 Found 415 hits with Last Name = 'goss' and Initial = 'j'
Found 415 hits with Last Name = 'goss' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287557

(CHEMBL4159568)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(C)cc1)C(=O)NCc1nc(C)no1 Show InChI InChI=1S/C19H20N4O5S/c1-12-4-7-15(8-5-12)23-29(25,26)17-10-14(6-9-16(17)27-3)19(24)20-11-18-21-13(2)22-28-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287554

(CHEMBL4162926)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C19H20N4O4S/c1-12-4-8-16(9-5-12)23-28(25,26)17-10-15(7-6-13(17)2)19(24)20-11-18-21-14(3)22-27-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287553
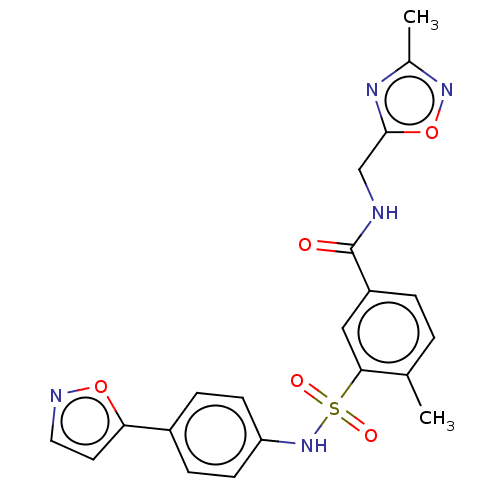
(CHEMBL4166791)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2ccno2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)22-12-20-24-14(2)25-31-20)11-19(13)32(28,29)26-17-7-5-15(6-8-17)18-9-10-23-30-18/h3-11,26H,12H2,1-2H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287559
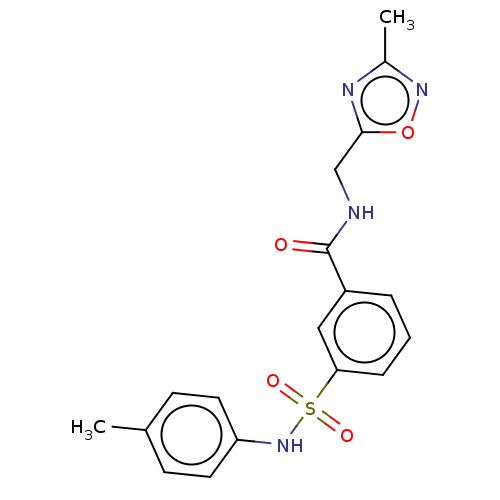
(CHEMBL4170197)Show SMILES Cc1noc(CNC(=O)c2cccc(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C18H18N4O4S/c1-12-6-8-15(9-7-12)22-27(24,25)16-5-3-4-14(10-16)18(23)19-11-17-20-13(2)21-26-17/h3-10,22H,11H2,1-2H3,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287556

(CHEMBL4161489)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(cc1)-c1ccno1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C21H20N6O4S/c1-14-3-4-16(21(28)22-12-18-13-27(2)26-24-18)11-20(14)32(29,30)25-17-7-5-15(6-8-17)19-9-10-23-31-19/h3-11,13,25H,12H2,1-2H3,(H,22,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287552
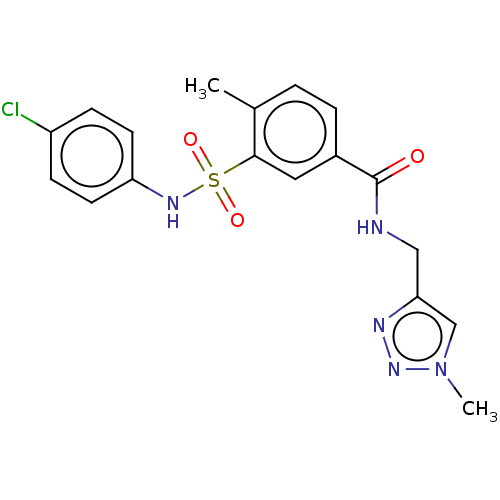
(CHEMBL4175004)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C18H18ClN5O3S/c1-12-3-4-13(18(25)20-10-16-11-24(2)23-21-16)9-17(12)28(26,27)22-15-7-5-14(19)6-8-15/h3-9,11,22H,10H2,1-2H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287558
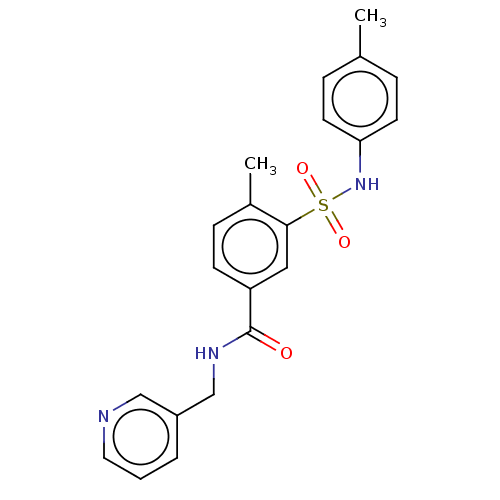
(CHEMBL1333494)Show SMILES Cc1ccc(NS(=O)(=O)c2cc(ccc2C)C(=O)NCc2cccnc2)cc1 Show InChI InChI=1S/C21H21N3O3S/c1-15-5-9-19(10-6-15)24-28(26,27)20-12-18(8-7-16(20)2)21(25)23-14-17-4-3-11-22-13-17/h3-13,24H,14H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287555
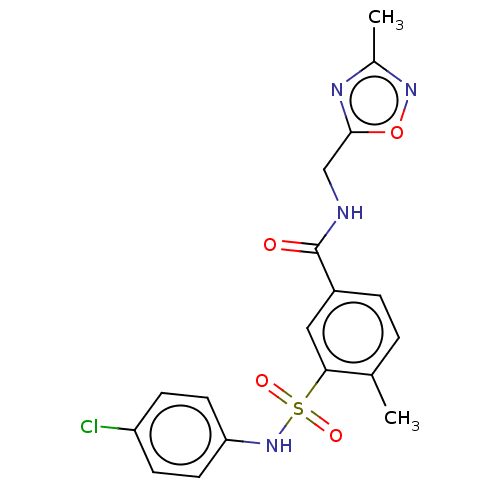
(CHEMBL4174695)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(Cl)cc2)n1 Show InChI InChI=1S/C18H17ClN4O4S/c1-11-3-4-13(18(24)20-10-17-21-12(2)22-27-17)9-16(11)28(25,26)23-15-7-5-14(19)6-8-15/h3-9,23H,10H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287519
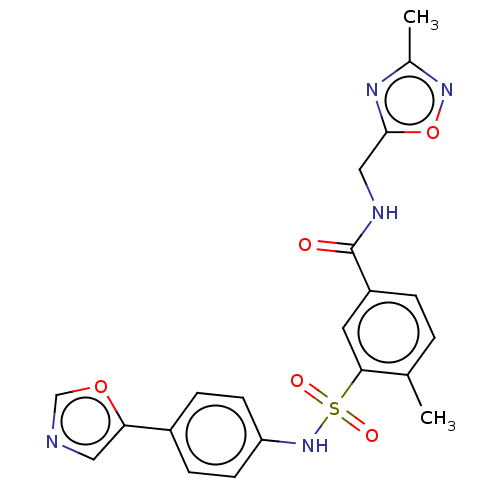
(CHEMBL4172084)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2cnco2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)23-11-20-24-14(2)25-31-20)9-19(13)32(28,29)26-17-7-5-15(6-8-17)18-10-22-12-30-18/h3-10,12,26H,11H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359779
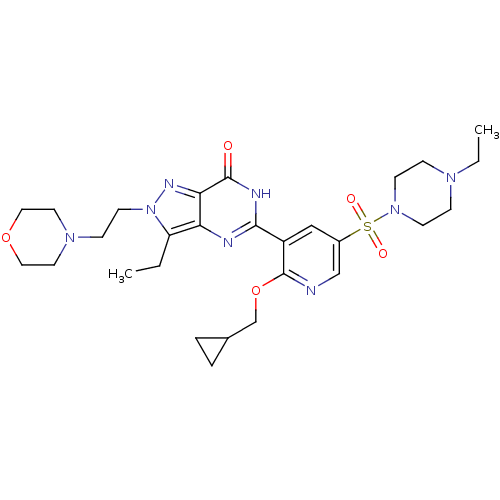
(CHEMBL1928271)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC2CC2)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C28H40N8O5S/c1-3-23-24-25(32-36(23)12-9-34-13-15-40-16-14-34)27(37)31-26(30-24)22-17-21(18-29-28(22)41-19-20-5-6-20)42(38,39)35-10-7-33(4-2)8-11-35/h17-18,20H,3-16,19H2,1-2H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50113443
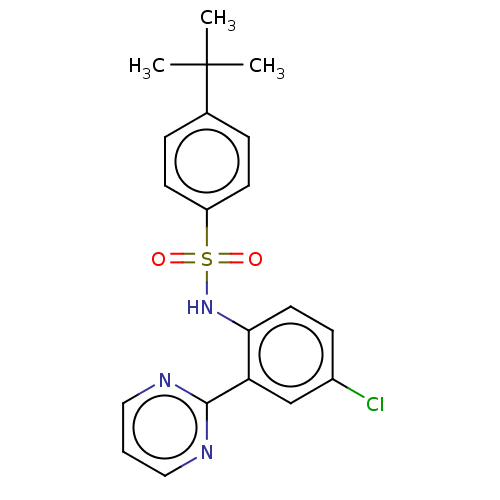
(CHEMBL3604484)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1-c1ncccn1 Show InChI InChI=1S/C20H20ClN3O2S/c1-20(2,3)14-5-8-16(9-6-14)27(25,26)24-18-10-7-15(21)13-17(18)19-22-11-4-12-23-19/h4-13,24H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 receptor (unknown origin) assessed as inhibition of TECK-induced calcium mobilization incubated for 10 mins prior to TECK... |
Bioorg Med Chem Lett 25: 3661-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.046
BindingDB Entry DOI: 10.7270/Q2RR2123 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359773
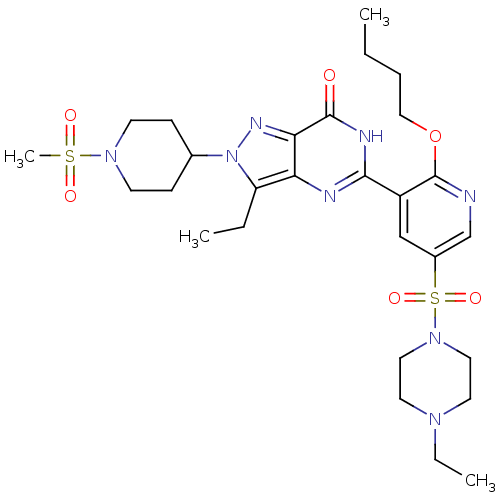
(CHEMBL1928265)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(CC1)S(C)(=O)=O)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O6S2/c1-5-8-17-42-28-22(18-21(19-29-28)44(40,41)35-15-13-33(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-34(12-10-20)43(4,38)39/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501764
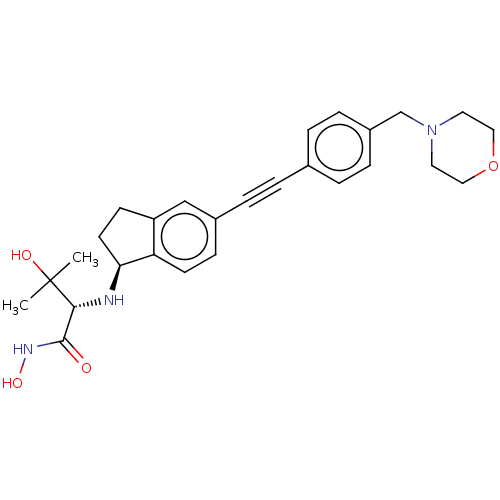
(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359795
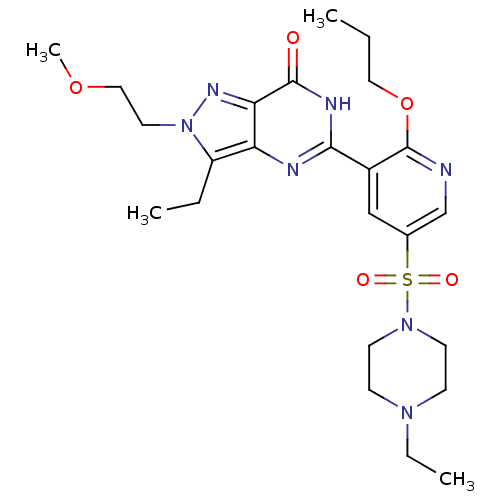
(CHEMBL1928258)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C24H35N7O5S/c1-5-13-36-24-18(15-17(16-25-24)37(33,34)30-10-8-29(7-3)9-11-30)22-26-20-19(6-2)31(12-14-35-4)28-21(20)23(32)27-22/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359767
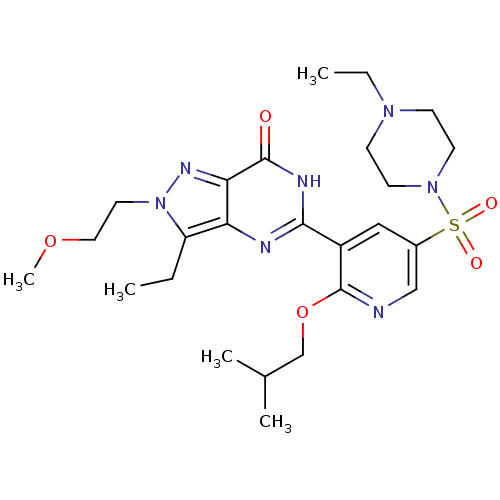
(CHEMBL1928259)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)12-13-36-5)24(33)28-23(27-21)19-14-18(15-26-25(19)37-16-17(3)4)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359774

(CHEMBL1928266)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(C)CC1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O4S/c1-5-8-17-40-28-22(18-21(19-29-28)41(38,39)35-15-13-34(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-33(4)12-10-20/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359768
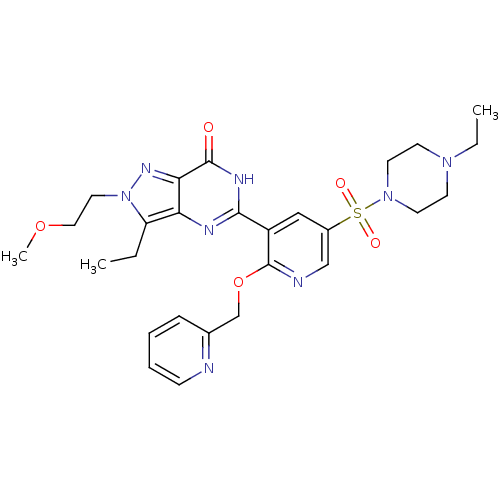
(CHEMBL1928260)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCc2ccccn2)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H34N8O5S/c1-4-22-23-24(32-35(22)14-15-39-3)26(36)31-25(30-23)21-16-20(41(37,38)34-12-10-33(5-2)11-13-34)17-29-27(21)40-18-19-8-6-7-9-28-19/h6-9,16-17H,4-5,10-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359777
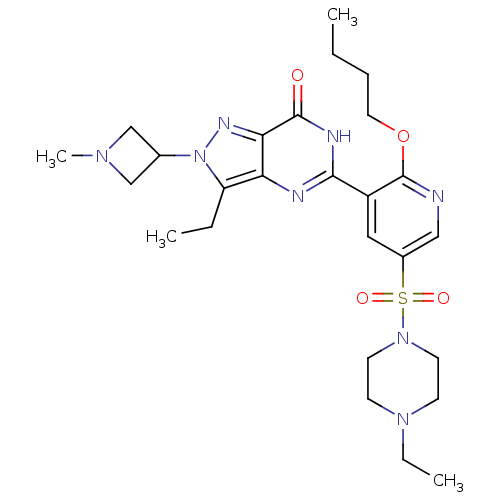
(CHEMBL1928269)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CN(C)C1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O4S/c1-5-8-13-38-26-20(14-19(15-27-26)39(36,37)33-11-9-32(7-3)10-12-33)24-28-22-21(6-2)34(18-16-31(4)17-18)30-23(22)25(35)29-24/h14-15,18H,5-13,16-17H2,1-4H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359778
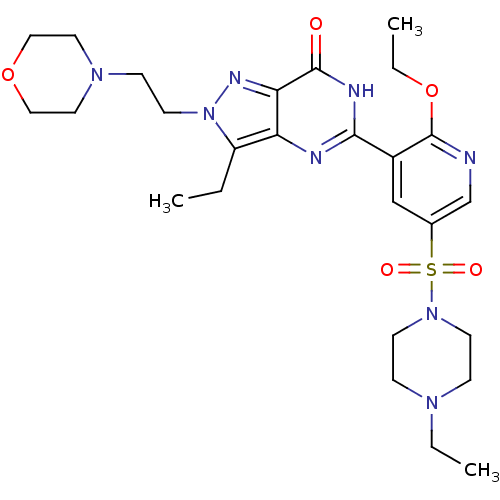
(CHEMBL1928270)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O5S/c1-4-21-22-23(30-34(21)12-9-32-13-15-38-16-14-32)25(35)29-24(28-22)20-17-19(18-27-26(20)39-6-3)40(36,37)33-10-7-31(5-2)8-11-33/h17-18H,4-16H2,1-3H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359771
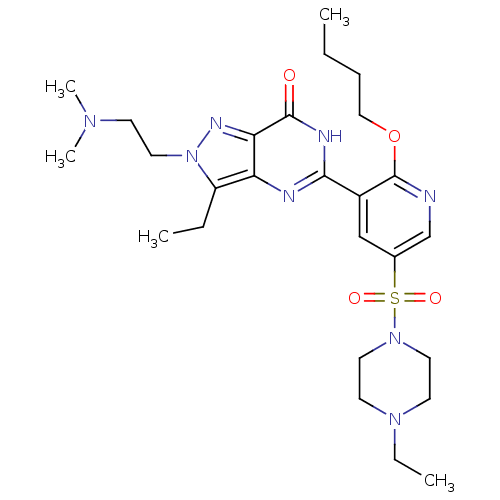
(CHEMBL1928263)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(CCN(C)C)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H40N8O4S/c1-6-9-16-38-26-20(17-19(18-27-26)39(36,37)33-13-11-32(8-3)12-14-33)24-28-22-21(7-2)34(15-10-31(4)5)30-23(22)25(35)29-24/h17-18H,6-16H2,1-5H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359790

(CHEMBL1928253)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC3CC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)16-17-6-7-17)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-3)38(34,35)31-10-8-30(5-2)9-11-31/h14-15,17H,4-13,16H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501762

(CHEMBL4081478)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2C)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O3/c1-17(31)25(26(32)29-33)28-24-12-10-22-15-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)16-30-14-13-27-18(30)2/h4-5,7-9,11,13-15,17,24-25,28,31,33H,10,12,16H2,1-2H3,(H,29,32)/t17-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501764

(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501779
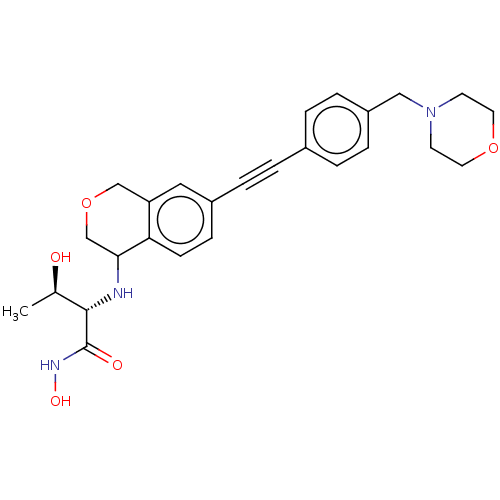
(CHEMBL4102207)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-24-17-34-16-22-14-20(8-9-23(22)24)5-2-19-3-6-21(7-4-19)15-29-10-12-33-13-11-29/h3-4,6-9,14,18,24-25,27,30,32H,10-13,15-17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501755
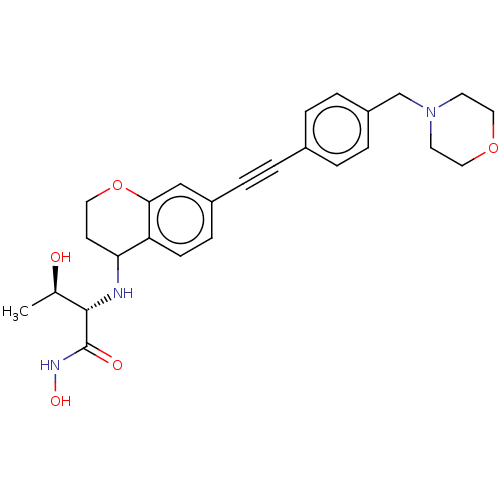
(CHEMBL4077947)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-23-10-13-34-24-16-20(8-9-22(23)24)5-2-19-3-6-21(7-4-19)17-29-11-14-33-15-12-29/h3-4,6-9,16,18,23,25,27,30,32H,10-15,17H2,1H3,(H,28,31)/t18-,23?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501782

(CHEMBL4087371)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H24N2O3/c1-15(25)21(22(26)24-27)23-20-9-5-8-18-14-17(12-13-19(18)20)11-10-16-6-3-2-4-7-16/h2-4,6-7,12-15,20-21,23,25,27H,5,8-9H2,1H3,(H,24,26)/t15-,20?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359789
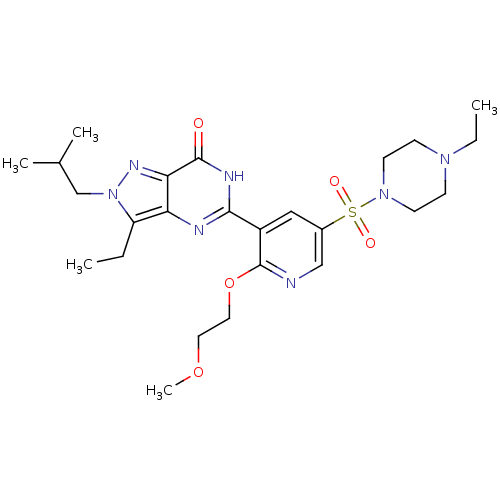
(CHEMBL1928252)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)16-17(3)4)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-5)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501780
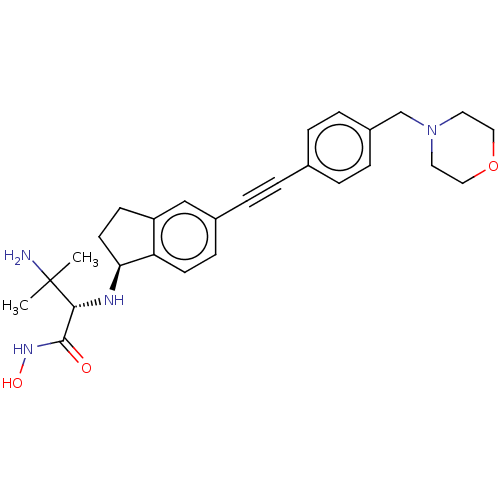
(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50246483
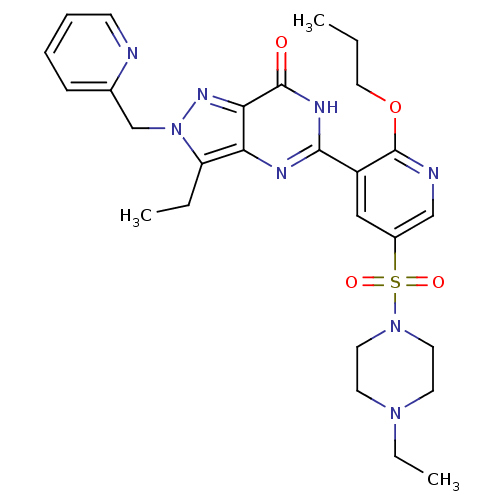
(3-ethyl-5-(5-(4-ethylpiperazin-1-ylsulfonyl)-2-pro...)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(Cc3ccccn3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C27H34N8O4S/c1-4-15-39-27-21(16-20(17-29-27)40(37,38)34-13-11-33(6-3)12-14-34)25-30-23-22(5-2)35(32-24(23)26(36)31-25)18-19-9-7-8-10-28-19/h7-10,16-17H,4-6,11-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
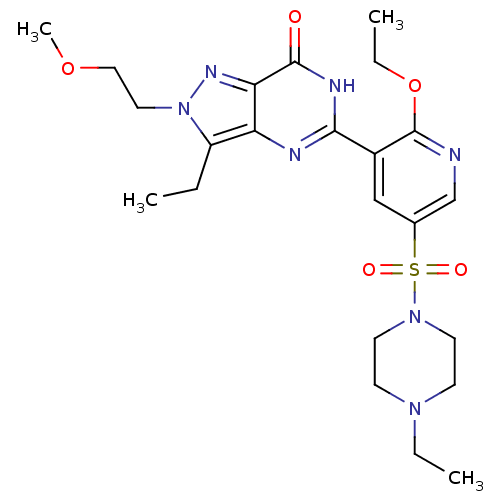
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359770
(CHEMBL1928262)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H33N7O5S/c1-5-18-19-20(27-30(18)12-13-34-4)22(31)26-21(25-19)17-14-16(15-24-23(17)35-7-3)36(32,33)29-10-8-28(6-2)9-11-29/h14-15H,5-13H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
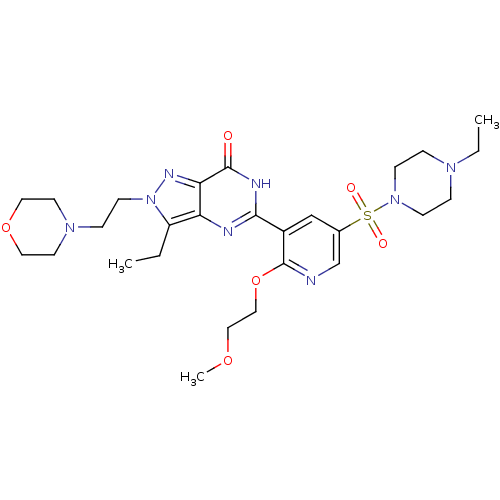
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359780
(CHEMBL1928272)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H40N8O6S/c1-4-22-23-24(31-35(22)11-8-33-12-14-40-15-13-33)26(36)30-25(29-23)21-18-20(19-28-27(21)41-17-16-39-3)42(37,38)34-9-6-32(5-2)7-10-34/h18-19H,4-17H2,1-3H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
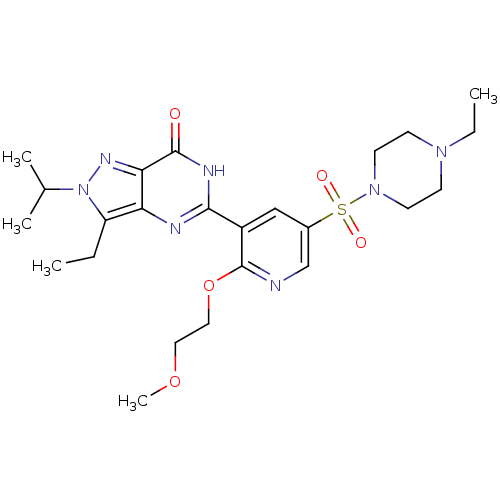
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359787
(CHEMBL1928250)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C(C)C Show InChI InChI=1S/C24H35N7O5S/c1-6-19-20-21(28-31(19)16(3)4)23(32)27-22(26-20)18-14-17(15-25-24(18)36-13-12-35-5)37(33,34)30-10-8-29(7-2)9-11-30/h14-16H,6-13H2,1-5H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50178705
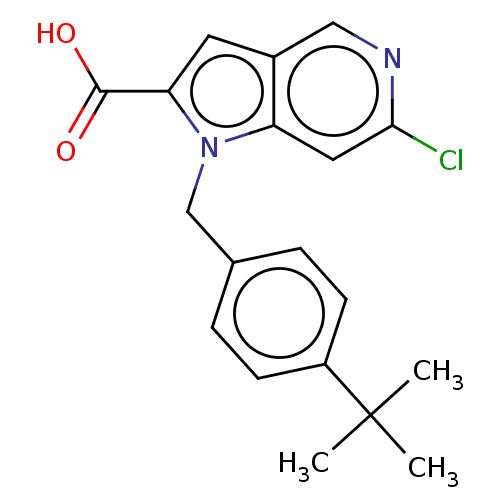
(CHEMBL3814804)Show SMILES CC(C)(C)c1ccc(Cn2c(cc3cnc(Cl)cc23)C(O)=O)cc1 Show InChI InChI=1S/C19H19ClN2O2/c1-19(2,3)14-6-4-12(5-7-14)11-22-15-9-17(20)21-10-13(15)8-16(22)18(23)24/h4-10H,11H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 (unknown origin) assessed as inhibition of TECK-stimulated calcium mobilization preincubated for 10 mins followed by agon... |
Bioorg Med Chem Lett 26: 3322-3325 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.043
BindingDB Entry DOI: 10.7270/Q2PZ5BQX |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359769

(CHEMBL1928261)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C24H35N7O6S/c1-5-19-20-21(28-31(19)11-12-35-3)23(32)27-22(26-20)18-15-17(16-25-24(18)37-14-13-36-4)38(33,34)30-9-7-29(6-2)8-10-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359791

(CHEMBL1928254)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C26H37N7O6S/c1-4-21-22-23(30-33(21)18-6-12-38-13-7-18)25(34)29-24(28-22)20-16-19(17-27-26(20)39-15-14-37-3)40(35,36)32-10-8-31(5-2)9-11-32/h16-18H,4-15H2,1-3H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359786

(CHEMBL1928249)Show SMILES CCCn1nc2c(nc([nH]c2=O)-c2cc(cnc2OCCOC)S(=O)(=O)N2CCN(CC)CC2)c1CC Show InChI InChI=1S/C24H35N7O5S/c1-5-8-31-19(6-2)20-21(28-31)23(32)27-22(26-20)18-15-17(16-25-24(18)36-14-13-35-4)37(33,34)30-11-9-29(7-3)10-12-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359788
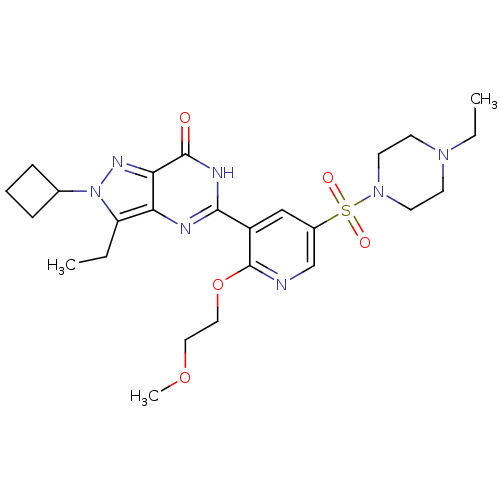
(CHEMBL1928251)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCC1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)17-7-6-8-17)24(33)28-23(27-21)19-15-18(16-26-25(19)37-14-13-36-3)38(34,35)31-11-9-30(5-2)10-12-31/h15-17H,4-14H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501752
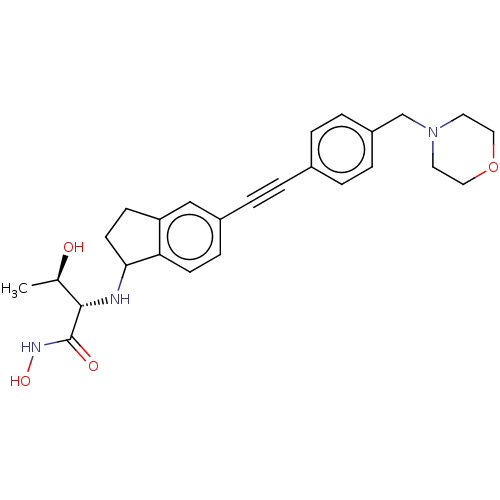
(CHEMBL4095059)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501770
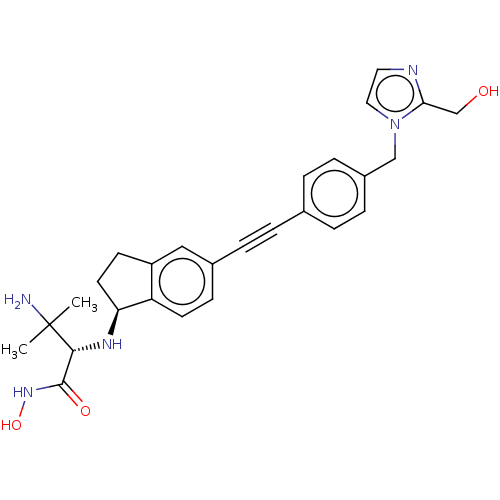
(CHEMBL4092719)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H31N5O3/c1-27(2,28)25(26(34)31-35)30-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-29-24(32)17-33/h4-5,7-9,11,13-15,23,25,30,33,35H,10,12,16-17,28H2,1-2H3,(H,31,34)/t23-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501756
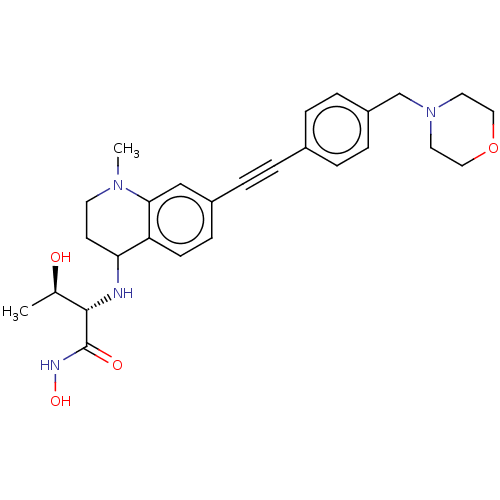
(CHEMBL4066982)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-19(32)26(27(33)29-34)28-24-11-12-30(2)25-17-21(9-10-23(24)25)6-3-20-4-7-22(8-5-20)18-31-13-15-35-16-14-31/h4-5,7-10,17,19,24,26,28,32,34H,11-16,18H2,1-2H3,(H,29,33)/t19-,24?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501778

(CHEMBL4098674)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-19-13-27-12-17-11-16(9-10-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9-11,14,19-20,22,24,26H,12-13H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501780

(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501768
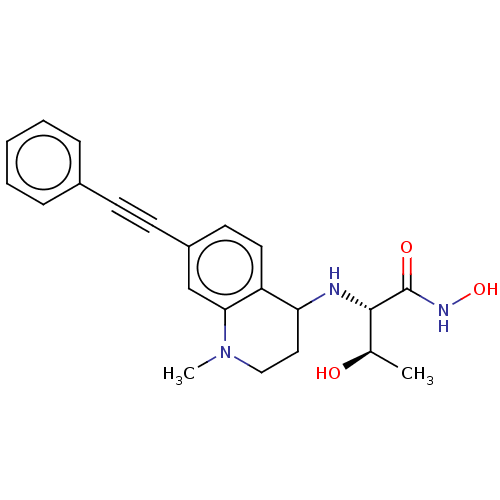
(CHEMBL4102818)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H25N3O3/c1-15(26)21(22(27)24-28)23-19-12-13-25(2)20-14-17(10-11-18(19)20)9-8-16-6-4-3-5-7-16/h3-7,10-11,14-15,19,21,23,26,28H,12-13H2,1-2H3,(H,24,27)/t15-,19?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359772

(CHEMBL1928264)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCCN(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H42N8O4S/c1-7-22-23-24(31-35(22)11-9-10-32(5)6)26(36)30-25(29-23)21-16-20(17-28-27(21)39-18-19(3)4)40(37,38)34-14-12-33(8-2)13-15-34/h16-17,19H,7-15,18H2,1-6H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data