 Found 108 hits with Last Name = 'glynne' and Initial = 'r'
Found 108 hits with Last Name = 'glynne' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538098

(CHEMBL4640031)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C(=O)Nc1cc(ccn1)-c1cn([C@H]2CC[C@@](C)(O)CC2)c2cnccc12 |r,wU:23.25,5.5,1.0,wD:20.21,(67.8,-7.39,;66.77,-6.25,;65.27,-6.57,;64.23,-5.42,;64.7,-3.97,;66.21,-3.64,;66.68,-2.18,;67.24,-4.79,;62.73,-5.75,;62.26,-7.22,;61.69,-4.61,;60.19,-4.94,;59.72,-6.41,;58.21,-6.74,;57.18,-5.6,;57.64,-4.14,;59.15,-3.8,;57.75,-8.2,;58.66,-9.44,;57.76,-10.69,;58.24,-12.15,;57.22,-13.3,;57.69,-14.75,;59.2,-15.07,;58.8,-16.55,;60.28,-16.15,;60.23,-13.92,;59.75,-12.45,;56.29,-10.22,;54.95,-11,;53.61,-10.23,;53.62,-8.69,;54.95,-7.92,;56.28,-8.68,)| Show InChI InChI=1S/C26H33N5O3/c1-17-14-30(15-18(2)34-17)25(32)29-24-12-19(6-11-28-24)22-16-31(23-13-27-10-7-21(22)23)20-4-8-26(3,33)9-5-20/h6-7,10-13,16-18,20,33H,4-5,8-9,14-15H2,1-3H3,(H,28,29,32)/t17-,18+,20-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538084

(CHEMBL4647659)Show SMILES CC(C)Oc1ccc(F)c(c1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1N1CCC[C@H](C1)C(O)=O |r| Show InChI InChI=1S/C25H27FN6O4/c1-14(2)36-16-5-6-18(26)17(10-16)19-12-29-23(27)22(30-19)24(33)31-20-11-28-8-7-21(20)32-9-3-4-15(13-32)25(34)35/h5-8,10-12,14-15H,3-4,9,13H2,1-2H3,(H2,27,29)(H,31,33)(H,34,35)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DYRK1A (129 to 509 residues) expressed in mammalian expression system by Kinomescan method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538099
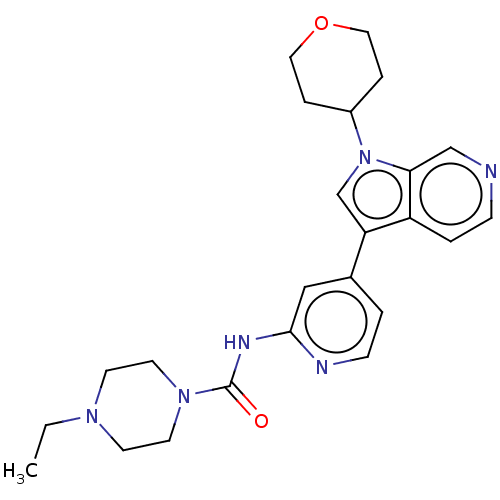
(CHEMBL4636064)Show SMILES CCN1CCN(CC1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 Show InChI InChI=1S/C24H30N6O2/c1-2-28-9-11-29(12-10-28)24(31)27-23-15-18(3-8-26-23)21-17-30(19-5-13-32-14-6-19)22-16-25-7-4-20(21)22/h3-4,7-8,15-17,19H,2,5-6,9-14H2,1H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538095

(CHEMBL4649473)Show SMILES CC1(C)CN(C1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 Show InChI InChI=1S/C23H27N5O2/c1-23(2)14-27(15-23)22(29)26-21-11-16(3-8-25-21)19-13-28(17-5-9-30-10-6-17)20-12-24-7-4-18(19)20/h3-4,7-8,11-13,17H,5-6,9-10,14-15H2,1-2H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538090
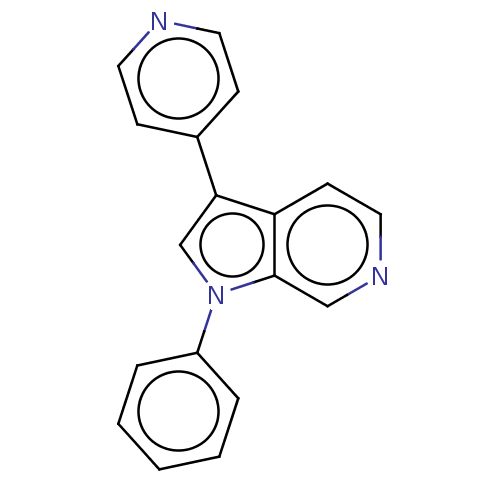
(CHEMBL4648742)Show InChI InChI=1S/C18H13N3/c1-2-4-15(5-3-1)21-13-17(14-6-9-19-10-7-14)16-8-11-20-12-18(16)21/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538093
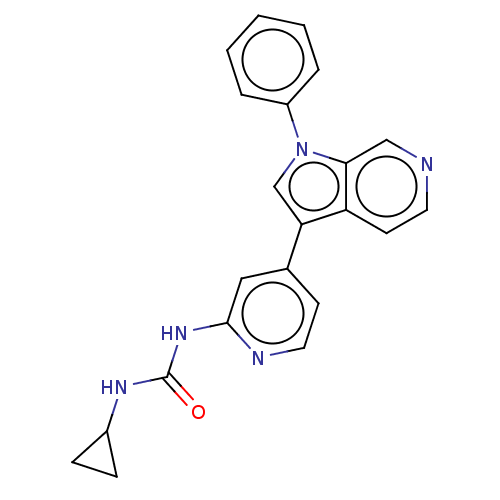
(CHEMBL4645474)Show SMILES O=C(NC1CC1)Nc1cc(ccn1)-c1cn(-c2ccccc2)c2cnccc12 Show InChI InChI=1S/C22H19N5O/c28-22(25-16-6-7-16)26-21-12-15(8-11-24-21)19-14-27(17-4-2-1-3-5-17)20-13-23-10-9-18(19)20/h1-5,8-14,16H,6-7H2,(H2,24,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538084

(CHEMBL4647659)Show SMILES CC(C)Oc1ccc(F)c(c1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1N1CCC[C@H](C1)C(O)=O |r| Show InChI InChI=1S/C25H27FN6O4/c1-14(2)36-16-5-6-18(26)17(10-16)19-12-29-23(27)22(30-19)24(33)31-20-11-28-8-7-21(20)32-9-3-4-15(13-32)25(34)35/h5-8,10-12,14-15H,3-4,9,13H2,1-2H3,(H2,27,29)(H,31,33)(H,34,35)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GSK3beta (1 to 433 residues) expressed in mammalian expression system by Kinomescan method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538094

(CHEMBL4641631)Show SMILES O=C(NC1CC1)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 Show InChI InChI=1S/C21H23N5O2/c27-21(24-15-1-2-15)25-20-11-14(3-8-23-20)18-13-26(16-5-9-28-10-6-16)19-12-22-7-4-17(18)19/h3-4,7-8,11-13,15-16H,1-2,5-6,9-10H2,(H2,23,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538091
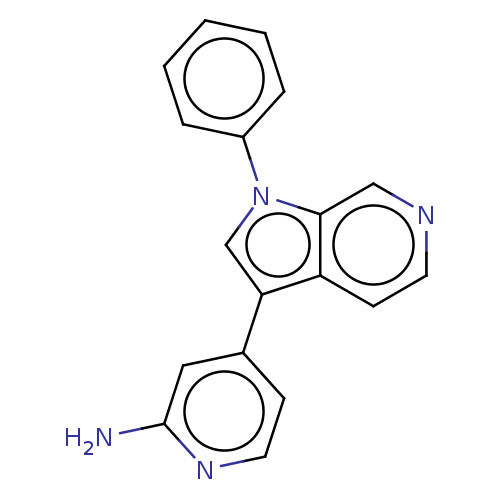
(CHEMBL4640465)Show InChI InChI=1S/C18H14N4/c19-18-10-13(6-9-21-18)16-12-22(14-4-2-1-3-5-14)17-11-20-8-7-15(16)17/h1-12H,(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538096

(CHEMBL4641672)Show SMILES O=C(Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12)N1CCOCC1 Show InChI InChI=1S/C22H25N5O3/c28-22(26-7-11-30-12-8-26)25-21-13-16(1-6-24-21)19-15-27(17-3-9-29-10-4-17)20-14-23-5-2-18(19)20/h1-2,5-6,13-15,17H,3-4,7-12H2,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538083

(CHEMBL4638292)Show SMILES Nc1ncc(nc1C(=O)Nc1cnccc1N1CCC(CC1)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C22H22N6O3/c23-20-19(26-16(13-25-20)14-4-2-1-3-5-14)21(29)27-17-12-24-9-6-18(17)28-10-7-15(8-11-28)22(30)31/h1-6,9,12-13,15H,7-8,10-11H2,(H2,23,25)(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GSK3beta (1 to 433 residues) expressed in mammalian expression system by Kinomescan method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538097

(CHEMBL4641839)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 |r| Show InChI InChI=1S/C24H29N5O3/c1-16-13-28(14-17(2)32-16)24(30)27-23-11-18(3-8-26-23)21-15-29(19-5-9-31-10-6-19)22-12-25-7-4-20(21)22/h3-4,7-8,11-12,15-17,19H,5-6,9-10,13-14H2,1-2H3,(H,26,27,30)/t16-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538085

(CHEMBL4634969)Show InChI InChI=1S/C16H17N3/c1-12(2)10-19-11-15(13-3-6-17-7-4-13)14-5-8-18-9-16(14)19/h3-9,11-12H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50375654

(CHEMBL99203 | US11633415, Compound 5-iodotubercidi...)Show SMILES Nc1ncnc2n(cc(I)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of DYRK1A (unknown origin) |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538083

(CHEMBL4638292)Show SMILES Nc1ncc(nc1C(=O)Nc1cnccc1N1CCC(CC1)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C22H22N6O3/c23-20-19(26-16(13-25-20)14-4-2-1-3-5-14)21(29)27-17-12-24-9-6-18(17)28-10-7-15(8-11-28)22(30)31/h1-6,9,12-13,15H,7-8,10-11H2,(H2,23,25)(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DYRK1A (129 to 509 residues) expressed in mammalian expression system by Kinomescan method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538089

(CHEMBL4638460)Show InChI InChI=1S/C19H15N3/c1-2-4-15(5-3-1)13-22-14-18(16-6-9-20-10-7-16)17-8-11-21-12-19(17)22/h1-12,14H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538086
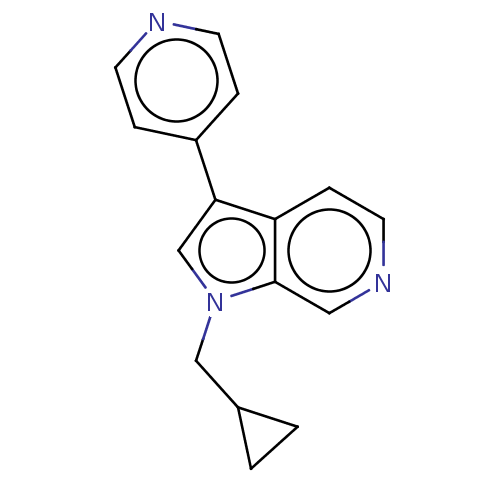
(CHEMBL4648945)Show InChI InChI=1S/C16H15N3/c1-2-12(1)10-19-11-15(13-3-6-17-7-4-13)14-5-8-18-9-16(14)19/h3-9,11-12H,1-2,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538092

(CHEMBL4648047)Show SMILES O=C(Nc1cc(ccn1)-c1cn(-c2ccccc2)c2cnccc12)C1CC1 Show InChI InChI=1S/C22H18N4O/c27-22(15-6-7-15)25-21-12-16(8-11-24-21)19-14-26(17-4-2-1-3-5-17)20-13-23-10-9-18(19)20/h1-5,8-15H,6-7H2,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50251436
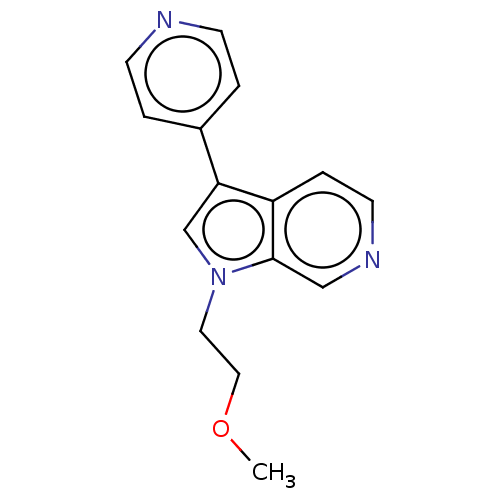
(CHEMBL4091320)Show InChI InChI=1S/C15H15N3O/c1-19-9-8-18-11-14(12-2-5-16-6-3-12)13-4-7-17-10-15(13)18/h2-7,10-11H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538092

(CHEMBL4648047)Show SMILES O=C(Nc1cc(ccn1)-c1cn(-c2ccccc2)c2cnccc12)C1CC1 Show InChI InChI=1S/C22H18N4O/c27-22(15-6-7-15)25-21-12-16(8-11-24-21)19-14-26(17-4-2-1-3-5-17)20-13-23-10-9-18(19)20/h1-5,8-15H,6-7H2,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 301 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538087
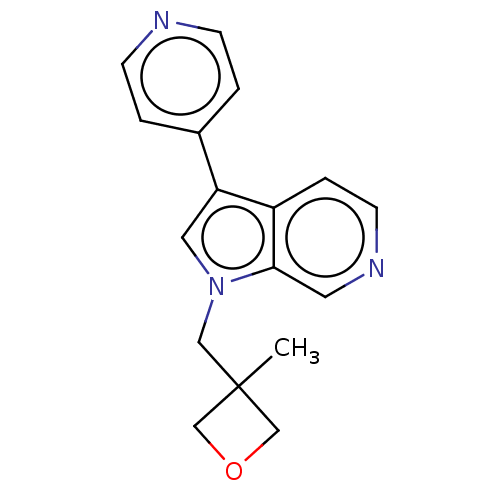
(CHEMBL4644898)Show InChI InChI=1S/C17H17N3O/c1-17(11-21-12-17)10-20-9-15(13-2-5-18-6-3-13)14-4-7-19-8-16(14)20/h2-9H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50538088
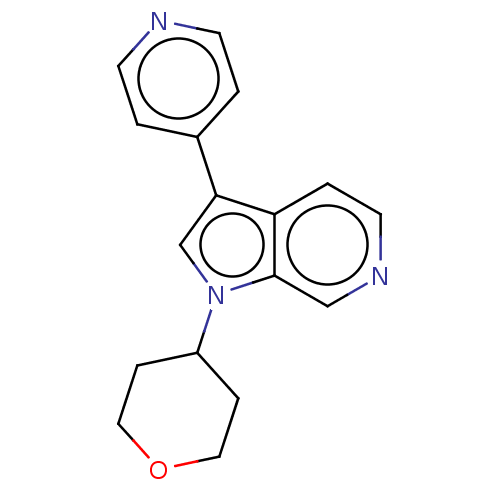
(CHEMBL4640598)Show InChI InChI=1S/C17H17N3O/c1-6-18-7-2-13(1)16-12-20(14-4-9-21-10-5-14)17-11-19-8-3-15(16)17/h1-3,6-8,11-12,14H,4-5,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538091

(CHEMBL4640465)Show InChI InChI=1S/C18H14N4/c19-18-10-13(6-9-21-18)16-12-22(14-4-2-1-3-5-14)17-11-20-8-7-15(16)17/h1-12H,(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538093

(CHEMBL4645474)Show SMILES O=C(NC1CC1)Nc1cc(ccn1)-c1cn(-c2ccccc2)c2cnccc12 Show InChI InChI=1S/C22H19N5O/c28-22(25-16-6-7-16)26-21-12-15(8-11-24-21)19-14-27(17-4-2-1-3-5-17)20-13-23-10-9-18(19)20/h1-5,8-14,16H,6-7H2,(H2,24,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Qpatch S8 assay |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538092

(CHEMBL4648047)Show SMILES O=C(Nc1cc(ccn1)-c1cn(-c2ccccc2)c2cnccc12)C1CC1 Show InChI InChI=1S/C22H18N4O/c27-22(15-6-7-15)25-21-12-16(8-11-24-21)19-14-26(17-4-2-1-3-5-17)20-13-23-10-9-18(19)20/h1-5,8-15H,6-7H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538093

(CHEMBL4645474)Show SMILES O=C(NC1CC1)Nc1cc(ccn1)-c1cn(-c2ccccc2)c2cnccc12 Show InChI InChI=1S/C22H19N5O/c28-22(25-16-6-7-16)26-21-12-15(8-11-24-21)19-14-27(17-4-2-1-3-5-17)20-13-23-10-9-18(19)20/h1-5,8-14,16H,6-7H2,(H2,24,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347943
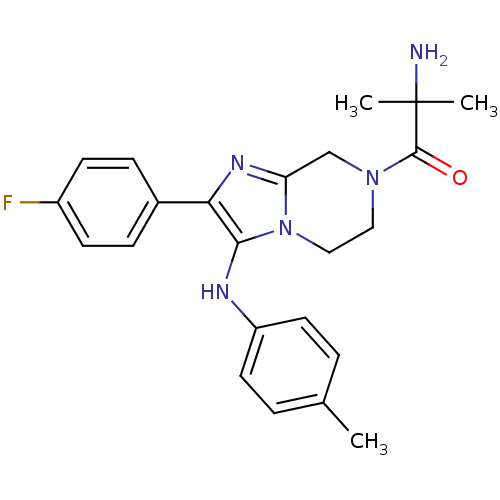
(CHEMBL1800770)Show SMILES Cc1ccc(Nc2c(nc3CN(CCn23)C(=O)C(C)(C)N)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H26FN5O/c1-15-4-10-18(11-5-15)26-21-20(16-6-8-17(24)9-7-16)27-19-14-28(12-13-29(19)21)22(30)23(2,3)25/h4-11,26H,12-14,25H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Q-patch assay |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50495687
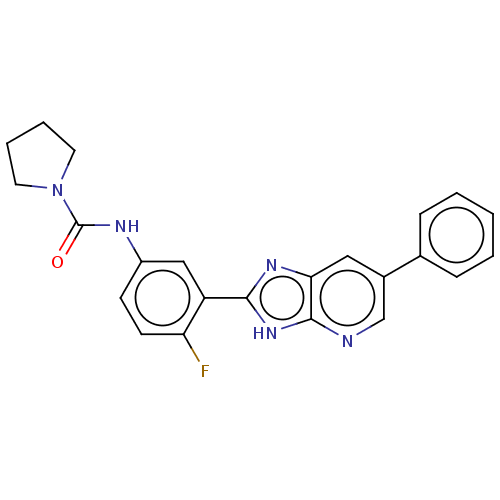
(CHEMBL3115821)Show SMILES Fc1ccc(NC(=O)N2CCCC2)cc1-c1nc2cc(cnc2[nH]1)-c1ccccc1 Show InChI InChI=1S/C23H20FN5O/c24-19-9-8-17(26-23(30)29-10-4-5-11-29)13-18(19)21-27-20-12-16(14-25-22(20)28-21)15-6-2-1-3-7-15/h1-3,6-9,12-14H,4-5,10-11H2,(H,26,30)(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 57: 828-35 (2014)
Article DOI: 10.1021/jm401178t
BindingDB Entry DOI: 10.7270/Q28D006X |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538091

(CHEMBL4640465)Show InChI InChI=1S/C18H14N4/c19-18-10-13(6-9-21-18)16-12-22(14-4-2-1-3-5-14)17-11-20-8-7-15(16)17/h1-12H,(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347944
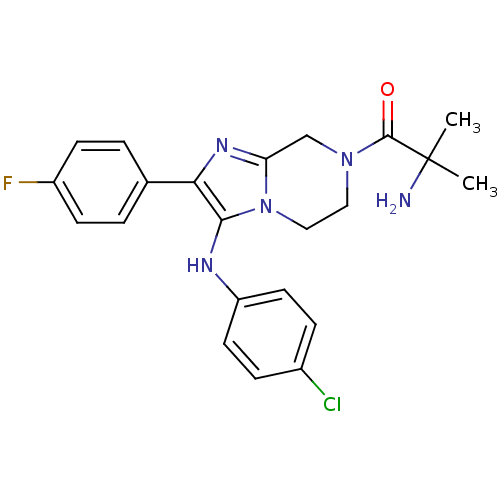
(CHEMBL1800772)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H23ClFN5O/c1-22(2,25)21(30)28-11-12-29-18(13-28)27-19(14-3-7-16(24)8-4-14)20(29)26-17-9-5-15(23)6-10-17/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Q-patch assay |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538095

(CHEMBL4649473)Show SMILES CC1(C)CN(C1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 Show InChI InChI=1S/C23H27N5O2/c1-23(2)14-27(15-23)22(29)26-21-11-16(3-8-25-21)19-13-28(17-5-9-30-10-6-17)20-12-24-7-4-18(19)20/h3-4,7-8,11-13,17H,5-6,9-10,14-15H2,1-2H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538094

(CHEMBL4641631)Show SMILES O=C(NC1CC1)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 Show InChI InChI=1S/C21H23N5O2/c27-21(24-15-1-2-15)25-20-11-14(3-8-23-20)18-13-26(16-5-9-28-10-6-16)19-12-22-7-4-17(18)19/h3-4,7-8,11-13,15-16H,1-2,5-6,9-10H2,(H2,23,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347945
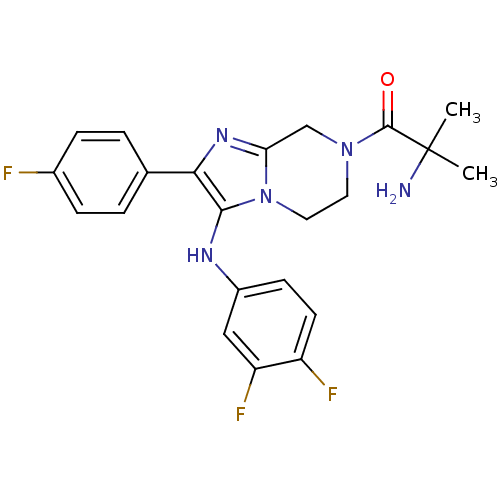
(CHEMBL1800774)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(F)c(F)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H22F3N5O/c1-22(2,26)21(31)29-9-10-30-18(12-29)28-19(13-3-5-14(23)6-4-13)20(30)27-15-7-8-16(24)17(25)11-15/h3-8,11,27H,9-10,12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Q-patch assay |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347939
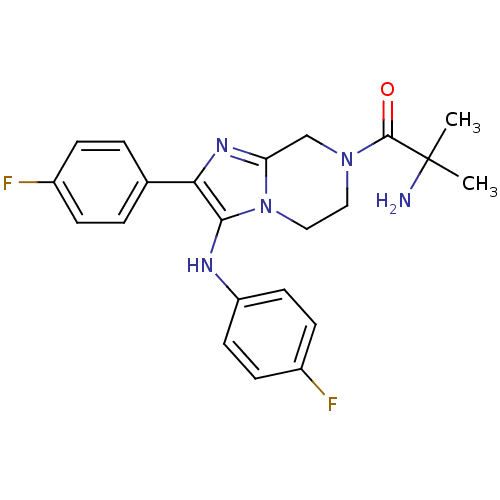
(CHEMBL1800137)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H23F2N5O/c1-22(2,25)21(30)28-11-12-29-18(13-28)27-19(14-3-5-15(23)6-4-14)20(29)26-17-9-7-16(24)8-10-17/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Q-patch assay |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347947

(CHEMBL1800776)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(Cl)c(F)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H22ClF2N5O/c1-22(2,26)21(31)29-9-10-30-18(12-29)28-19(13-3-5-14(24)6-4-13)20(30)27-15-7-8-16(23)17(25)11-15/h3-8,11,27H,9-10,12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Q-patch assay |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347939

(CHEMBL1800137)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H23F2N5O/c1-22(2,25)21(30)28-11-12-29-18(13-28)27-19(14-3-5-15(23)6-4-14)20(29)26-17-9-7-16(24)8-10-17/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in CHO cells by patch clamp assay |
J Med Chem 55: 4244-73 (2012)
Article DOI: 10.1021/jm300041e
BindingDB Entry DOI: 10.7270/Q21V5G1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538094

(CHEMBL4641631)Show SMILES O=C(NC1CC1)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 Show InChI InChI=1S/C21H23N5O2/c27-21(24-15-1-2-15)25-20-11-14(3-8-23-20)18-13-26(16-5-9-28-10-6-16)19-12-22-7-4-17(18)19/h3-4,7-8,11-13,15-16H,1-2,5-6,9-10H2,(H2,23,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538089

(CHEMBL4638460)Show InChI InChI=1S/C19H15N3/c1-2-4-15(5-3-1)13-22-14-18(16-6-9-20-10-7-16)17-8-11-21-12-19(17)22/h1-12,14H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50347941
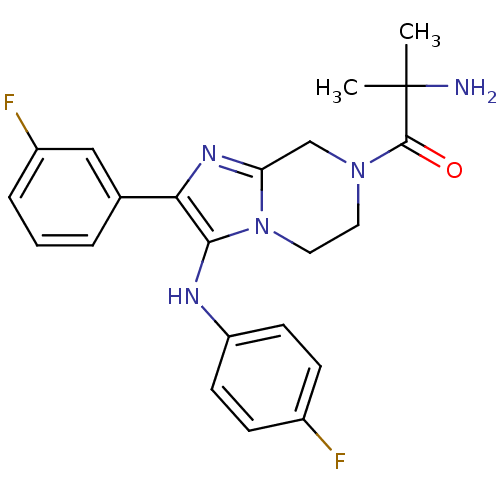
(CHEMBL1800768)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(F)cc1)-c1cccc(F)c1 Show InChI InChI=1S/C22H23F2N5O/c1-22(2,25)21(30)28-10-11-29-18(13-28)27-19(14-4-3-5-16(24)12-14)20(29)26-17-8-6-15(23)7-9-17/h3-9,12,26H,10-11,13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Q-patch assay |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50388532
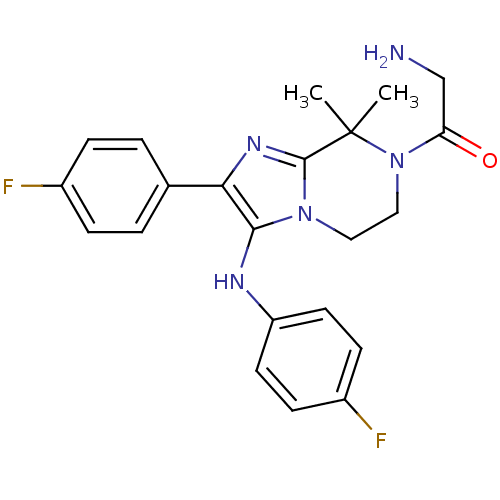
(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 55: 4244-73 (2012)
Article DOI: 10.1021/jm300041e
BindingDB Entry DOI: 10.7270/Q21V5G1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50388532

(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 55: 4244-73 (2012)
Article DOI: 10.1021/jm300041e
BindingDB Entry DOI: 10.7270/Q21V5G1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388532

(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 55: 4244-73 (2012)
Article DOI: 10.1021/jm300041e
BindingDB Entry DOI: 10.7270/Q21V5G1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50388532

(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 55: 4244-73 (2012)
Article DOI: 10.1021/jm300041e
BindingDB Entry DOI: 10.7270/Q21V5G1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50388532

(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 55: 4244-73 (2012)
Article DOI: 10.1021/jm300041e
BindingDB Entry DOI: 10.7270/Q21V5G1P |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50538097

(CHEMBL4641839)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 |r| Show InChI InChI=1S/C24H29N5O3/c1-16-13-28(14-17(2)32-16)24(30)27-23-11-18(3-8-26-23)21-15-29(19-5-9-31-10-6-19)22-12-25-7-4-20(21)22/h3-4,7-8,11-12,15-17,19H,5-6,9-10,13-14H2,1-2H3,(H,26,27,30)/t16-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Qpatch S8 assay |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538090

(CHEMBL4648742)Show InChI InChI=1S/C18H13N3/c1-2-4-15(5-3-1)21-13-17(14-6-9-19-10-7-14)16-8-11-20-12-18(16)21/h1-13H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538085

(CHEMBL4634969)Show InChI InChI=1S/C16H17N3/c1-12(2)10-19-11-15(13-3-6-17-7-4-13)14-5-8-18-9-16(14)19/h3-9,11-12H,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50538086

(CHEMBL4648945)Show InChI InChI=1S/C16H15N3/c1-2-12(1)10-19-11-15(13-3-6-17-7-4-13)14-5-8-18-9-16(14)19/h3-9,11-12H,1-2,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... |
J Med Chem 63: 2958-2973 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01624
BindingDB Entry DOI: 10.7270/Q2WQ079V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50347939

(CHEMBL1800137)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H23F2N5O/c1-22(2,25)21(30)28-11-12-29-18(13-28)27-19(14-3-5-15(23)6-4-14)20(29)26-17-9-7-16(24)8-10-17/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP 1A2 |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50347945

(CHEMBL1800774)Show SMILES CC(C)(N)C(=O)N1CCn2c(C1)nc(c2Nc1ccc(F)c(F)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H22F3N5O/c1-22(2,26)21(31)29-9-10-30-18(12-29)28-19(13-3-5-14(23)6-4-13)20(30)27-15-7-8-16(24)17(25)11-15/h3-8,11,27H,9-10,12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP 1A2 |
J Med Chem 54: 5116-30 (2011)
Article DOI: 10.1021/jm2003359
BindingDB Entry DOI: 10.7270/Q2FX79SN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data