

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170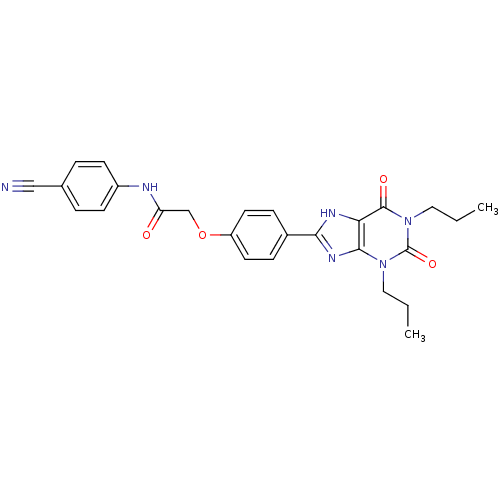 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50336977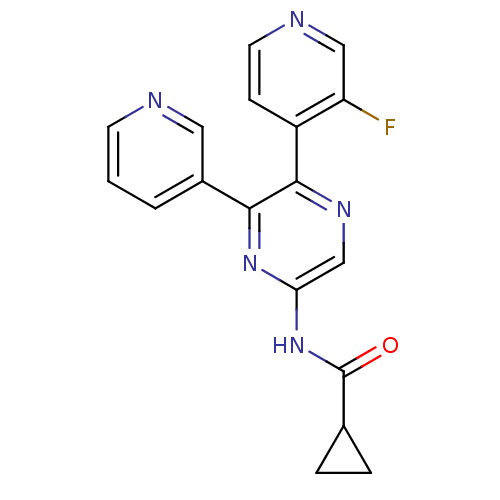 (CHEMBL1672627 | N-[5-(3-Fluoropyridin-4-yl)-6-pyri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM10849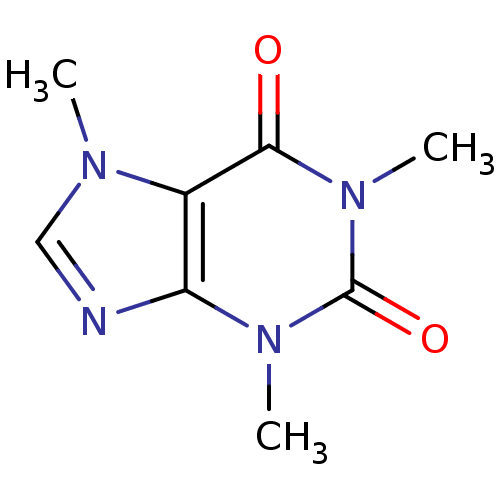 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254888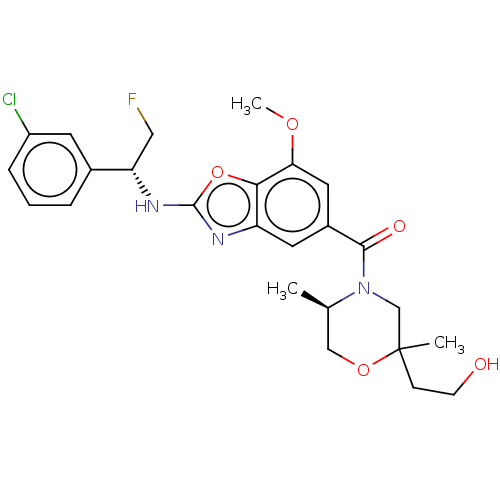 (US9493472, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254889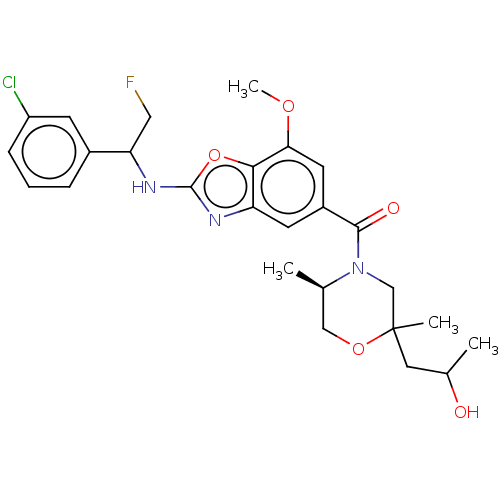 (US9493472, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254905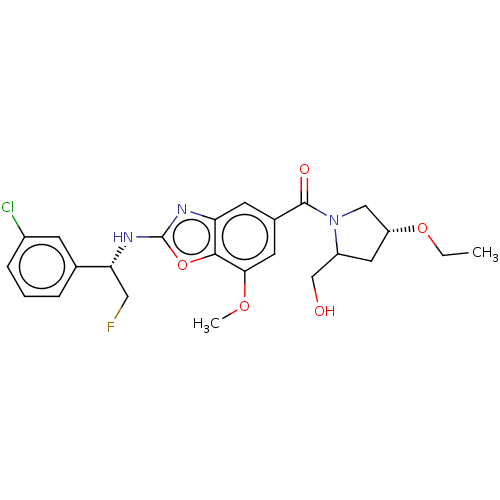 (US9493472, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254904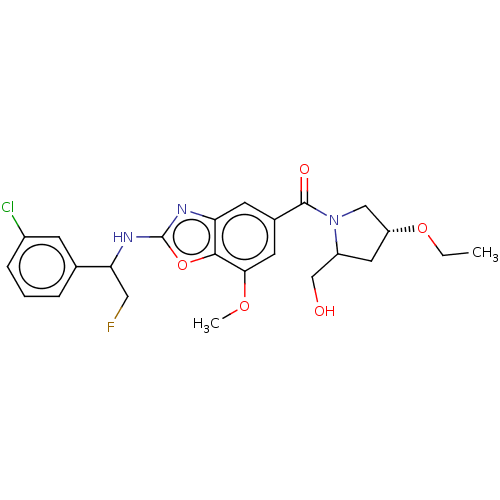 (US9493472, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254886 (US9493472, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254896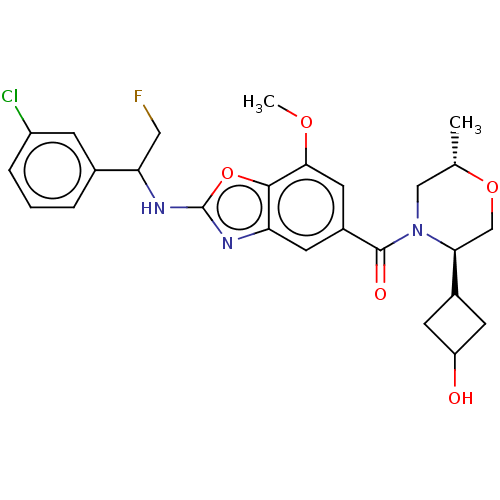 (US9493472, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254895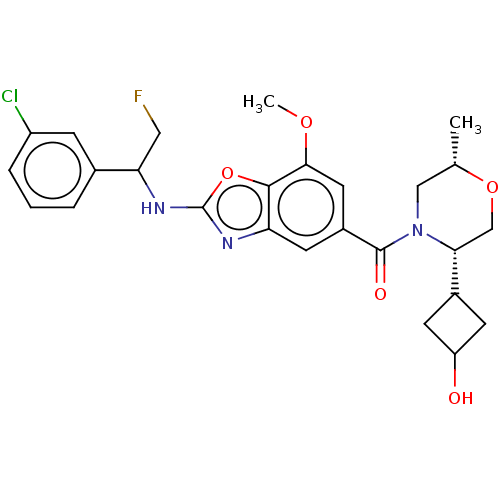 (US9493472, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254911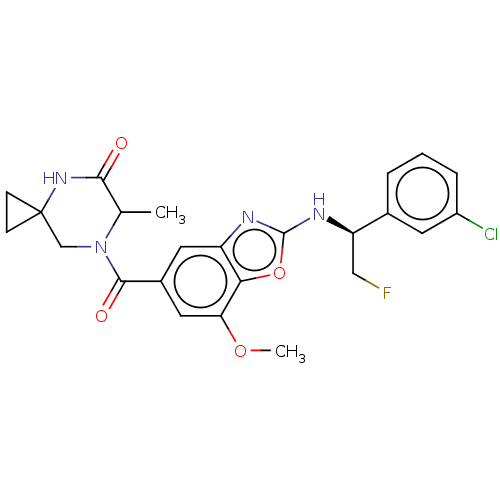 (US9493472, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254894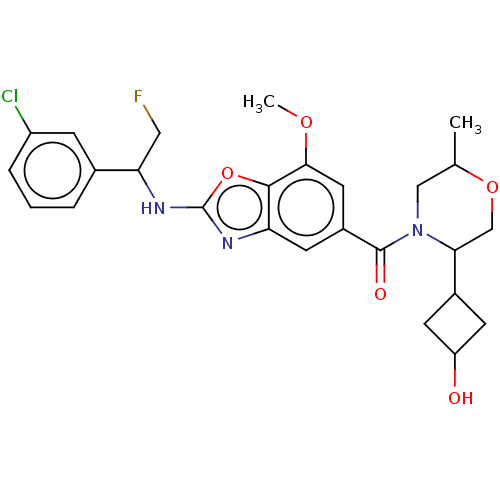 (US9493472, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907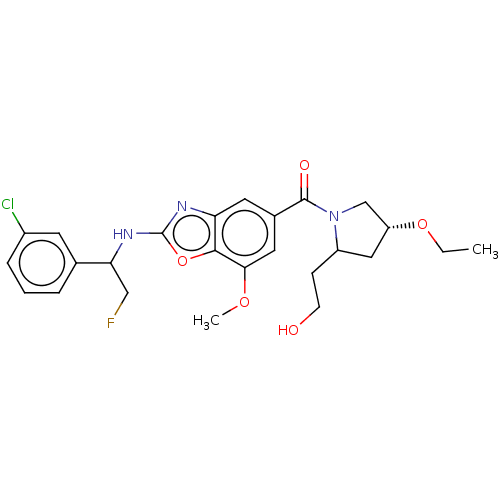 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254910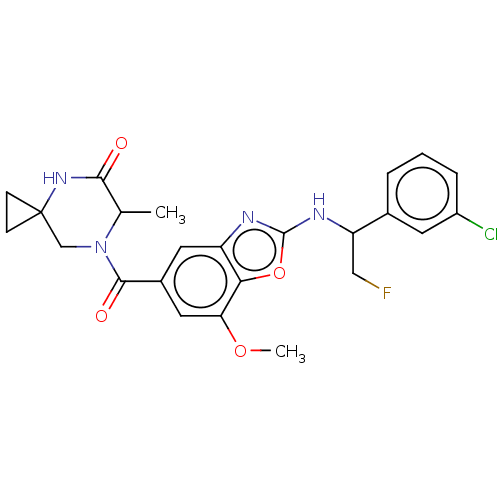 (US9493472, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254892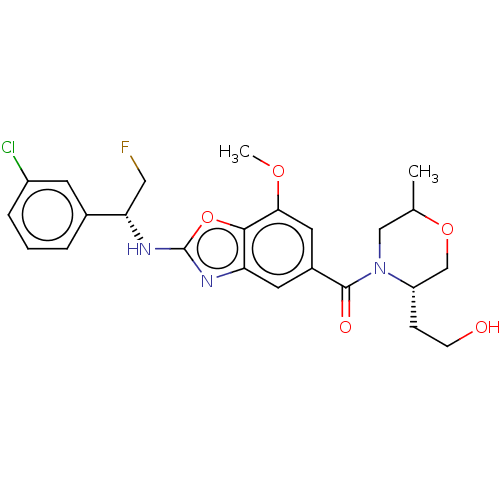 (US9493472, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254897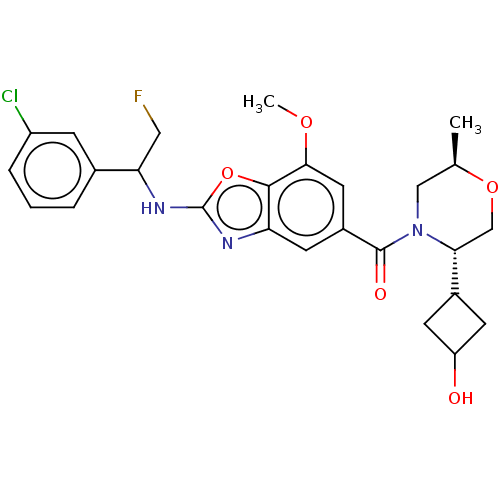 (US9493472, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254882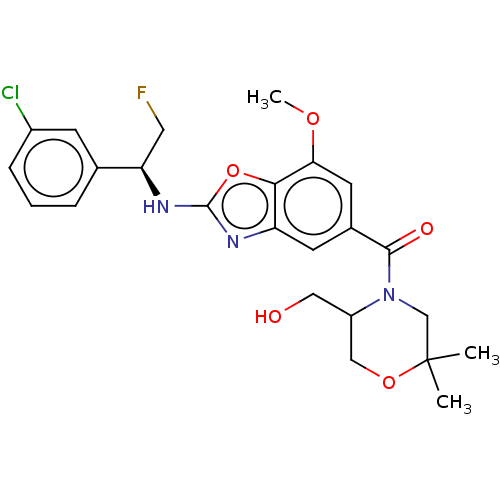 (US9493472, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254890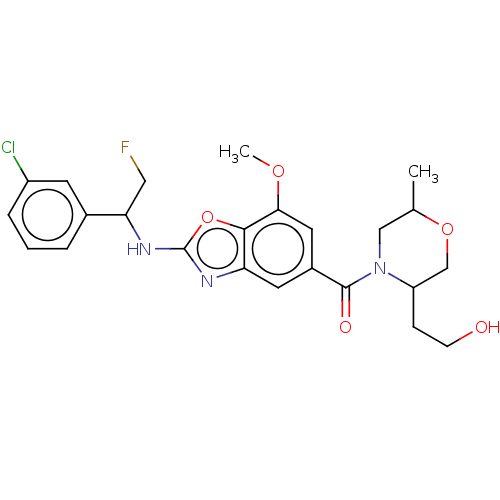 (US9493472, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254899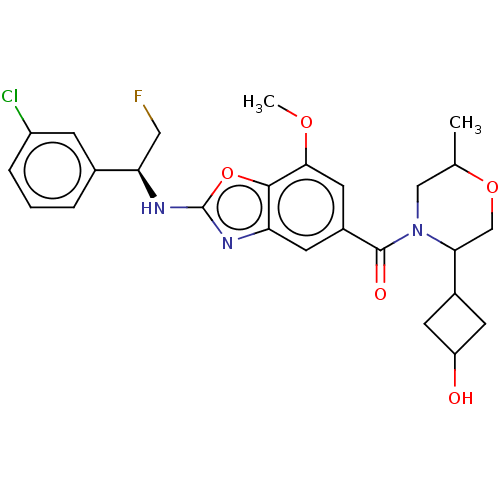 (US9493472, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254898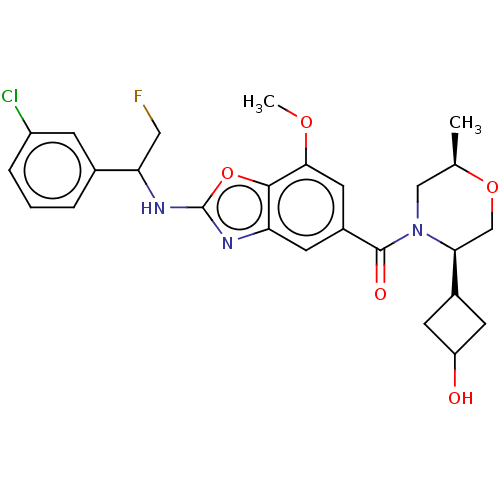 (US9493472, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254900 (US9493472, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254881 (US9493472, 4 | US9493472, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254901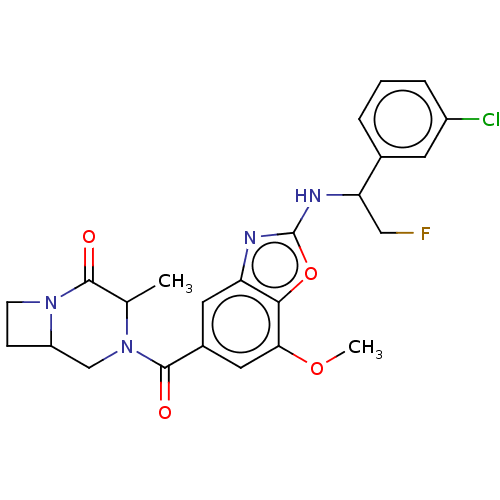 (US9493472, 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254891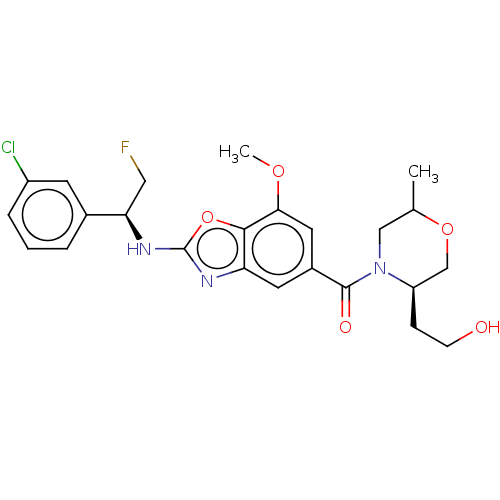 (US9493472, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254906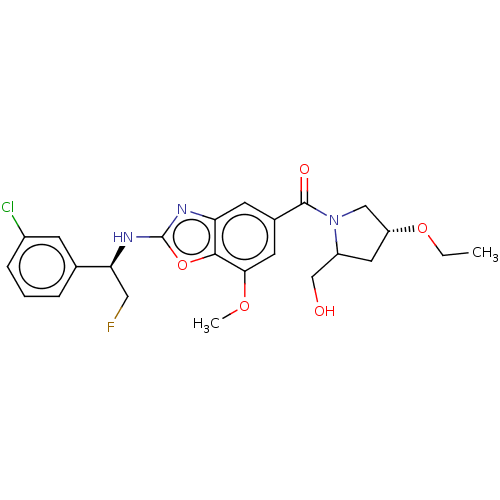 (US9493472, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254880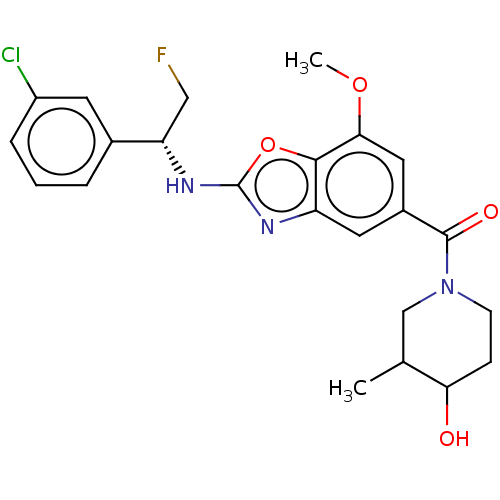 (US9493472, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50531447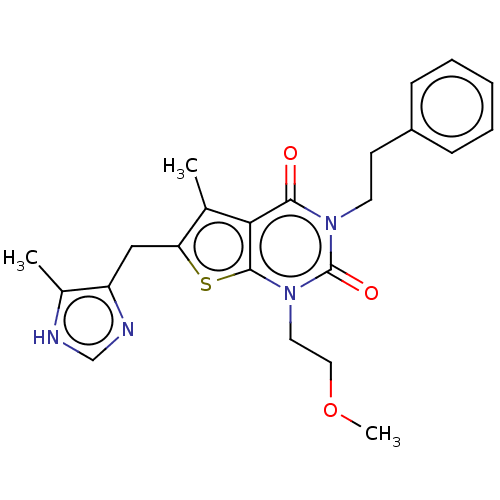 (CHEMBL4569909) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A2B adenosine receptor expressed in HEK293 cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM97722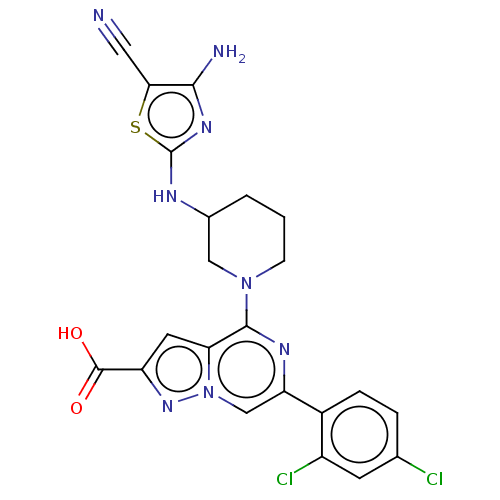 (US8476267, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 30 |
Bayer Intellectual Property GmbH US Patent | Assay Description The inhibitory activity of active substance is determined in a biochemical assay. | US Patent US8476267 (2013) BindingDB Entry DOI: 10.7270/Q20863X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254878 (US9493472, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254903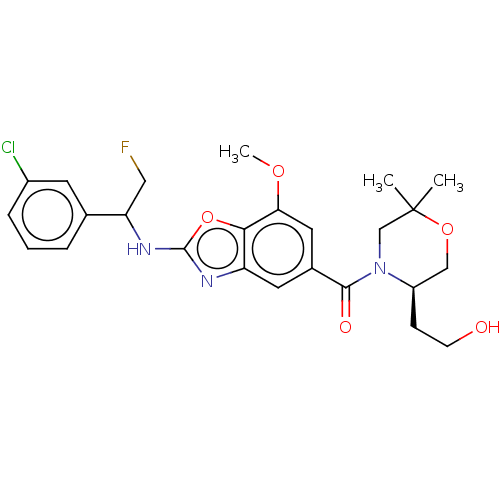 (US9493472, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254902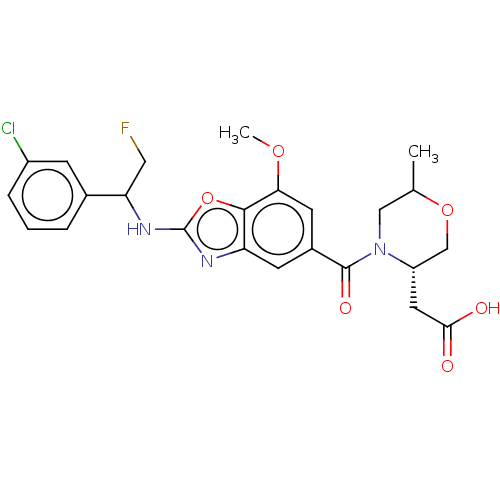 (US9493472, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM97723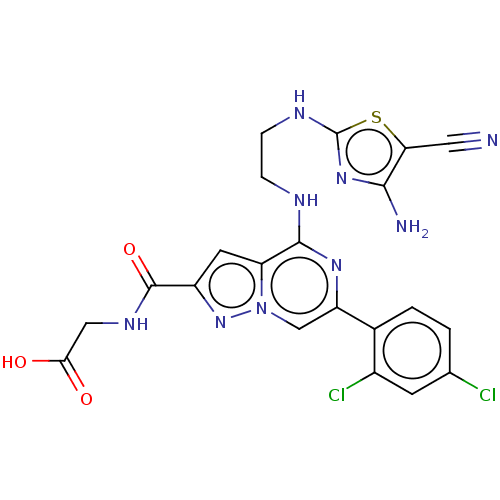 (US8476267, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 30 |
Bayer Intellectual Property GmbH US Patent | Assay Description The inhibitory activity of active substance is determined in a biochemical assay. | US Patent US8476267 (2013) BindingDB Entry DOI: 10.7270/Q20863X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254884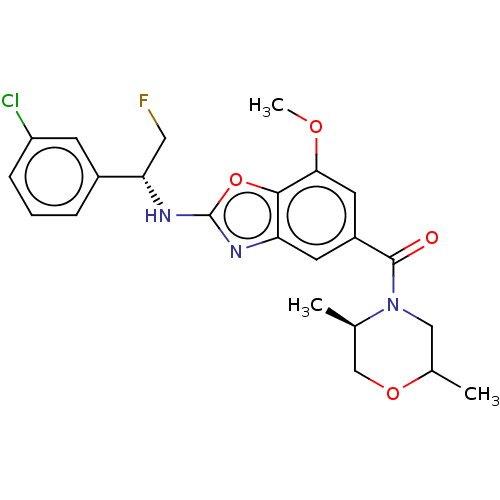 (US9493472, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM150313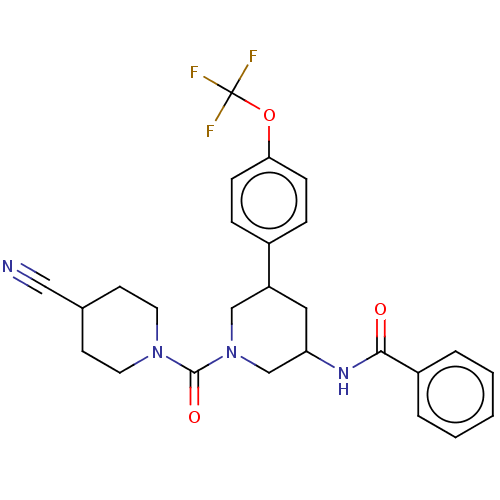 (US8987248, 106) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.05 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description Platelet membranes are incubated with 12 nM [3H]haTRAP and test substance in different concentrations in a buffer (50 mM Tris pH 7.5, 10 mM magnesium... | US Patent US8987248 (2015) BindingDB Entry DOI: 10.7270/Q2QC027W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM97721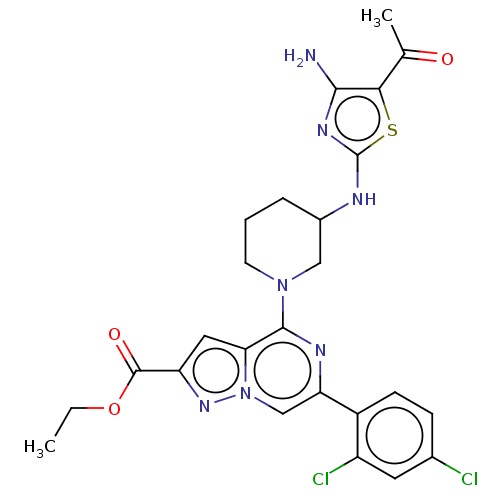 (US8476267, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 30 |
Bayer Intellectual Property GmbH US Patent | Assay Description The inhibitory activity of active substance is determined in a biochemical assay. | US Patent US8476267 (2013) BindingDB Entry DOI: 10.7270/Q20863X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120247 (3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human recombinant Poly (ADP-ribose) polymerase 1 was determined using doseresponse inhibition | Bioorg Med Chem Lett 12: 3187-90 (2002) BindingDB Entry DOI: 10.7270/Q2VX0FTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50531478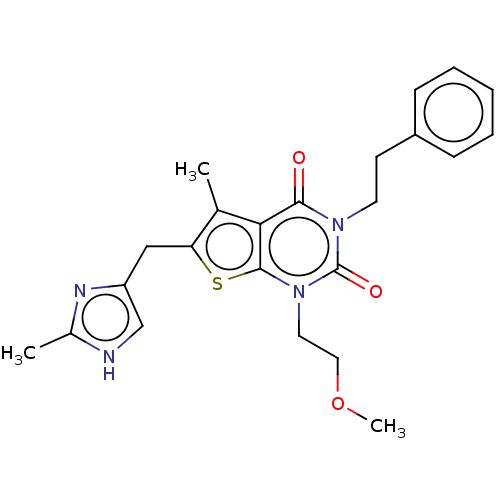 (CHEMBL4576817) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A2B adenosine receptor expressed in HEK293 cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254881 (US9493472, 4 | US9493472, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254885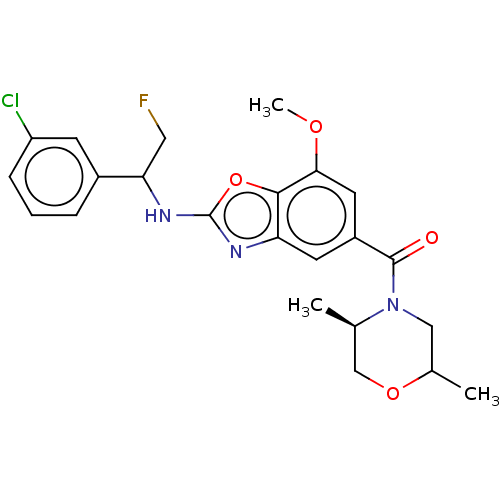 (US9493472, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM150314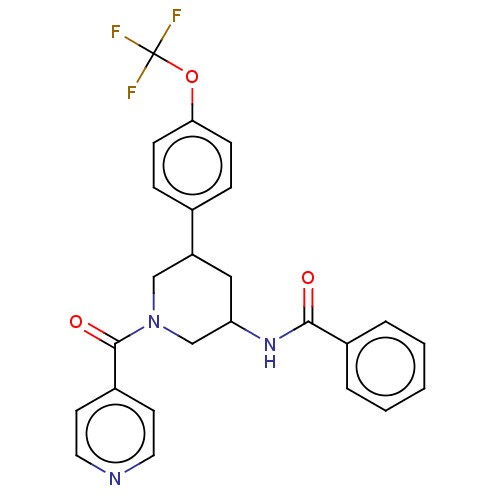 (US8987248, 115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description Platelet membranes are incubated with 12 nM [3H]haTRAP and test substance in different concentrations in a buffer (50 mM Tris pH 7.5, 10 mM magnesium... | US Patent US8987248 (2015) BindingDB Entry DOI: 10.7270/Q2QC027W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM150322 (US8987248, 156) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description Platelet membranes are incubated with 12 nM [3H]haTRAP and test substance in different concentrations in a buffer (50 mM Tris pH 7.5, 10 mM magnesium... | US Patent US8987248 (2015) BindingDB Entry DOI: 10.7270/Q2QC027W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120246 (3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant PARP-1 | Bioorg Med Chem Lett 12: 3187-90 (2002) BindingDB Entry DOI: 10.7270/Q2VX0FTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50531452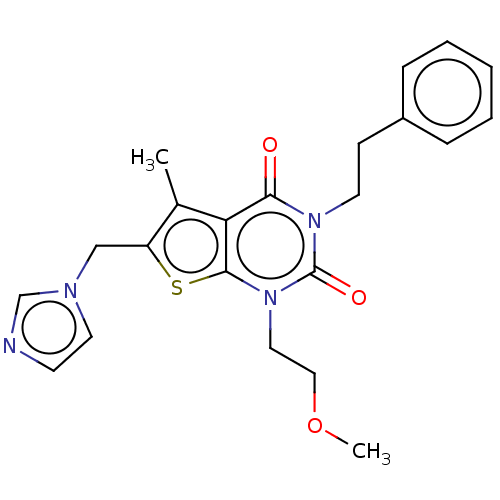 (CHEMBL4521489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A2B adenosine receptor expressed in HEK293 cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120236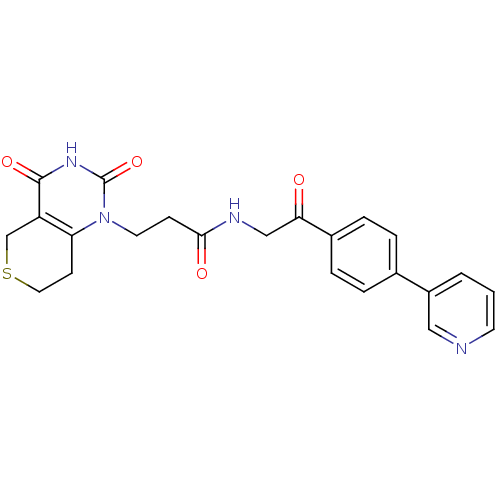 (3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant PARP-1 | Bioorg Med Chem Lett 12: 3187-90 (2002) BindingDB Entry DOI: 10.7270/Q2VX0FTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50531434 (CHEMBL4458223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A2B adenosine receptor expressed in HEK293 cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 247 total ) | Next | Last >> |