 Found 342 hits with Last Name = 'griffith' and Initial = 'e'
Found 342 hits with Last Name = 'griffith' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50247012
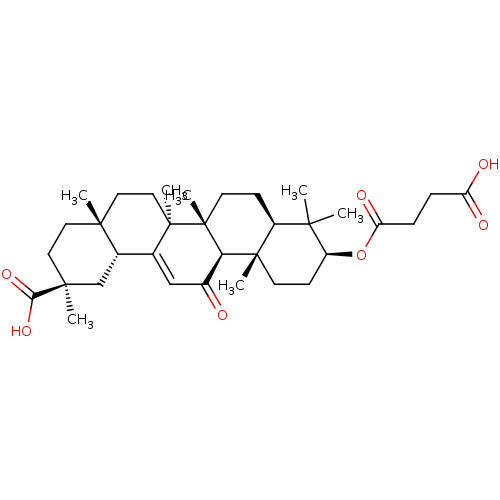
(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504051

(CHEMBL4450502)Show SMILES CC1=CC[C@]2(C[C@@H]1O)[C@@H](CC[C@@H]2C(O)=O)C1CCCCC1 |r,t:1| Show InChI InChI=1S/C18H28O3/c1-12-9-10-18(11-16(12)19)14(7-8-15(18)17(20)21)13-5-3-2-4-6-13/h9,13-16,19H,2-8,10-11H2,1H3,(H,20,21)/t14-,15+,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504050

(CHEMBL4436767)Show SMILES CC1=CC[C@]2(C=C1)[C@@H](CC[C@@H]2C(O)=O)C1CCCCC1 |r,c:5,t:1| Show InChI InChI=1S/C18H26O2/c1-13-9-11-18(12-10-13)15(7-8-16(18)17(19)20)14-5-3-2-4-6-14/h9-11,14-16H,2-8,12H2,1H3,(H,19,20)/t15-,16+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
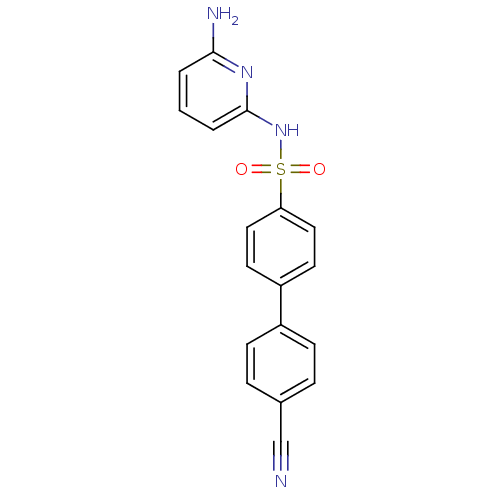
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Homoserine kinase
(Escherichia coli) | BDBM85124
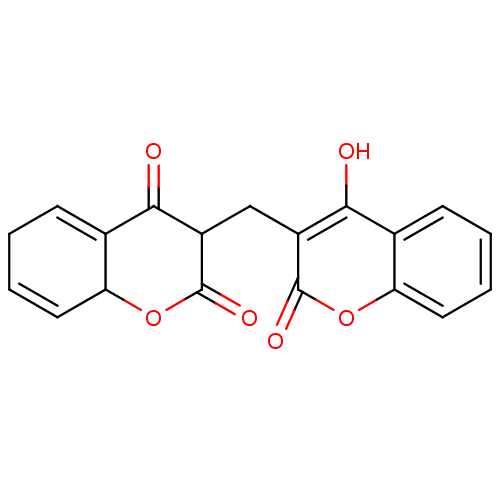
(Thr1 inhibitor, 4)Show SMILES Oc1c(CC2C(=O)OC3C=CCC=C3C2=O)c(=O)oc2ccccc12 |c:9,12| Show InChI InChI=1S/C19H14O6/c20-16-10-5-1-3-7-14(10)24-18(22)12(16)9-13-17(21)11-6-2-4-8-15(11)25-19(13)23/h1,3-8,13,15,20H,2,9H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 460 | -37.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
McMaster University
| Assay Description
The phosphorylation of L-Hse was monitored by coupling the formation of ADP with pyruvate kinase and lactate dehydrogenase (PK/LDH). The resulting o... |
Chembiochem 12: 1179-82 (2011)
Article DOI: 10.1002/cbic.201100121
BindingDB Entry DOI: 10.7270/Q24T6GWV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504041

(CHEMBL4529787)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CC=C(C)[C@@H](O)C1 |r,t:13| Show InChI InChI=1S/C15H24O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)13(16)8-15/h6,9,11-13,16H,4-5,7-8H2,1-3H3,(H,17,18)/t11-,12+,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504049
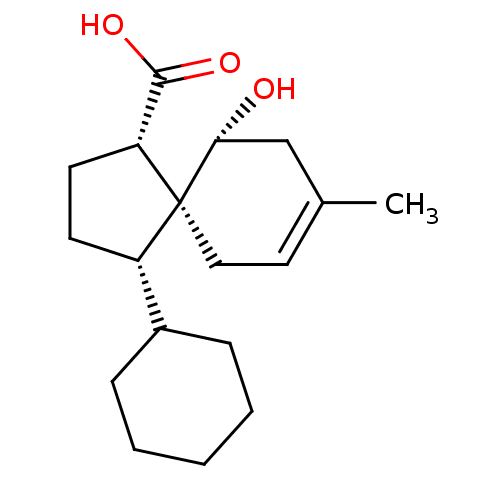
(CHEMBL4450320)Show SMILES CC1=CC[C@@]2([C@@H](CC[C@@H]2C(O)=O)C2CCCCC2)[C@H](O)C1 |r,t:1| Show InChI InChI=1S/C18H28O3/c1-12-9-10-18(16(19)11-12)14(7-8-15(18)17(20)21)13-5-3-2-4-6-13/h9,13-16,19H,2-8,10-11H2,1H3,(H,20,21)/t14-,15+,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504048
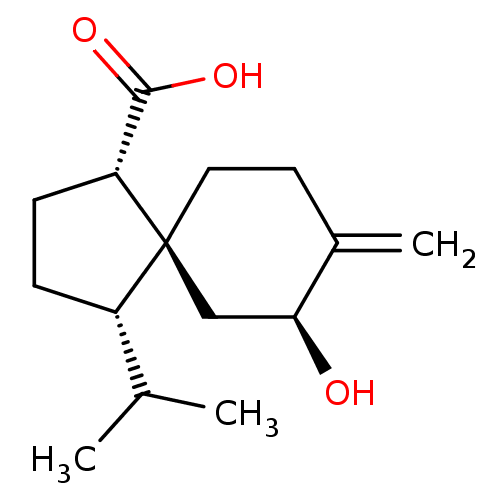
(CHEMBL4566781)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CCC(=C)[C@@H](O)C1 |r| Show InChI InChI=1S/C15H24O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)13(16)8-15/h9,11-13,16H,3-8H2,1-2H3,(H,17,18)/t11-,12+,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504053
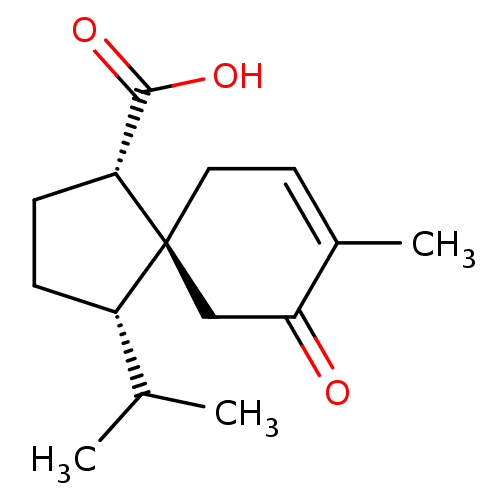
(CHEMBL4536375)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CC=C(C)C(=O)C1 |r,t:13| Show InChI InChI=1S/C15H22O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)13(16)8-15/h6,9,11-12H,4-5,7-8H2,1-3H3,(H,17,18)/t11-,12+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504052
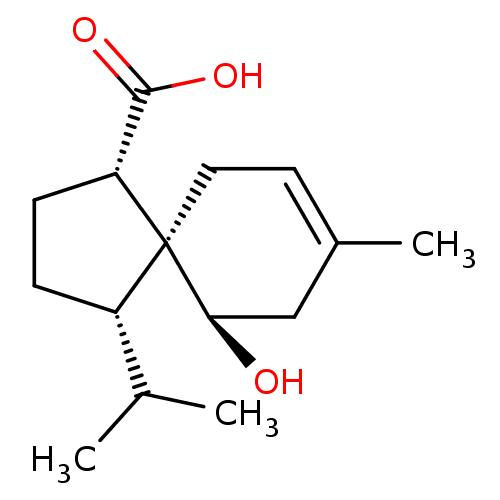
(CHEMBL4552570)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CC=C(C)C[C@H]1O |r,t:13| Show InChI InChI=1S/C15H24O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)8-13(15)16/h6,9,11-13,16H,4-5,7-8H2,1-3H3,(H,17,18)/t11-,12+,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Homoserine kinase
(Escherichia coli) | BDBM85126
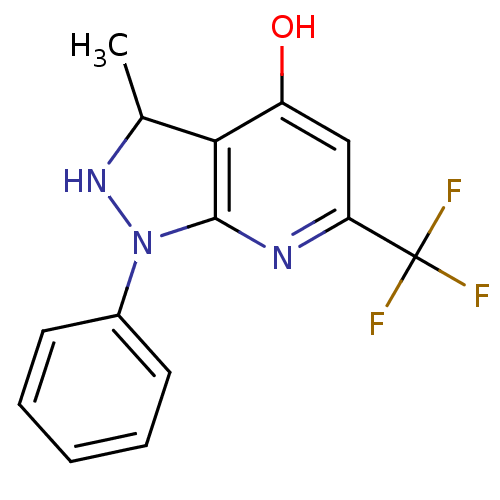
(Thr1 inhibitor, 6)Show InChI InChI=1S/C14H12F3N3O/c1-8-12-10(21)7-11(14(15,16)17)18-13(12)20(19-8)9-5-3-2-4-6-9/h2-8,19H,1H3,(H,18,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.60E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
McMaster University
| Assay Description
The phosphorylation of L-Hse was monitored by coupling the formation of ADP with pyruvate kinase and lactate dehydrogenase (PK/LDH). The resulting o... |
Chembiochem 12: 1179-82 (2011)
Article DOI: 10.1002/cbic.201100121
BindingDB Entry DOI: 10.7270/Q24T6GWV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504047
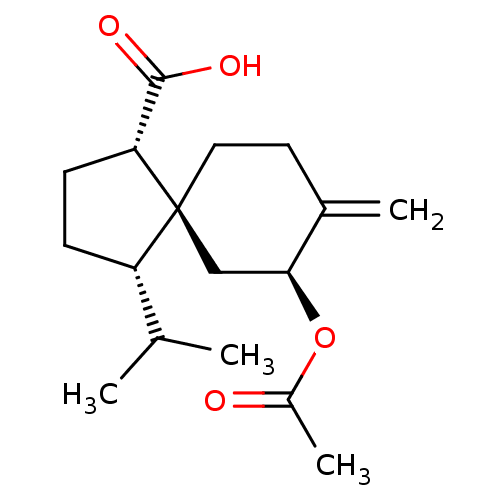
(CHEMBL4475775)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CCC(=C)[C@H](C1)OC(C)=O |r| Show InChI InChI=1S/C17H26O4/c1-10(2)13-5-6-14(16(19)20)17(13)8-7-11(3)15(9-17)21-12(4)18/h10,13-15H,3,5-9H2,1-2,4H3,(H,19,20)/t13-,14+,15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Homoserine kinase
(Escherichia coli) | BDBM24941

((2Z)-2-{[(2,5-dibromophenyl)amino](hydroxy)methyli...)Show InChI InChI=1S/C11H8Br2N2O2/c1-6(16)8(5-14)11(17)15-10-4-7(12)2-3-9(10)13/h2-4,8H,1H3,(H,15,17) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
McMaster University
| Assay Description
The phosphorylation of L-Hse was monitored by coupling the formation of ADP with pyruvate kinase and lactate dehydrogenase (PK/LDH). The resulting o... |
Chembiochem 12: 1179-82 (2011)
Article DOI: 10.1002/cbic.201100121
BindingDB Entry DOI: 10.7270/Q24T6GWV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504044
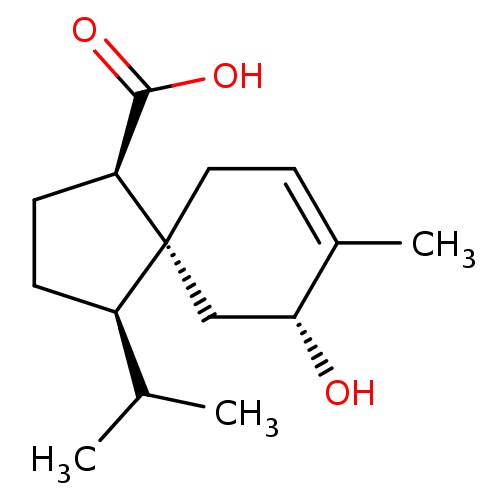
(CHEMBL4548901)Show SMILES CC(C)[C@H]1CC[C@@H](C(O)=O)[C@@]11CC=C(C)[C@H](O)C1 |r,t:13| Show InChI InChI=1S/C15H24O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)13(16)8-15/h6,9,11-13,16H,4-5,7-8H2,1-3H3,(H,17,18)/t11-,12+,13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504054

(CHEMBL4588470)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CC=C(C)[C@H](C1)OC(C)=O |r,t:13| Show InChI InChI=1S/C17H26O4/c1-10(2)13-5-6-14(16(19)20)17(13)8-7-11(3)15(9-17)21-12(4)18/h7,10,13-15H,5-6,8-9H2,1-4H3,(H,19,20)/t13-,14+,15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504042
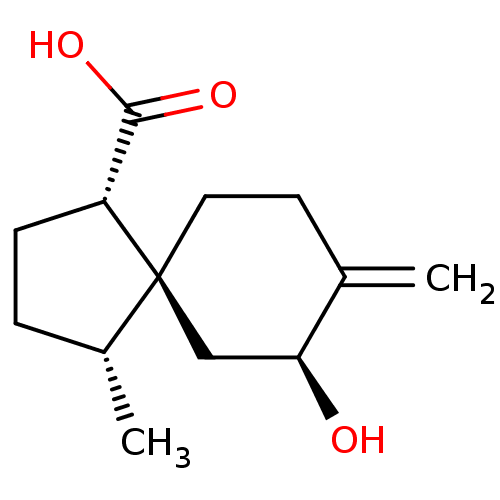
(CHEMBL4545661)Show SMILES C[C@@H]1CC[C@H](C(O)=O)[C@]11CCC(=C)[C@@H](O)C1 |r| Show InChI InChI=1S/C13H20O3/c1-8-5-6-13(7-11(8)14)9(2)3-4-10(13)12(15)16/h9-11,14H,1,3-7H2,2H3,(H,15,16)/t9-,10-,11+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Homoserine kinase
(Escherichia coli) | BDBM85123

(Thr1 inhibitor, 3 | cid_235240)Show InChI InChI=1S/C15H12O7/c16-8-2-1-6(3-9(8)17)15-14(21)13(20)7-4-10(18)11(19)5-12(7)22-15/h1-5,13,15-20H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.71E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
McMaster University
| Assay Description
The phosphorylation of L-Hse was monitored by coupling the formation of ADP with pyruvate kinase and lactate dehydrogenase (PK/LDH). The resulting o... |
Chembiochem 12: 1179-82 (2011)
Article DOI: 10.1002/cbic.201100121
BindingDB Entry DOI: 10.7270/Q24T6GWV |
More data for this
Ligand-Target Pair | |
Homoserine kinase
(Escherichia coli) | BDBM85122

(Thr1 inhibitor, 1)Show InChI InChI=1S/C13H10O/c1-2-13(14)12-8-7-10-5-3-4-6-11(10)9-12/h2-9H,1H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20E+4 | -26.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
McMaster University
| Assay Description
The phosphorylation of L-Hse was monitored by coupling the formation of ADP with pyruvate kinase and lactate dehydrogenase (PK/LDH). The resulting o... |
Chembiochem 12: 1179-82 (2011)
Article DOI: 10.1002/cbic.201100121
BindingDB Entry DOI: 10.7270/Q24T6GWV |
More data for this
Ligand-Target Pair | |
Homoserine kinase
(Escherichia coli) | BDBM85125
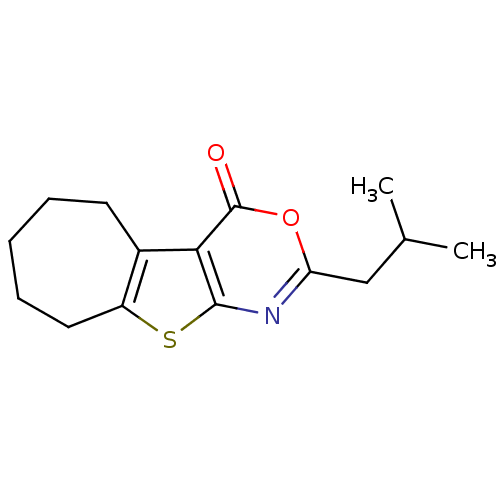
(Thr1 inhibitor, 5)Show InChI InChI=1S/C15H19NO2S/c1-9(2)8-12-16-14-13(15(17)18-12)10-6-4-3-5-7-11(10)19-14/h9H,3-8H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.58E+4 | -26.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
McMaster University
| Assay Description
The phosphorylation of L-Hse was monitored by coupling the formation of ADP with pyruvate kinase and lactate dehydrogenase (PK/LDH). The resulting o... |
Chembiochem 12: 1179-82 (2011)
Article DOI: 10.1002/cbic.201100121
BindingDB Entry DOI: 10.7270/Q24T6GWV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504056
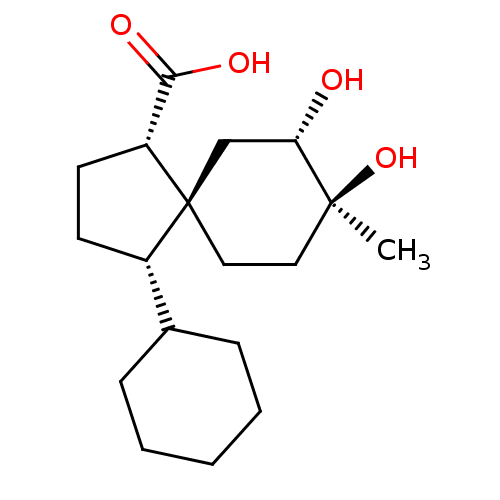
(CHEMBL4580823)Show SMILES C[C@]1(O)CC[C@]2(C[C@@H]1O)[C@@H](CC[C@@H]2C(O)=O)C1CCCCC1 |r| Show InChI InChI=1S/C18H30O4/c1-17(22)9-10-18(11-15(17)19)13(7-8-14(18)16(20)21)12-5-3-2-4-6-12/h12-15,19,22H,2-11H2,1H3,(H,20,21)/t13-,14+,15-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504058

(CHEMBL4528896)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@@]11C[C@H](O)[C@](C)(O)[C@H](O)C1 |r| Show InChI InChI=1S/C15H26O5/c1-8(2)9-4-5-10(13(18)19)15(9)6-11(16)14(3,20)12(17)7-15/h8-12,16-17,20H,4-7H2,1-3H3,(H,18,19)/t9-,10+,11-,12+,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504043
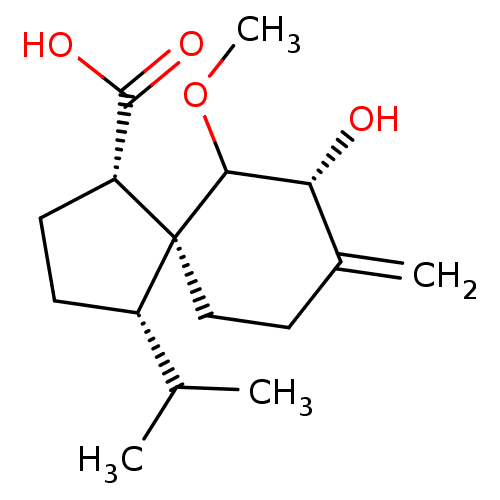
(CHEMBL4448701)Show SMILES COC1[C@H](O)C(=C)CC[C@@]11[C@@H](CC[C@@H]1C(O)=O)C(C)C |r| Show InChI InChI=1S/C16H26O4/c1-9(2)11-5-6-12(15(18)19)16(11)8-7-10(3)13(17)14(16)20-4/h9,11-14,17H,3,5-8H2,1-2,4H3,(H,18,19)/t11-,12+,13+,14?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504055
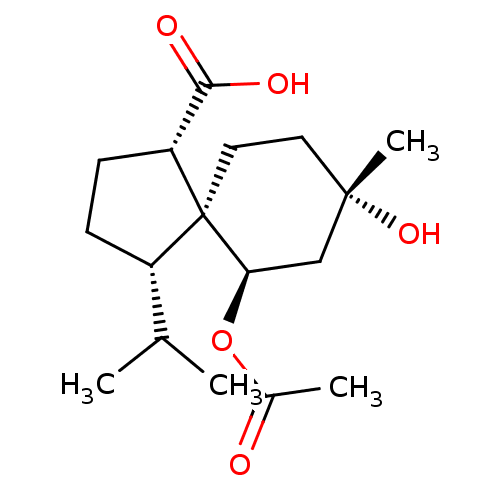
(CHEMBL4457718)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CC[C@](C)(O)C[C@H]1OC(C)=O |r| Show InChI InChI=1S/C17H28O5/c1-10(2)12-5-6-13(15(19)20)17(12)8-7-16(4,21)9-14(17)22-11(3)18/h10,12-14,21H,5-9H2,1-4H3,(H,19,20)/t12-,13+,14+,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504046
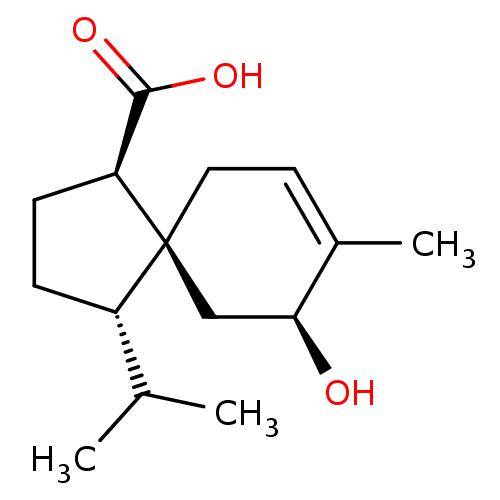
(CHEMBL4441254)Show SMILES CC(C)[C@@H]1CC[C@@H](C(O)=O)[C@]11CC=C(C)[C@@H](O)C1 |r,t:13| Show InChI InChI=1S/C15H24O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)13(16)8-15/h6,9,11-13,16H,4-5,7-8H2,1-3H3,(H,17,18)/t11-,12-,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504045
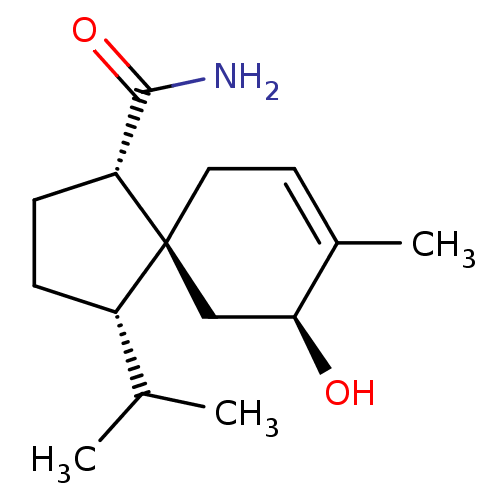
(CHEMBL4571180)Show SMILES CC(C)[C@@H]1CC[C@H](C(N)=O)[C@]11CC=C(C)[C@@H](O)C1 |r,t:13| Show InChI InChI=1S/C15H25NO2/c1-9(2)11-4-5-12(14(16)18)15(11)7-6-10(3)13(17)8-15/h6,9,11-13,17H,4-5,7-8H2,1-3H3,(H2,16,18)/t11-,12+,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504057
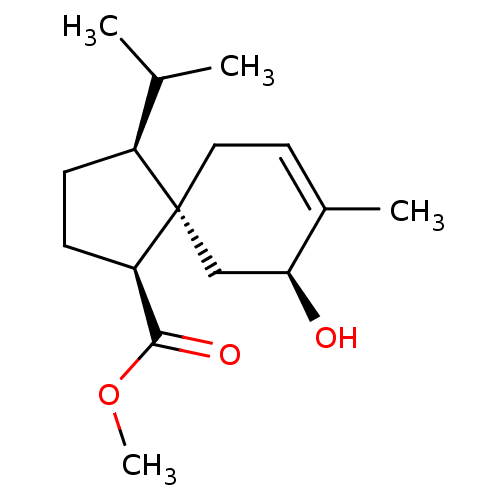
(CHEMBL4531837)Show SMILES COC(=O)[C@H]1CC[C@@H](C(C)C)[C@@]11CC=C(C)[C@@H](O)C1 |r,t:14| Show InChI InChI=1S/C16H26O3/c1-10(2)12-5-6-13(15(18)19-4)16(12)8-7-11(3)14(17)9-16/h7,10,12-14,17H,5-6,8-9H2,1-4H3/t12-,13+,14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36597
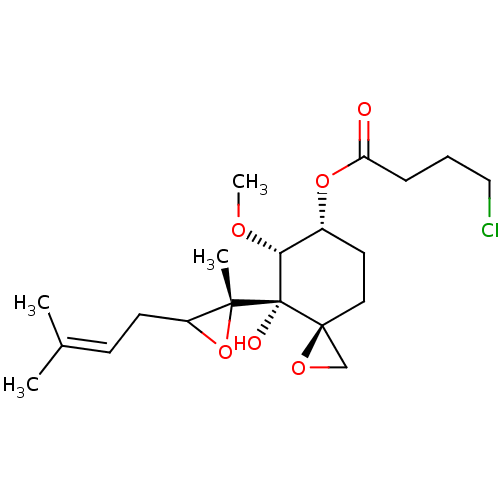
(FOS-69)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#6]-[#6]-[#6]Cl |r| Show InChI InChI=1S/C20H31ClO6/c1-13(2)7-8-15-18(3,27-15)20(23)17(24-4)14(9-10-19(20)12-25-19)26-16(22)6-5-11-21/h7,14-15,17,23H,5-6,8-12H2,1-4H3/t14-,15?,17-,18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36589
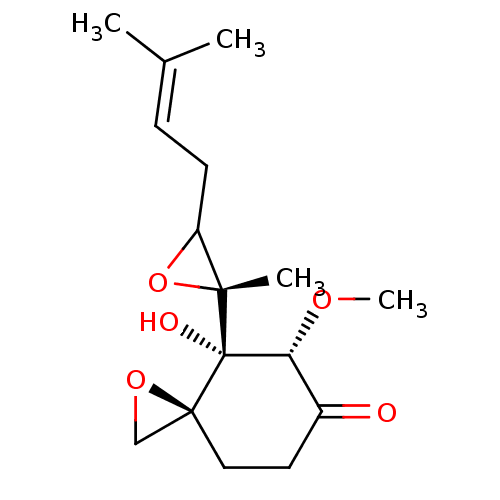
(Ovalicin)Show SMILES [#6]-[#8]-[#6@@H]1-[#6](=O)-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H24O5/c1-10(2)5-6-12-14(3,21-12)16(18)13(19-4)11(17)7-8-15(16)9-20-15/h5,12-13,18H,6-9H2,1-4H3/t12?,13-,14-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36588

(AGM-1470)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl |r| Show InChI InChI=1S/C19H28ClNO6/c1-11(2)5-6-13-18(3,27-13)16-15(24-4)12(7-8-19(16)10-25-19)26-17(23)21-14(22)9-20/h5,12-13,15-16H,6-10H2,1-4H3,(H,21,22,23)/t12-,13?,15-,16?,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36595
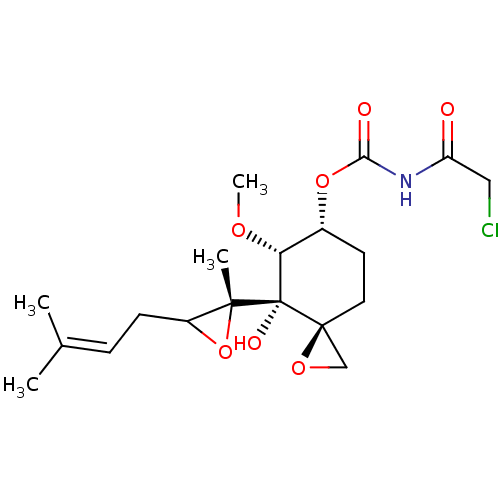
(FOS-68)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl |r| Show InChI InChI=1S/C19H28ClNO7/c1-11(2)5-6-13-17(3,28-13)19(24)15(25-4)12(7-8-18(19)10-26-18)27-16(23)21-14(22)9-20/h5,12-13,15,24H,6-10H2,1-4H3,(H,21,22,23)/t12-,13?,15-,17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36592
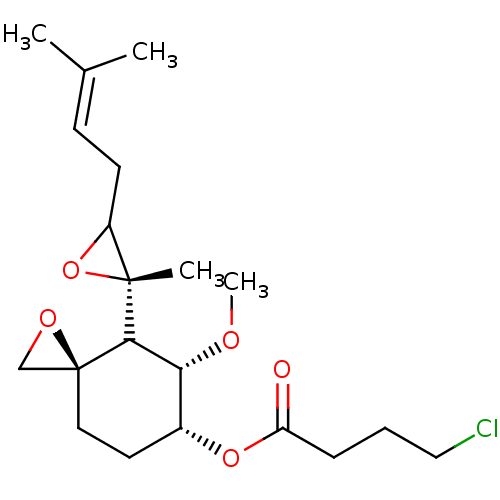
(FOS-70)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#6]-[#6]-[#6]Cl |r| Show InChI InChI=1S/C20H31ClO5/c1-13(2)7-8-15-19(3,26-15)18-17(23-4)14(9-10-20(18)12-24-20)25-16(22)6-5-11-21/h7,14-15,17-18H,5-6,8-12H2,1-4H3/t14-,15?,17-,18?,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36596
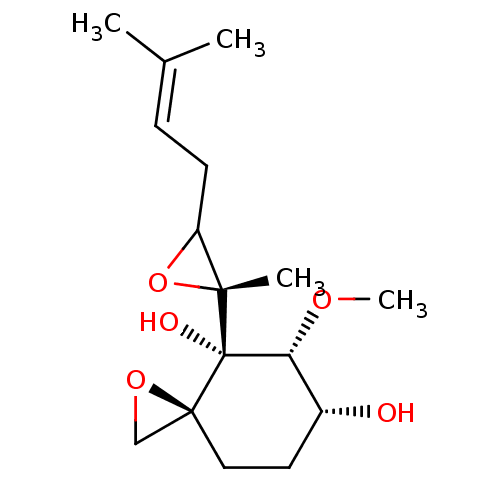
(FOS-34)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@H](-[#8])-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H26O5/c1-10(2)5-6-12-14(3,21-12)16(18)13(19-4)11(17)7-8-15(16)9-20-15/h5,11-13,17-18H,6-9H2,1-4H3/t11-,12?,13-,14-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291219
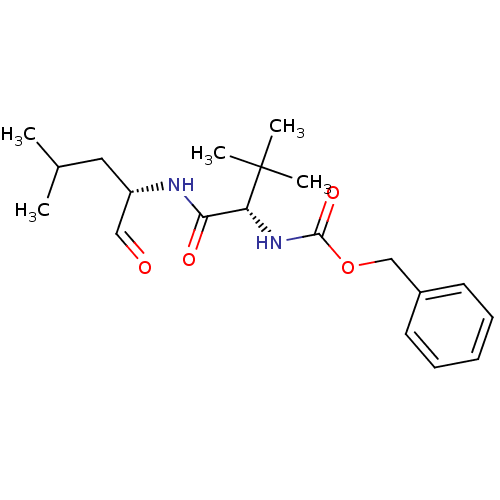
(CHEMBL151521 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)C)C=O Show InChI InChI=1S/C20H30N2O4/c1-14(2)11-16(12-23)21-18(24)17(20(3,4)5)22-19(25)26-13-15-9-7-6-8-10-15/h6-10,12,14,16-17H,11,13H2,1-5H3,(H,21,24)(H,22,25)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291234
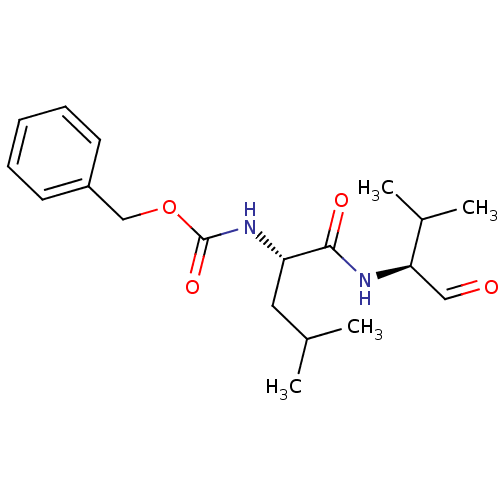
(CHEMBL150600 | [(S)-1-((S)-1-Formyl-2-methyl-propy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](C=O)C(C)C Show InChI InChI=1S/C19H28N2O4/c1-13(2)10-16(18(23)20-17(11-22)14(3)4)21-19(24)25-12-15-8-6-5-7-9-15/h5-9,11,13-14,16-17H,10,12H2,1-4H3,(H,20,23)(H,21,24)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50084655
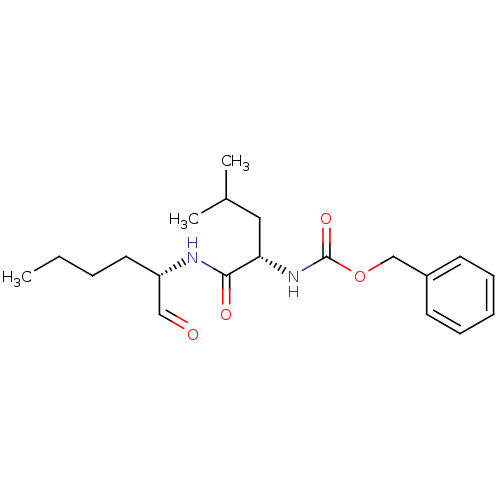
(CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C20H30N2O4/c1-4-5-11-17(13-23)21-19(24)18(12-15(2)3)22-20(25)26-14-16-9-7-6-8-10-16/h6-10,13,15,17-18H,4-5,11-12,14H2,1-3H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291225
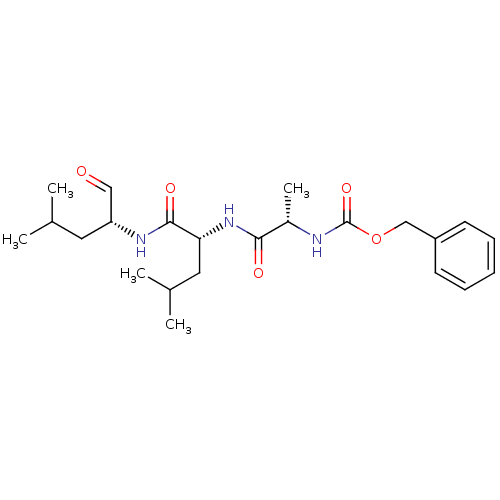
(CHEMBL357595 | {(S)-1-[(R)-1-((R)-1-Formyl-3-methy...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C23H35N3O5/c1-15(2)11-19(13-27)25-22(29)20(12-16(3)4)26-21(28)17(5)24-23(30)31-14-18-9-7-6-8-10-18/h6-10,13,15-17,19-20H,11-12,14H2,1-5H3,(H,24,30)(H,25,29)(H,26,28)/t17-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291188

(((S)-1-{(R)-1-[(S)-1-((S)-1-Formyl-3-methyl-butylc...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H46N4O6/c1-18(2)13-23(16-34)31-27(36)24(14-19(3)4)33-28(37)25(15-20(5)6)32-26(35)21(7)30-29(38)39-17-22-11-9-8-10-12-22/h8-12,16,18-21,23-25H,13-15,17H2,1-7H3,(H,30,38)(H,31,36)(H,32,35)(H,33,37)/t21-,23-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36590
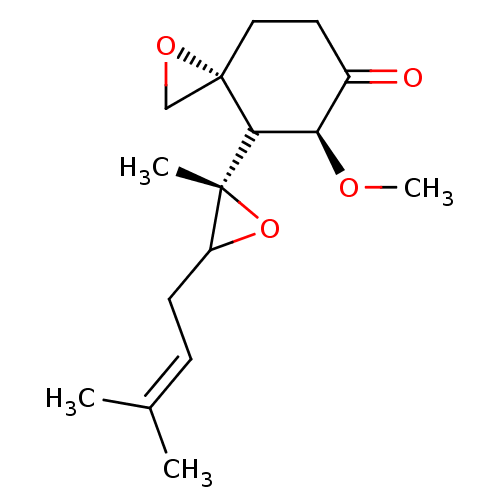
(FOS-72)Show SMILES [#6]-[#8]-[#6@H]-1-[#6]([C@]2([#6])[#8]-[#6]2-[#6]\[#6]=[#6](\[#6])-[#6])[C@@]2([#6]-[#8]2)[#6]-[#6]-[#6]-1=O |r| Show InChI InChI=1S/C16H24O4/c1-10(2)5-6-12-15(3,20-12)14-13(18-4)11(17)7-8-16(14)9-19-16/h5,12-14H,6-9H2,1-4H3/t12?,13-,14?,15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291222
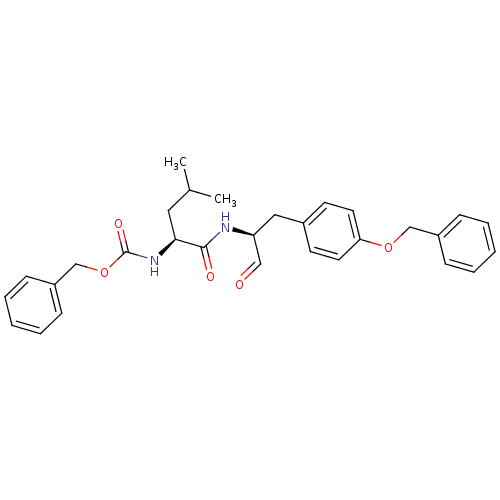
(CHEMBL423222 | {(S)-1-[(S)-1-(4-Benzyloxy-benzyl)-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C=O Show InChI InChI=1S/C30H34N2O5/c1-22(2)17-28(32-30(35)37-21-25-11-7-4-8-12-25)29(34)31-26(19-33)18-23-13-15-27(16-14-23)36-20-24-9-5-3-6-10-24/h3-16,19,22,26,28H,17-18,20-21H2,1-2H3,(H,31,34)(H,32,35)/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
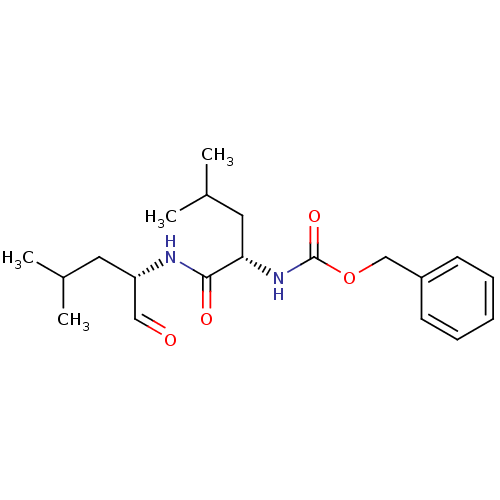
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291236
(CHEMBL356841 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C20H30N2O4/c1-14(2)10-17(12-23)21-19(24)18(11-15(3)4)22-20(25)26-13-16-8-6-5-7-9-16/h5-9,12,14-15,17-18H,10-11,13H2,1-4H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
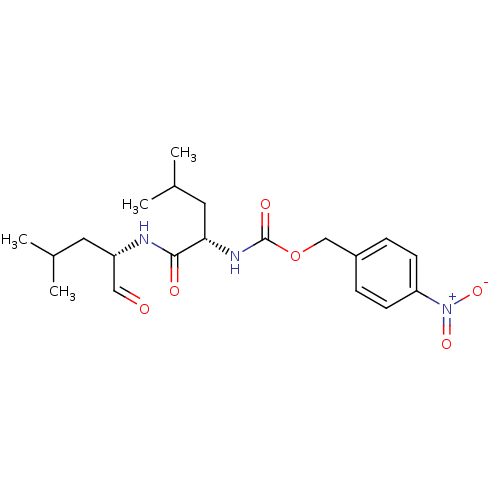
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291228
(CHEMBL347742 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccc(cc1)[N+]([O-])=O)C=O Show InChI InChI=1S/C20H29N3O6/c1-13(2)9-16(11-24)21-19(25)18(10-14(3)4)22-20(26)29-12-15-5-7-17(8-6-15)23(27)28/h5-8,11,13-14,16,18H,9-10,12H2,1-4H3,(H,21,25)(H,22,26)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
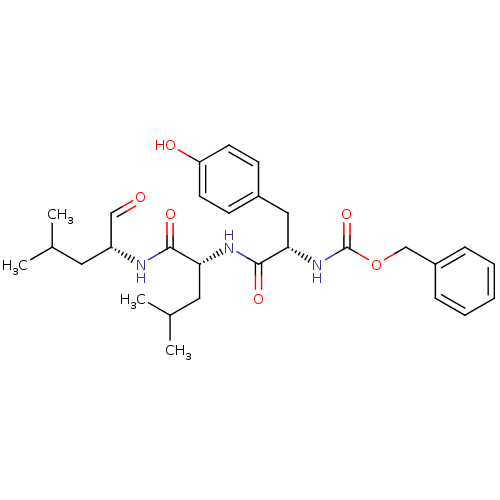
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291192
(CHEMBL345337 | [(S)-1-[(R)-1-((R)-1-Formyl-3-methy...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H39N3O6/c1-19(2)14-23(17-33)30-27(35)25(15-20(3)4)31-28(36)26(16-21-10-12-24(34)13-11-21)32-29(37)38-18-22-8-6-5-7-9-22/h5-13,17,19-20,23,25-26,34H,14-16,18H2,1-4H3,(H,30,35)(H,31,36)(H,32,37)/t23-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36591
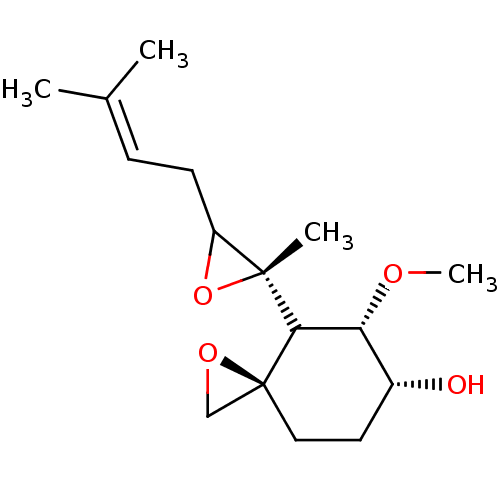
(FOS-37)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@H](-[#8])-[#6]-[#6][C@]2([#6]-[#8]2)[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H26O4/c1-10(2)5-6-12-15(3,20-12)14-13(18-4)11(17)7-8-16(14)9-19-16/h5,11-14,17H,6-9H2,1-4H3/t11-,12?,13-,14?,15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291218
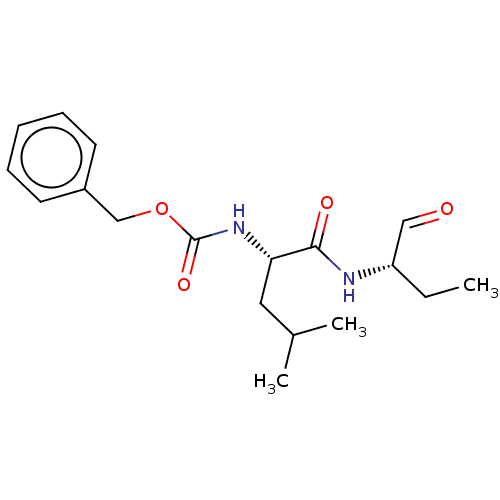
(CHEMBL2371036 | [(S)-1-(1-Formyl-propylcarbamoyl)-...)Show SMILES CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C18H26N2O4/c1-4-15(11-21)19-17(22)16(10-13(2)3)20-18(23)24-12-14-8-6-5-7-9-14/h5-9,11,13,15-16H,4,10,12H2,1-3H3,(H,19,22)(H,20,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50137397
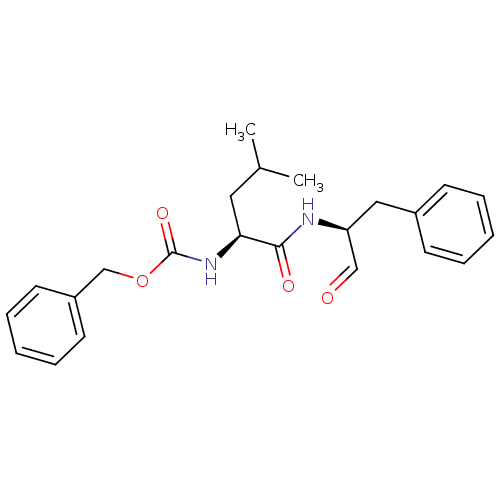
(CHEMBL341014 | [(S)-1-((S)-1-Benzyl-2-oxo-ethylcar...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O4/c1-17(2)13-21(25-23(28)29-16-19-11-7-4-8-12-19)22(27)24-20(15-26)14-18-9-5-3-6-10-18/h3-12,15,17,20-21H,13-14,16H2,1-2H3,(H,24,27)(H,25,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291223
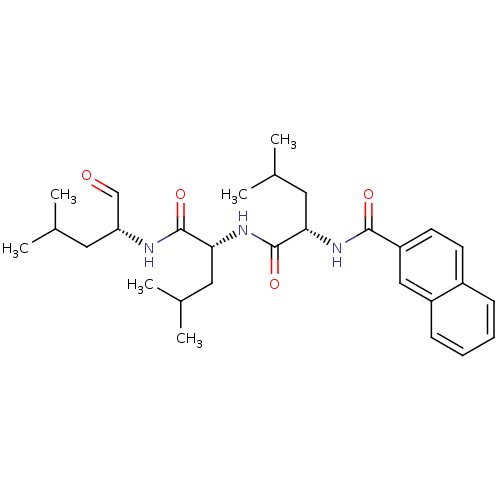
(CHEMBL150134 | Naphthalene-2-carboxylic acid {(S)-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C29H41N3O4/c1-18(2)13-24(17-33)30-28(35)25(14-19(3)4)32-29(36)26(15-20(5)6)31-27(34)23-12-11-21-9-7-8-10-22(21)16-23/h7-12,16-20,24-26H,13-15H2,1-6H3,(H,30,35)(H,31,34)(H,32,36)/t24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291194
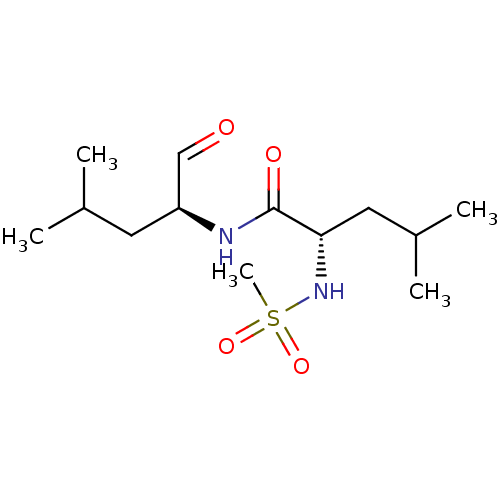
((S)-2-Methanesulfonylamino-4-methyl-pentanoic acid...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NS(C)(=O)=O)C=O Show InChI InChI=1S/C13H26N2O4S/c1-9(2)6-11(8-16)14-13(17)12(7-10(3)4)15-20(5,18)19/h8-12,15H,6-7H2,1-5H3,(H,14,17)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291226
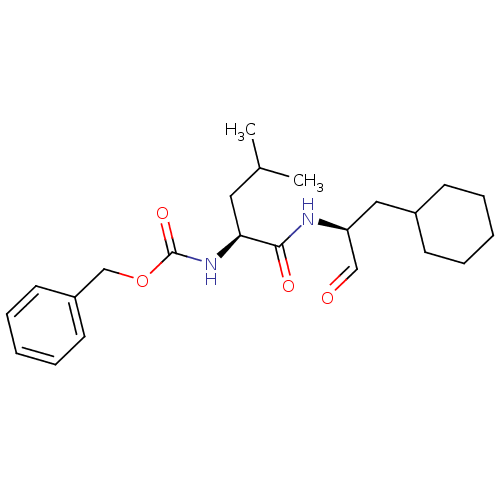
(CHEMBL151829 | [(S)-1-((S)-1-Cyclohexylmethyl-2-ox...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C=O Show InChI InChI=1S/C23H34N2O4/c1-17(2)13-21(25-23(28)29-16-19-11-7-4-8-12-19)22(27)24-20(15-26)14-18-9-5-3-6-10-18/h4,7-8,11-12,15,17-18,20-21H,3,5-6,9-10,13-14,16H2,1-2H3,(H,24,27)(H,25,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291187
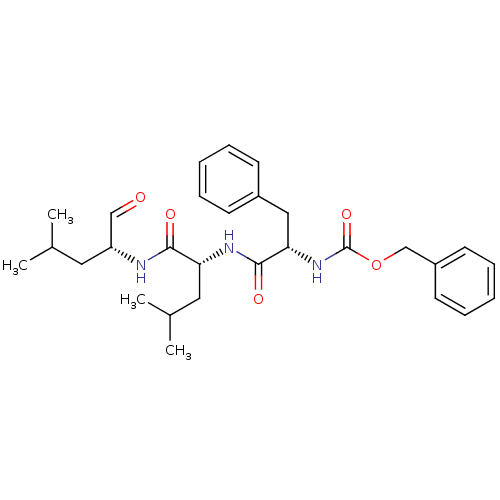
(CHEMBL151370 | {(S)-1-[(R)-1-((R)-1-Formyl-3-methy...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H39N3O5/c1-20(2)15-24(18-33)30-27(34)25(16-21(3)4)31-28(35)26(17-22-11-7-5-8-12-22)32-29(36)37-19-23-13-9-6-10-14-23/h5-14,18,20-21,24-26H,15-17,19H2,1-4H3,(H,30,34)(H,31,35)(H,32,36)/t24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50142287
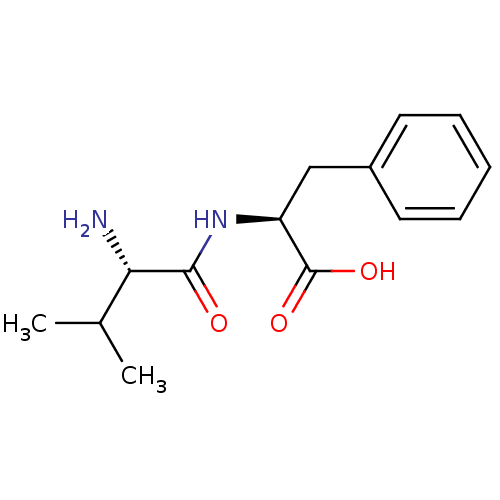
((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-phenyl...)Show InChI InChI=1S/C14H20N2O3/c1-9(2)12(15)13(17)16-11(14(18)19)8-10-6-4-3-5-7-10/h3-7,9,11-12H,8,15H2,1-2H3,(H,16,17)(H,18,19)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was evaluated against recombinant human calpain I |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data