

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50396534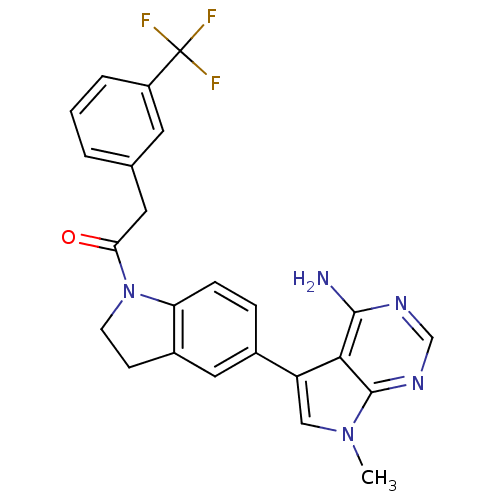 (CHEMBL2171124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442165 (CHEMBL2441341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442162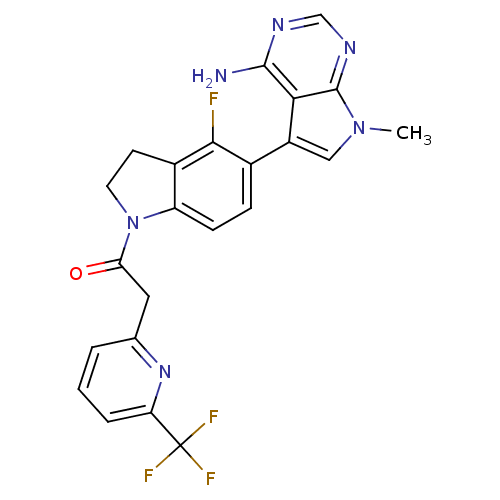 (CHEMBL2441342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442166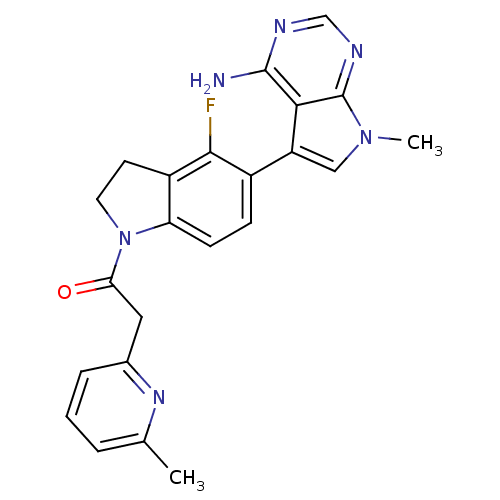 (CHEMBL2441340) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442164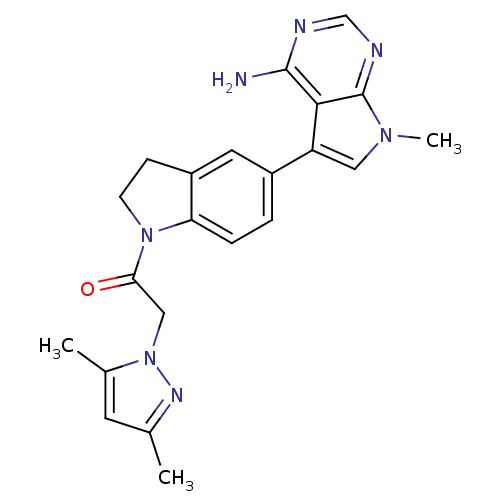 (CHEMBL2441345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442167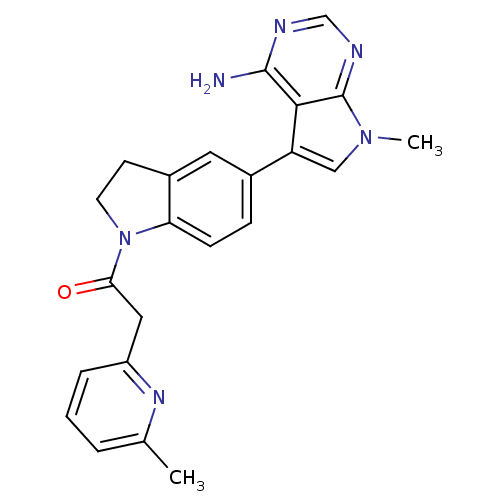 (CHEMBL2441339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442163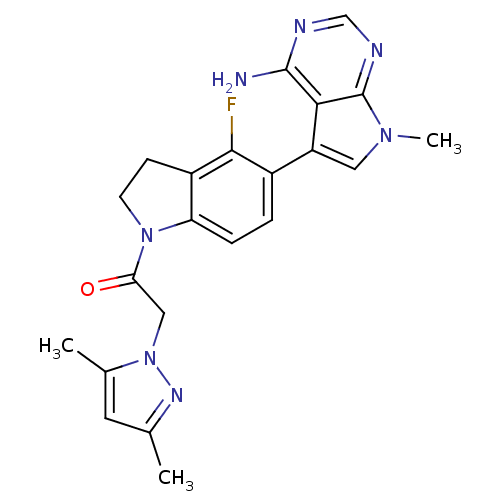 (CHEMBL2441346) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442168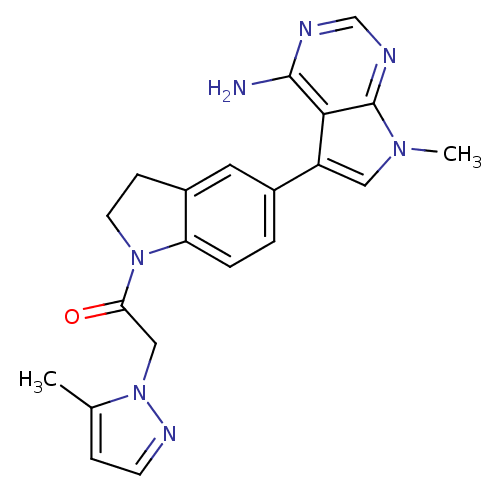 (CHEMBL2441344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442172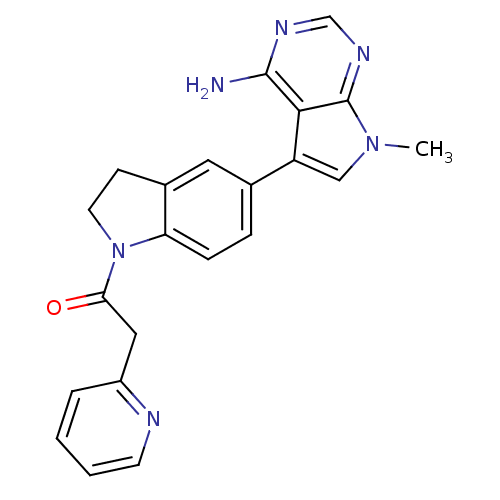 (CHEMBL2441336) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442171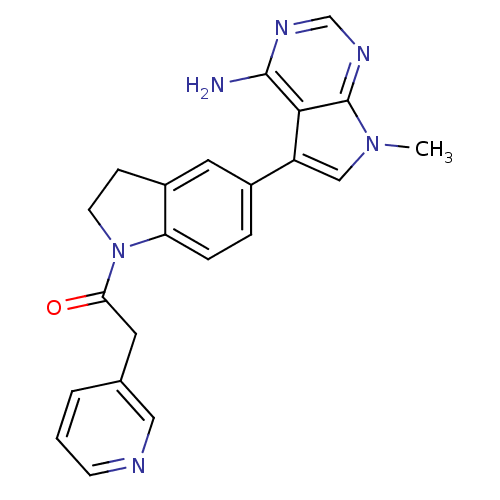 (CHEMBL2441337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of thapsigargin-induced autophosphorylation of PERK in human A549 cells preincubated for 1 hr followed by thapsigargin-induction measured ... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of thapsigargin-induced autophosphorylation of PERK in human A549 cells preincubated for 1 hr followed by thapsigargin-induction measured ... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442165 (CHEMBL2441341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of thapsigargin-induced autophosphorylation of PERK in human A549 cells preincubated for 1 hr followed by thapsigargin-induction measured ... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 1 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HRI (unknown origin) assessed as EIF2AK1 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442169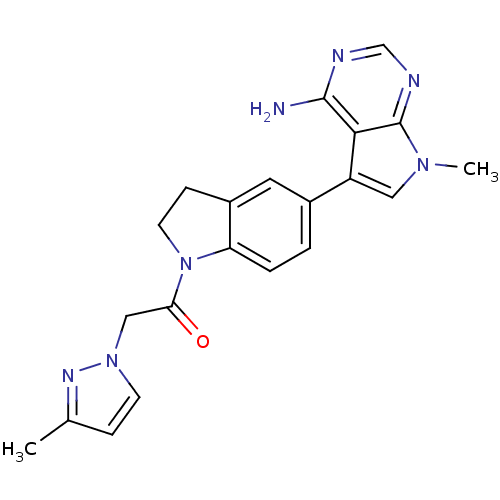 (CHEMBL2441343) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 1 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HRI (unknown origin) assessed as EIF2AK1 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442170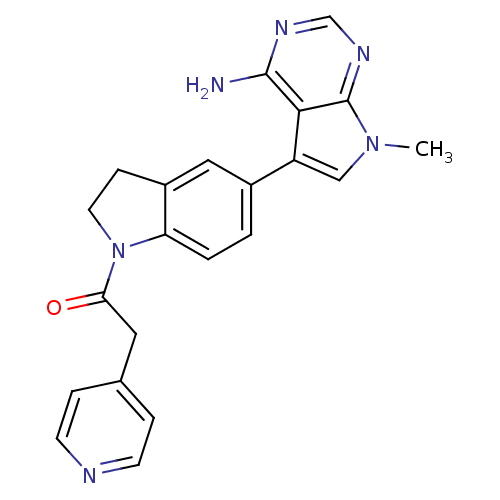 (CHEMBL2441338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interferon-induced, double-stranded RNA-activated protein kinase (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of PKR (unknown origin) assessed as EIF2AK2 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442167 (CHEMBL2441339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of thapsigargin-induced autophosphorylation of PERK in human A549 cells preincubated for 1 hr followed by thapsigargin-induction measured ... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50442164 (CHEMBL2441345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of thapsigargin-induced autophosphorylation of PERK in human A549 cells preincubated for 1 hr followed by thapsigargin-induction measured ... | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interferon-induced, double-stranded RNA-activated protein kinase (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of PKR (unknown origin) assessed as EIF2AK2 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 1 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HRI (unknown origin) assessed as EIF2AK1 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| eIF-2-alpha kinase GCN2 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 776 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GCN2 (unknown origin) assessed as EIF2AK4 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50396534 (CHEMBL2171124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interferon-induced, double-stranded RNA-activated protein kinase (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 905 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of PKR (unknown origin) assessed as EIF2AK2 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| eIF-2-alpha kinase GCN2 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GCN2 (unknown origin) assessed as EIF2AK4 phosphorylation | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50396534 (CHEMBL2171124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50396534 (CHEMBL2171124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50396534 (CHEMBL2171124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50396534 (CHEMBL2171124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50442162 (CHEMBL2441342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50442166 (CHEMBL2441340) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50442163 (CHEMBL2441346) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 60 mins by LC-MS/MS analysis | ACS Med Chem Lett 4: 964-8 (2013) Article DOI: 10.1021/ml400228e BindingDB Entry DOI: 10.7270/Q24X597X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||