

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50060321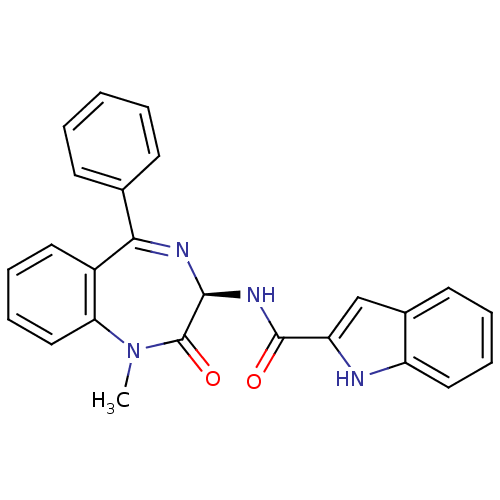 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060322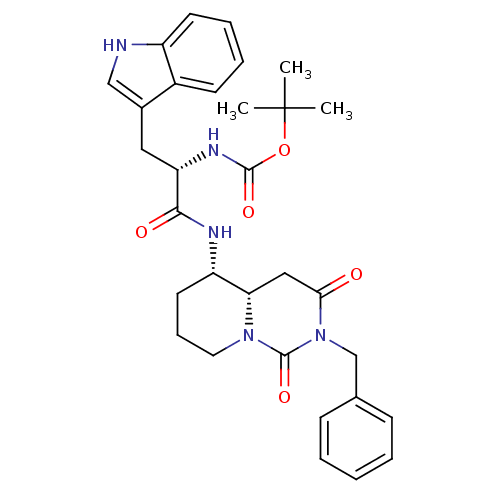 (CHEMBL332261 | [(S)-1-((4aS,5S)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50138784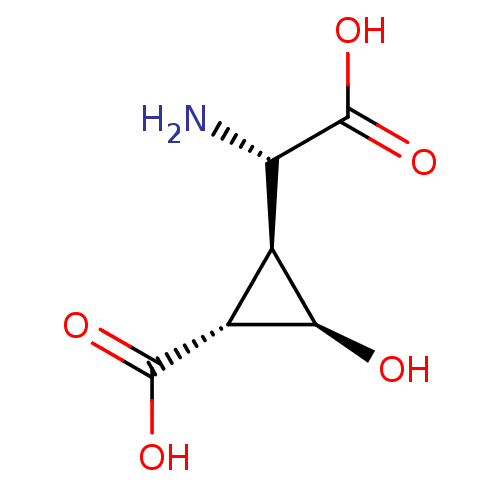 ((1R,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly, SA Curated by ChEMBL | Assay Description Binding affinity of the compound towards metabotropic glutamate receptor 3 was determined | J Med Chem 47: 456-66 (2004) Article DOI: 10.1021/jm030967o BindingDB Entry DOI: 10.7270/Q2K936Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060323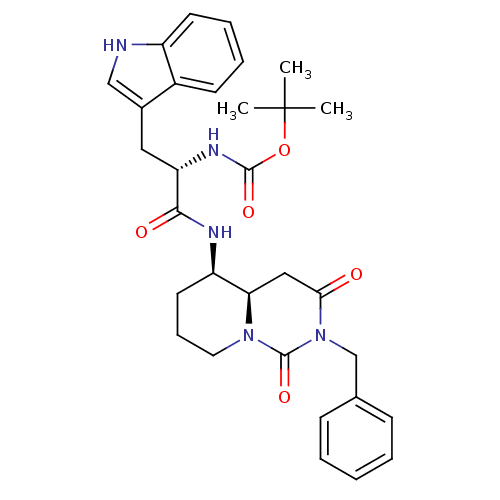 (CHEMBL431858 | [(S)-1-((4aR,5R)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50138784 ((1R,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly, SA Curated by ChEMBL | Assay Description Binding affinity of the compound towards metabotropic glutamate receptor 2 was determined | J Med Chem 47: 456-66 (2004) Article DOI: 10.1021/jm030967o BindingDB Entry DOI: 10.7270/Q2K936Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060326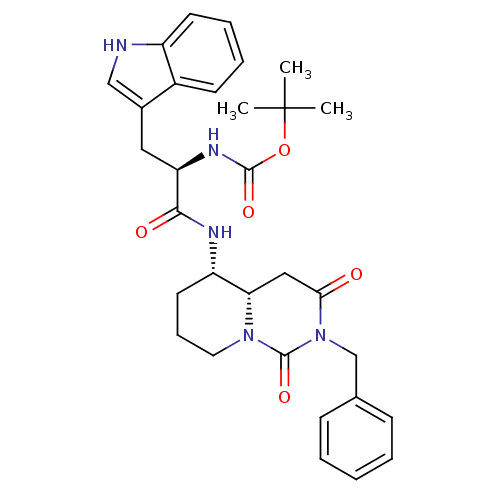 (CHEMBL116001 | [(R)-1-((4aS,5S)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060327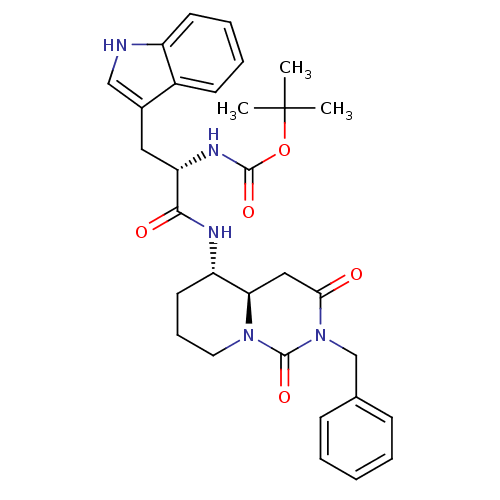 (CHEMBL116127 | [(S)-1-((4aR,5S)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060320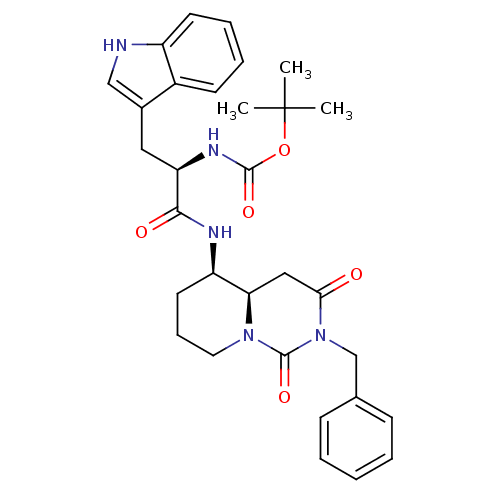 (CHEMBL326064 | [(R)-1-((4aR,5R)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060325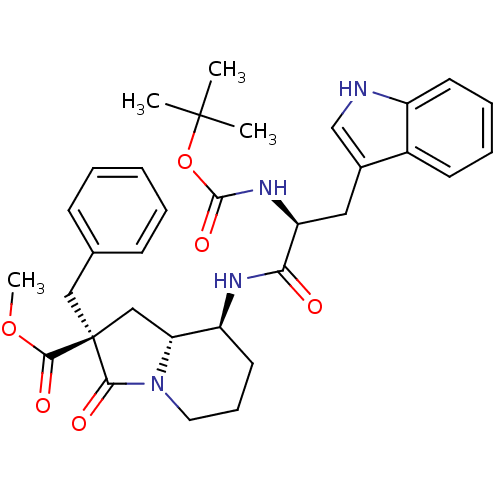 ((2S,8S,8aR)-2-Benzyl-8-[(S)-2-tert-butoxycarbonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060328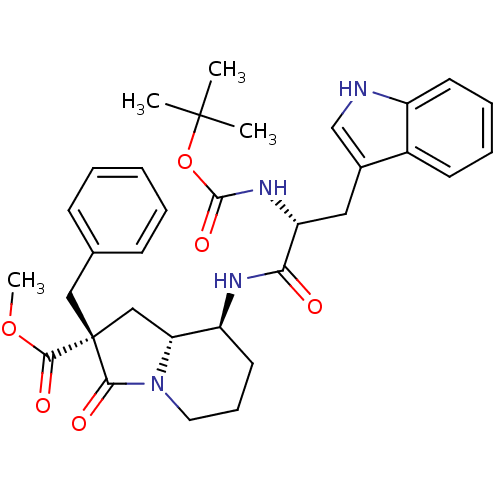 ((2R,8S,8aR)-2-Benzyl-8-[(R)-2-tert-butoxycarbonyla...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060318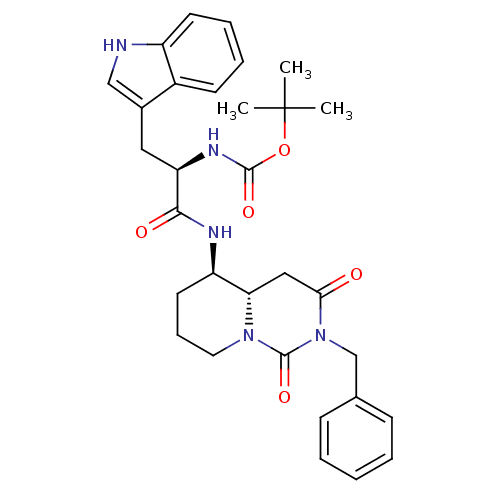 (CHEMBL324294 | [(R)-1-((4aS,5R)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060323 (CHEMBL431858 | [(S)-1-((4aR,5R)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060319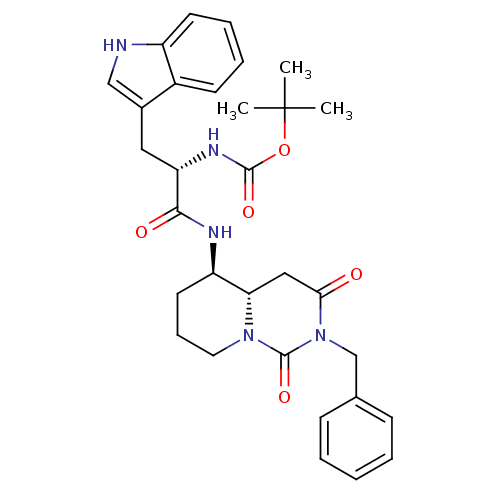 (CHEMBL113718 | [(S)-1-((4aS,5R)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060319 (CHEMBL113718 | [(S)-1-((4aS,5R)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060326 (CHEMBL116001 | [(R)-1-((4aS,5S)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060328 ((2R,8S,8aR)-2-Benzyl-8-[(R)-2-tert-butoxycarbonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060318 (CHEMBL324294 | [(R)-1-((4aS,5R)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060327 (CHEMBL116127 | [(S)-1-((4aR,5S)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060325 ((2S,8S,8aR)-2-Benzyl-8-[(S)-2-tert-butoxycarbonyla...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060324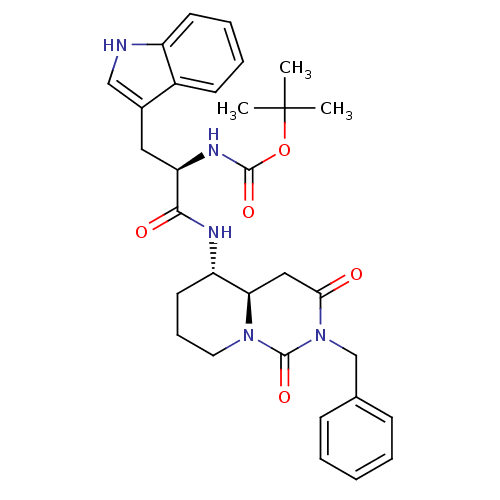 (CHEMBL114517 | [(R)-1-((4aR,5S)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060324 (CHEMBL114517 | [(R)-1-((4aR,5S)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060322 (CHEMBL332261 | [(S)-1-((4aS,5S)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060320 (CHEMBL326064 | [(R)-1-((4aR,5R)-2-Benzyl-1,3-dioxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50138785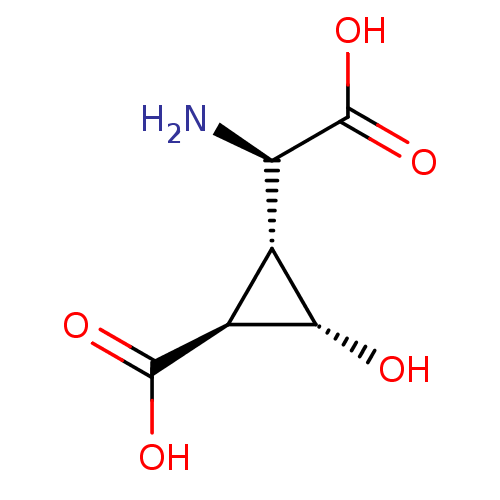 ((1S,2R,3S)-2-((R)-Amino-carboxy-methyl)-3-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly, SA Curated by ChEMBL | Assay Description Binding affinity of the compound towards Metabotropic glutamate receptor 3 was determined | J Med Chem 47: 456-66 (2004) Article DOI: 10.1021/jm030967o BindingDB Entry DOI: 10.7270/Q2K936Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50138785 ((1S,2R,3S)-2-((R)-Amino-carboxy-methyl)-3-hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly, SA Curated by ChEMBL | Assay Description Binding affinity of the compound towards metabotropic glutamate receptor 2 was determined | J Med Chem 47: 456-66 (2004) Article DOI: 10.1021/jm030967o BindingDB Entry DOI: 10.7270/Q2K936Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170765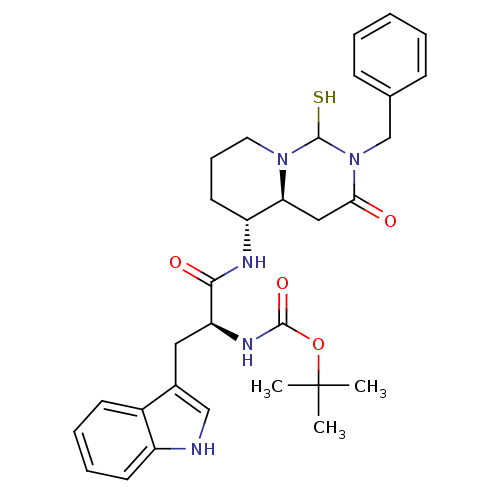 (CHEMBL363916 | [(S)-1-((4aS,5R)-2-Benzyl-1-mercapt...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50106593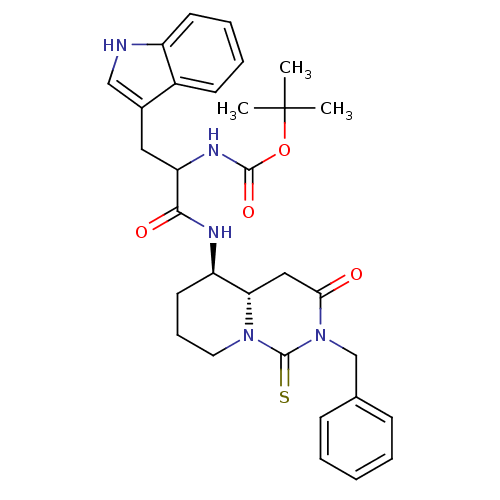 (CHEMBL135602 | [1-(2-Benzyl-3-oxo-1-thioxo-octahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 binding to cholecystokinin type A receptor of rat pancreas | J Med Chem 44: 4196-206 (2001) BindingDB Entry DOI: 10.7270/Q2TD9Z23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170765 (CHEMBL363916 | [(S)-1-((4aS,5R)-2-Benzyl-1-mercapt...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170764 (CHEMBL366344 | [(S)-2-(1H-Indol-3-yl)-1-((4aS,5R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170764 (CHEMBL366344 | [(S)-2-(1H-Indol-3-yl)-1-((4aS,5R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Insituto de Qu�mica M�dica (CSIC), Juan de la Cierva 3, E-28006 Madrid, Spain. Curated by ChEMBL | Assay Description Binding affinity by competitive inhibition of the radioligand [3H]pCCK-8 at Cholecystokinin type A receptor from rat pancreas | J Med Chem 42: 4659-68 (1999) BindingDB Entry DOI: 10.7270/Q24T6K2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description In vitro inhibition of [3H]propionyl-CCK-8 binding to rat pancreatic membranes at Cholecystokinin type A receptor. | J Med Chem 43: 3770-7 (2000) BindingDB Entry DOI: 10.7270/Q21J9BG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Insituto de Qu�mica M�dica (CSIC), Juan de la Cierva 3, E-28006 Madrid, Spain. Curated by ChEMBL | Assay Description Binding affinity by competitive inhibition of the radioligand [3H]pCCK-8 at Cholecystokinin type A receptor from rat pancreas | J Med Chem 42: 4659-68 (1999) BindingDB Entry DOI: 10.7270/Q24T6K2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 specific binding to cholecystokinin type A receptor in rat pancreas | J Med Chem 44: 2219-28 (2001) BindingDB Entry DOI: 10.7270/Q2M32WG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 binding to cholecystokinin type A receptor of rat pancreas | J Med Chem 44: 4196-206 (2001) BindingDB Entry DOI: 10.7270/Q2TD9Z23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089106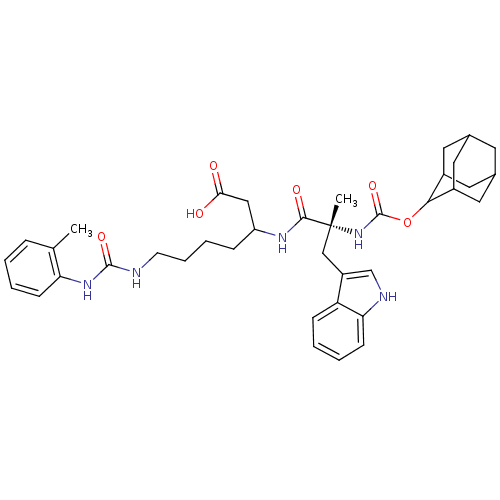 ((S)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-propionyl-CCK-8 specific binding to rat pancreatic Cholecystokinin type A receptor | Bioorg Med Chem Lett 12: 109-12 (2001) BindingDB Entry DOI: 10.7270/Q2P84B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50106588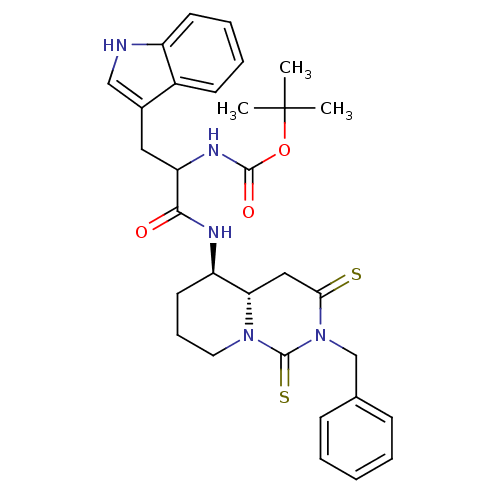 (CHEMBL133446 | [1-(2-Benzyl-1,3-dithioxo-octahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 binding to cholecystokinin type A receptor of rat pancreas | J Med Chem 44: 4196-206 (2001) BindingDB Entry DOI: 10.7270/Q2TD9Z23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060319 (CHEMBL113718 | [(S)-1-((4aS,5R)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50106591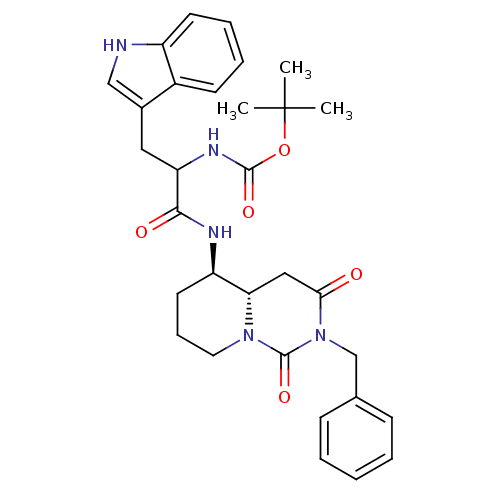 (CHEMBL337620 | [1-(2-Benzyl-1,3-dioxo-4a(S)-octahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 binding to cholecystokinin type A receptor of rat pancreas | J Med Chem 44: 4196-206 (2001) BindingDB Entry DOI: 10.7270/Q2TD9Z23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092567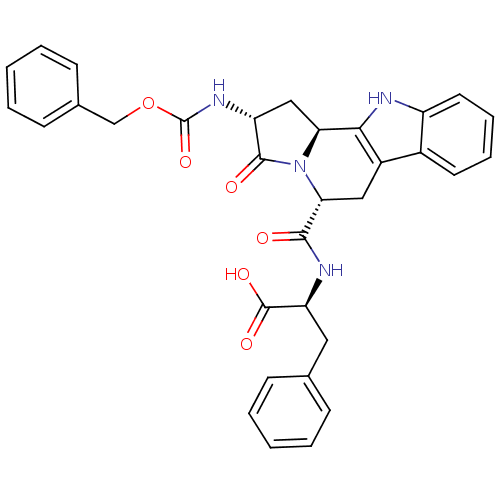 ((2R,5R,11bS,1'S)-2-[(2-Benzyloxycarbonylamino-3-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description In vitro inhibition of [3H]propionyl-CCK-8 binding to rat pancreatic membranes at Cholecystokinin type A receptor. | J Med Chem 43: 3770-7 (2000) BindingDB Entry DOI: 10.7270/Q21J9BG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092567 ((2R,5R,11bS,1'S)-2-[(2-Benzyloxycarbonylamino-3-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]propionylCCK8 from rat pancreatic CCK1 | J Med Chem 48: 7667-74 (2005) Article DOI: 10.1021/jm050689o BindingDB Entry DOI: 10.7270/Q2QJ7GV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092567 ((2R,5R,11bS,1'S)-2-[(2-Benzyloxycarbonylamino-3-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-propionyl-CCK-8 specific binding to rat pancreatic Cholecystokinin type A receptor | Bioorg Med Chem Lett 12: 109-12 (2001) BindingDB Entry DOI: 10.7270/Q2P84B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006875 (CHEMBL263969 | N-{(S)-2-[(R)-2-(Adamantan-2-yloxyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-propionyl-CCK-8 binding to mouse cerebral cortex membrane cholecystokinin-B (CCK-B) receptor | Bioorg Med Chem Lett 9: 43-8 (1999) BindingDB Entry DOI: 10.7270/Q2XP743J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50170764 (CHEMBL366344 | [(S)-2-(1H-Indol-3-yl)-1-((4aS,5R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of CCK binding to Cos-7 cells expressing human cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50073729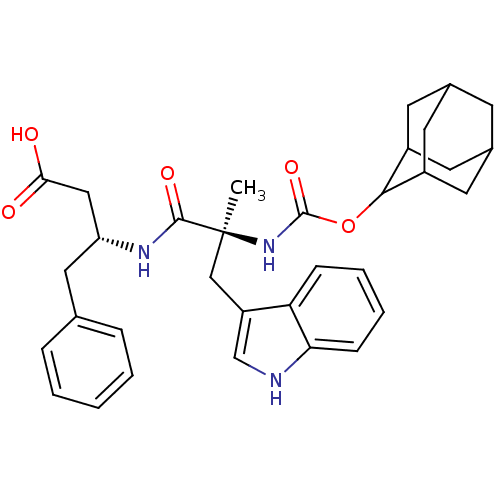 ((R)-3-[(S)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-propionyl-CCK-8 binding to rat pancreas cholecystokinin-A (CCK-A) receptor | Bioorg Med Chem Lett 9: 43-8 (1999) BindingDB Entry DOI: 10.7270/Q2XP743J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50106597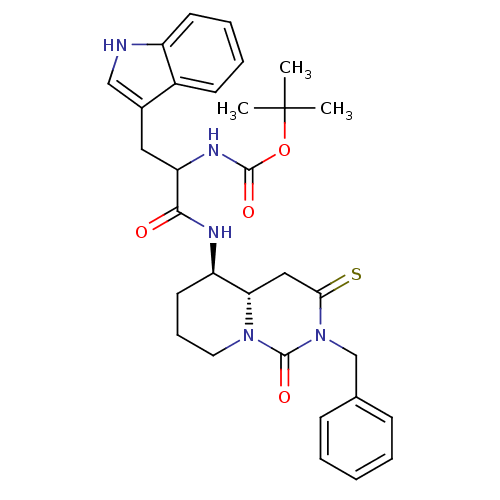 (CHEMBL133983 | [1-(2-Benzyl-1-oxo-3-thioxo-octahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 binding to cholecystokinin type A receptor of rat pancreas | J Med Chem 44: 4196-206 (2001) BindingDB Entry DOI: 10.7270/Q2TD9Z23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50170764 (CHEMBL366344 | [(S)-2-(1H-Indol-3-yl)-1-((4aS,5R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of CCK-Induced inositol phosphate in Cos-7 cells expressing mutant CCK1R (S348A) | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 391 total ) | Next | Last >> |