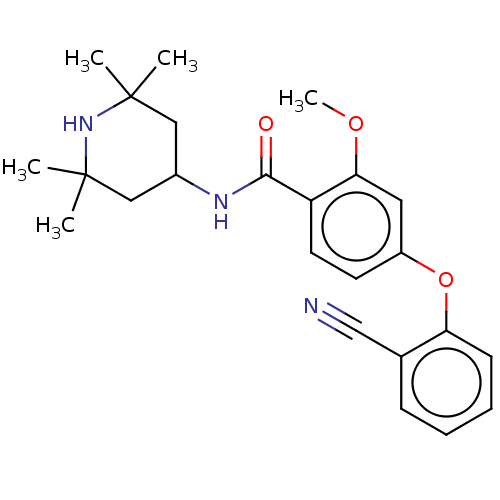
(CHEMBL3264773)Show SMILES COc1cc(Oc2ccccc2C#N)ccc1C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C24H29N3O3/c1-23(2)13-17(14-24(3,4)27-23)26-22(28)19-11-10-18(12-21(19)29-5)30-20-9-7-6-8-16(20)15-25/h6-12,17,27H,13-14H2,1-5H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010670
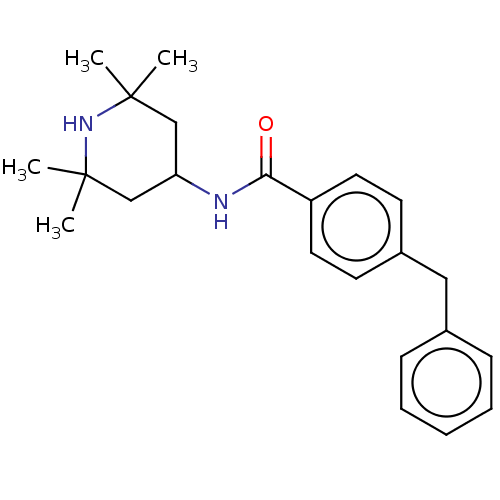
(CHEMBL3264759)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Cc2ccccc2)cc1 Show InChI InChI=1S/C23H30N2O/c1-22(2)15-20(16-23(3,4)25-22)24-21(26)19-12-10-18(11-13-19)14-17-8-6-5-7-9-17/h5-13,20,25H,14-16H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010671
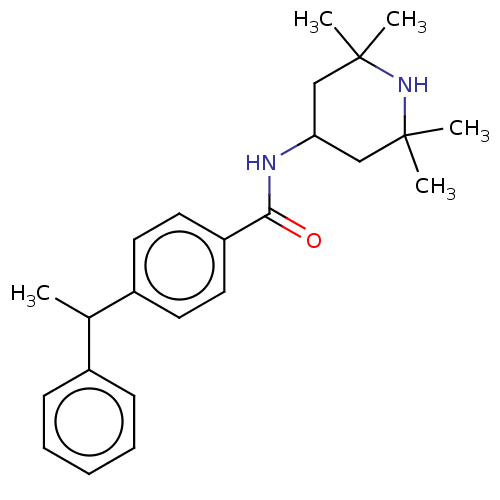
(CHEMBL3264760)Show SMILES CC(c1ccccc1)c1ccc(cc1)C(=O)NC1CC(C)(C)NC(C)(C)C1 Show InChI InChI=1S/C24H32N2O/c1-17(18-9-7-6-8-10-18)19-11-13-20(14-12-19)22(27)25-21-15-23(2,3)26-24(4,5)16-21/h6-14,17,21,26H,15-16H2,1-5H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010672
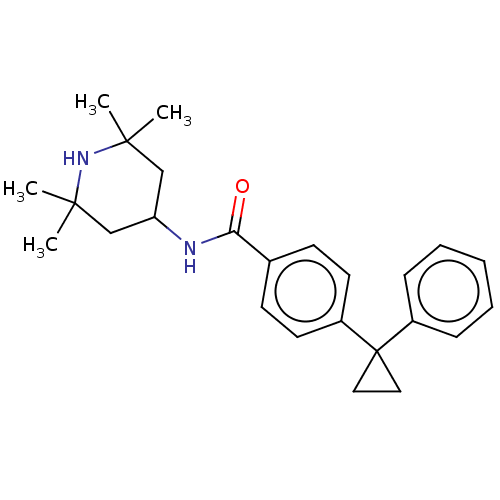
(CHEMBL3264761)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(cc1)C1(CC1)c1ccccc1 Show InChI InChI=1S/C25H32N2O/c1-23(2)16-21(17-24(3,4)27-23)26-22(28)18-10-12-20(13-11-18)25(14-15-25)19-8-6-5-7-9-19/h5-13,21,27H,14-17H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010675
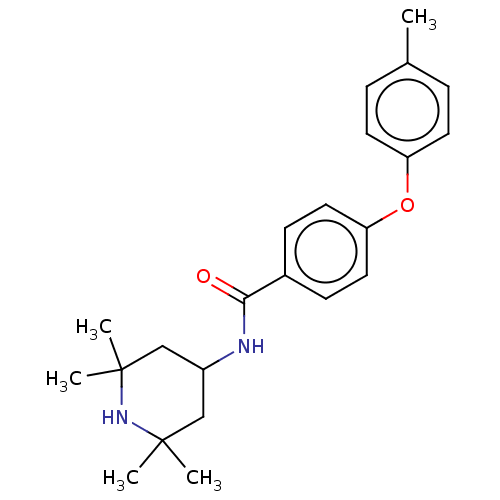
(CHEMBL3264764)Show SMILES Cc1ccc(Oc2ccc(cc2)C(=O)NC2CC(C)(C)NC(C)(C)C2)cc1 Show InChI InChI=1S/C23H30N2O2/c1-16-6-10-19(11-7-16)27-20-12-8-17(9-13-20)21(26)24-18-14-22(2,3)25-23(4,5)15-18/h6-13,18,25H,14-15H2,1-5H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010826
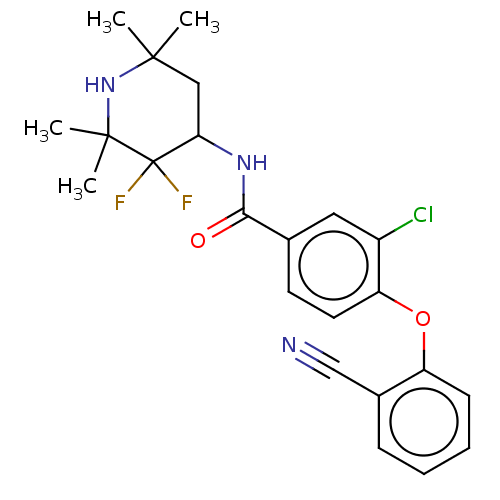
(CHEMBL3264790)Show SMILES CC1(C)CC(NC(=O)c2ccc(Oc3ccccc3C#N)c(Cl)c2)C(F)(F)C(C)(C)N1 Show InChI InChI=1S/C23H24ClF2N3O2/c1-21(2)12-19(23(25,26)22(3,4)29-21)28-20(30)14-9-10-18(16(24)11-14)31-17-8-6-5-7-15(17)13-27/h5-11,19,29H,12H2,1-4H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data