 Found 500 hits with Last Name = 'grimm' and Initial = 'el'
Found 500 hits with Last Name = 'grimm' and Initial = 'el' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340424
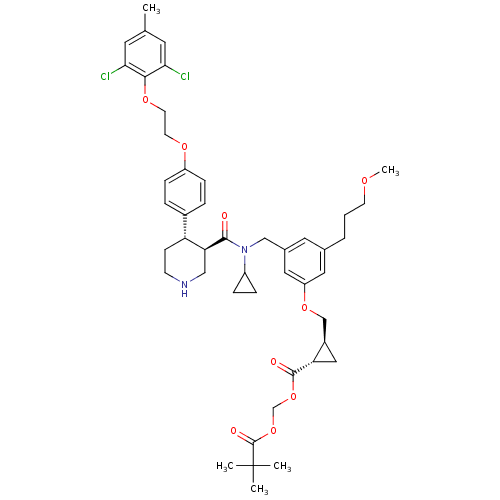
((1S,2S)-pivaloyloxymethyl 2-((3-(((3R,4S)-N-cyclop...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)OCOC(=O)C(C)(C)C)c1 |r| Show InChI InChI=1S/C46H58Cl2N2O9/c1-29-19-40(47)42(41(48)20-29)56-18-17-55-35-12-8-32(9-13-35)37-14-15-49-25-39(37)43(51)50(34-10-11-34)26-31-21-30(7-6-16-54-5)22-36(23-31)57-27-33-24-38(33)44(52)58-28-59-45(53)46(2,3)4/h8-9,12-13,19-23,33-34,37-39,49H,6-7,10-11,14-18,24-28H2,1-5H3/t33-,37-,38+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340406
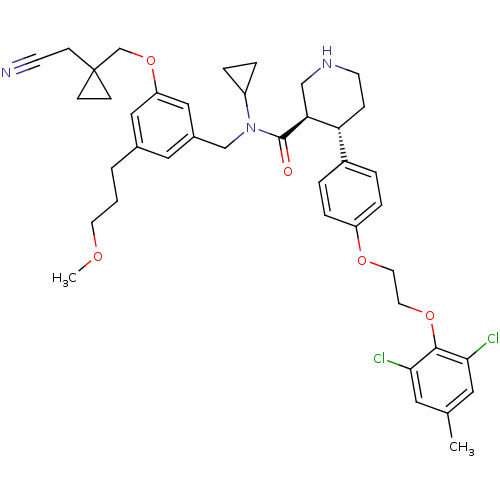
((3R,4S)-N-(3-((1-(cyanomethyl)cyclopropyl)methoxy)...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(CC#N)CC2)c1 |r| Show InChI InChI=1S/C41H49Cl2N3O5/c1-28-20-37(42)39(38(43)21-28)50-19-18-49-33-9-5-31(6-10-33)35-11-16-45-25-36(35)40(47)46(32-7-8-32)26-30-22-29(4-3-17-48-2)23-34(24-30)51-27-41(12-13-41)14-15-44/h5-6,9-10,20-24,32,35-36,45H,3-4,7-8,11-14,16-19,25-27H2,1-2H3/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340407
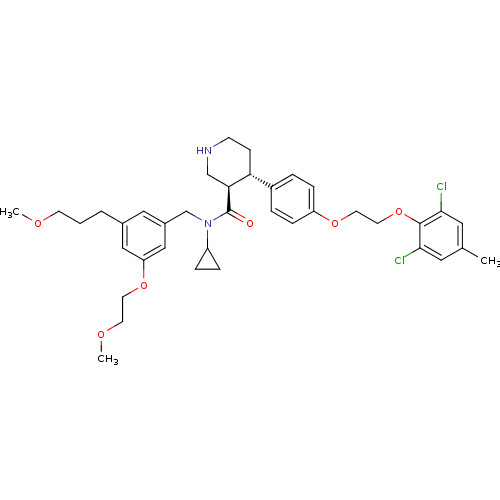
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCOC)c1 |r| Show InChI InChI=1S/C38H48Cl2N2O6/c1-26-19-35(39)37(36(40)20-26)48-18-17-46-31-10-6-29(7-11-31)33-12-13-41-24-34(33)38(43)42(30-8-9-30)25-28-21-27(5-4-14-44-2)22-32(23-28)47-16-15-45-3/h6-7,10-11,19-23,30,33-34,41H,4-5,8-9,12-18,24-25H2,1-3H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340412
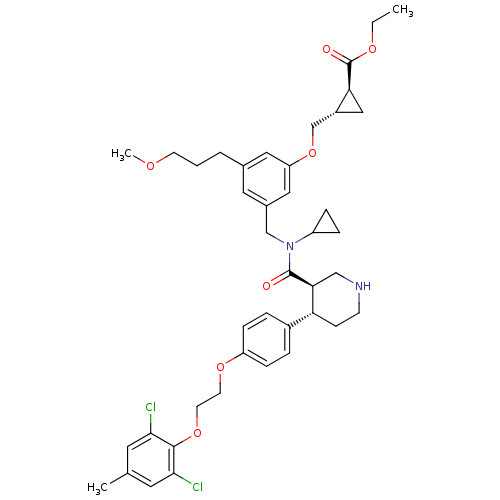
((1S,2S)-ethyl 2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(...)Show SMILES CCOC(=O)[C@H]1C[C@@H]1COc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C42H52Cl2N2O7/c1-4-50-42(48)36-23-31(36)26-53-34-21-28(6-5-15-49-3)20-29(22-34)25-46(32-9-10-32)41(47)37-24-45-14-13-35(37)30-7-11-33(12-8-30)51-16-17-52-40-38(43)18-27(2)19-39(40)44/h7-8,11-12,18-22,31-32,35-37,45H,4-6,9-10,13-17,23-26H2,1-3H3/t31-,35-,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340409
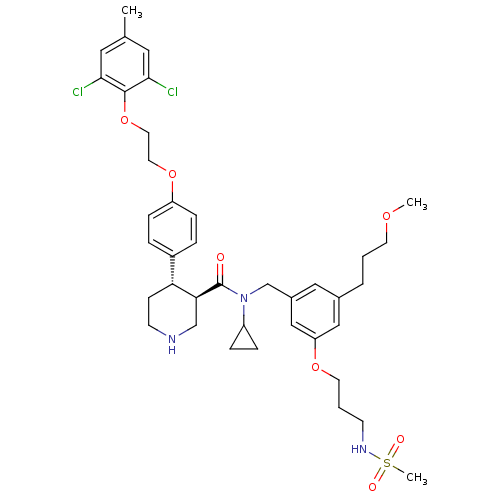
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCNS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O7S/c1-27-20-36(40)38(37(41)21-27)51-19-18-50-32-11-7-30(8-12-32)34-13-15-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-4-16-48-2)23-33(24-29)49-17-5-14-43-52(3,46)47/h7-8,11-12,20-24,31,34-35,42-43H,4-6,9-10,13-19,25-26H2,1-3H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340411
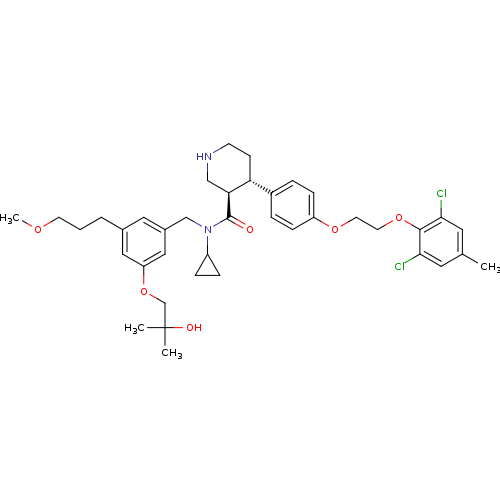
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)O)c1 |r| Show InChI InChI=1S/C39H50Cl2N2O6/c1-26-18-35(40)37(36(41)19-26)48-17-16-47-31-11-7-29(8-12-31)33-13-14-42-23-34(33)38(44)43(30-9-10-30)24-28-20-27(6-5-15-46-4)21-32(22-28)49-25-39(2,3)45/h7-8,11-12,18-22,30,33-34,42,45H,5-6,9-10,13-17,23-25H2,1-4H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340410
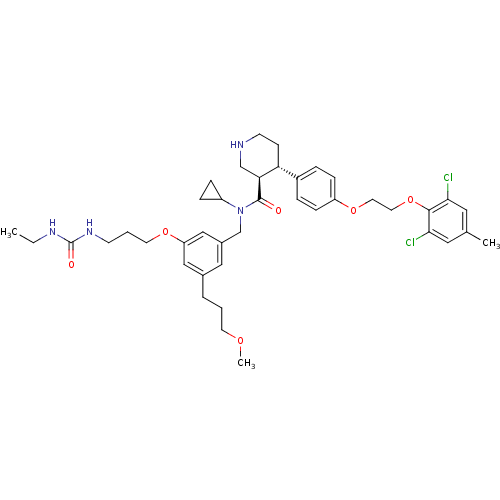
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES CCNC(=O)NCCCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C41H54Cl2N4O6/c1-4-45-41(49)46-15-6-18-51-34-24-29(7-5-17-50-3)23-30(25-34)27-47(32-10-11-32)40(48)36-26-44-16-14-35(36)31-8-12-33(13-9-31)52-19-20-53-39-37(42)21-28(2)22-38(39)43/h8-9,12-13,21-25,32,35-36,44H,4-7,10-11,14-20,26-27H2,1-3H3,(H2,45,46,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340408
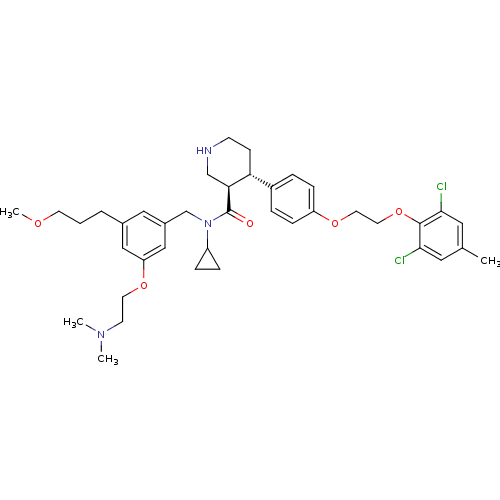
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCN(C)C)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O5/c1-27-20-36(40)38(37(41)21-27)49-19-18-48-32-11-7-30(8-12-32)34-13-14-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-5-16-46-4)23-33(24-29)47-17-15-43(2)3/h7-8,11-12,20-24,31,34-35,42H,5-6,9-10,13-19,25-26H2,1-4H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340423
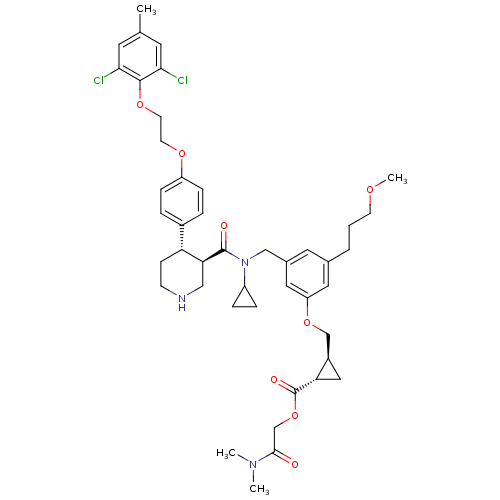
((1S,2S)-2-(dimethylamino)-2-oxoethyl 2-((3-(((3R,4...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)OCC(=O)N(C)C)c1 |r| Show InChI InChI=1S/C44H55Cl2N3O8/c1-28-18-39(45)42(40(46)19-28)55-17-16-54-34-11-7-31(8-12-34)36-13-14-47-24-38(36)43(51)49(33-9-10-33)25-30-20-29(6-5-15-53-4)21-35(22-30)56-26-32-23-37(32)44(52)57-27-41(50)48(2)3/h7-8,11-12,18-22,32-33,36-38,47H,5-6,9-10,13-17,23-27H2,1-4H3/t32-,36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340417
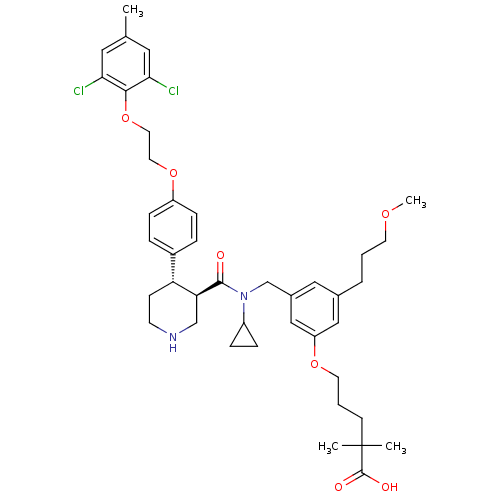
(5-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C42H54Cl2N2O7/c1-28-21-37(43)39(38(44)22-28)53-20-19-52-33-12-8-31(9-13-33)35-14-16-45-26-36(35)40(47)46(32-10-11-32)27-30-23-29(7-5-17-50-4)24-34(25-30)51-18-6-15-42(2,3)41(48)49/h8-9,12-13,21-25,32,35-36,45H,5-7,10-11,14-20,26-27H2,1-4H3,(H,48,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340416
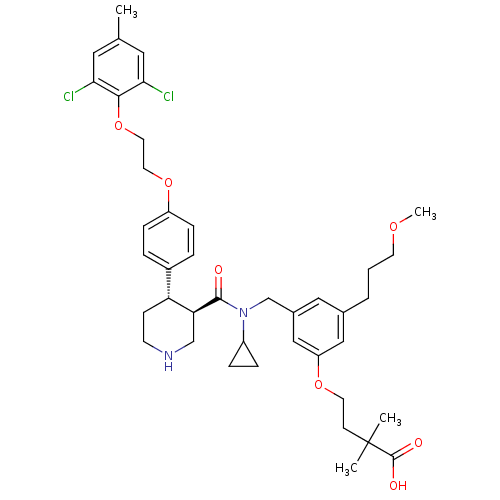
(4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C41H52Cl2N2O7/c1-27-20-36(42)38(37(43)21-27)52-19-18-51-32-11-7-30(8-12-32)34-13-15-44-25-35(34)39(46)45(31-9-10-31)26-29-22-28(6-5-16-49-4)23-33(24-29)50-17-14-41(2,3)40(47)48/h7-8,11-12,20-24,31,34-35,44H,5-6,9-10,13-19,25-26H2,1-4H3,(H,47,48)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340418
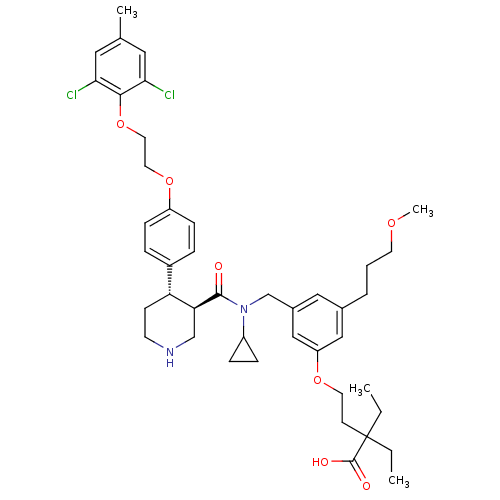
(4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES CCC(CC)(CCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1)C(O)=O |r| Show InChI InChI=1S/C43H56Cl2N2O7/c1-5-43(6-2,42(49)50)16-19-52-35-25-30(8-7-18-51-4)24-31(26-35)28-47(33-11-12-33)41(48)37-27-46-17-15-36(37)32-9-13-34(14-10-32)53-20-21-54-40-38(44)22-29(3)23-39(40)45/h9-10,13-14,22-26,33,36-37,46H,5-8,11-12,15-21,27-28H2,1-4H3,(H,49,50)/t36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340419
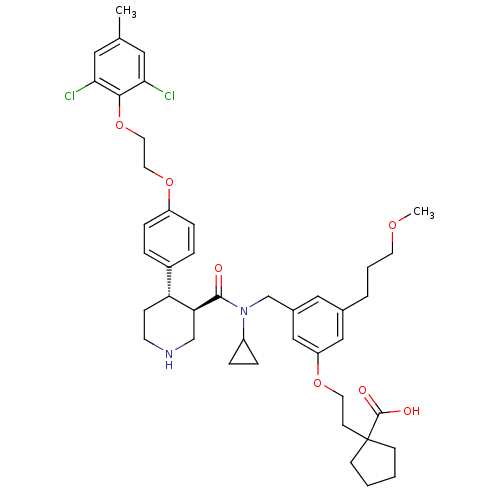
(1-(2-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichl...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCC2(CCCC2)C(O)=O)c1 |r| Show InChI InChI=1S/C43H54Cl2N2O7/c1-29-22-38(44)40(39(45)23-29)54-21-20-53-34-11-7-32(8-12-34)36-13-17-46-27-37(36)41(48)47(33-9-10-33)28-31-24-30(6-5-18-51-2)25-35(26-31)52-19-16-43(42(49)50)14-3-4-15-43/h7-8,11-12,22-26,33,36-37,46H,3-6,9-10,13-21,27-28H2,1-2H3,(H,49,50)/t36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340421
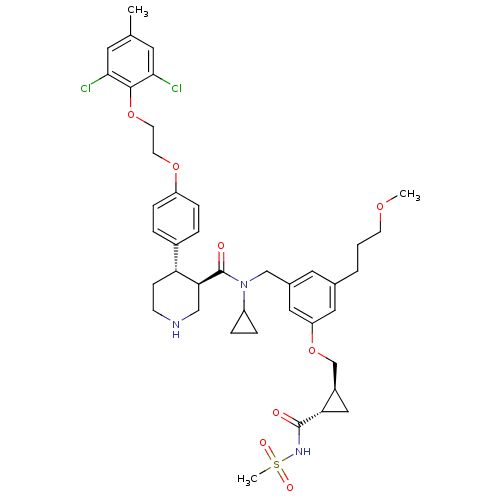
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C41H51Cl2N3O8S/c1-26-17-37(42)39(38(43)18-26)53-16-15-52-32-10-6-29(7-11-32)34-12-13-44-23-36(34)41(48)46(31-8-9-31)24-28-19-27(5-4-14-51-2)20-33(21-28)54-25-30-22-35(30)40(47)45-55(3,49)50/h6-7,10-11,17-21,30-31,34-36,44H,4-5,8-9,12-16,22-25H2,1-3H3,(H,45,47)/t30-,34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340422
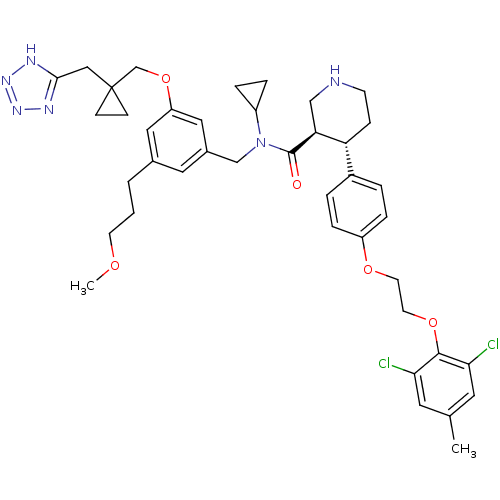
((3R,4S)-N-(3-((1-((2H-tetrazol-5-yl)methyl)cyclopr...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(Cc3nnn[nH]3)CC2)c1 |r| Show InChI InChI=1S/C41H50Cl2N6O5/c1-27-18-36(42)39(37(43)19-27)53-17-16-52-32-9-5-30(6-10-32)34-11-14-44-24-35(34)40(50)49(31-7-8-31)25-29-20-28(4-3-15-51-2)21-33(22-29)54-26-41(12-13-41)23-38-45-47-48-46-38/h5-6,9-10,18-22,31,34-35,44H,3-4,7-8,11-17,23-26H2,1-2H3,(H,45,46,47,48)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340415
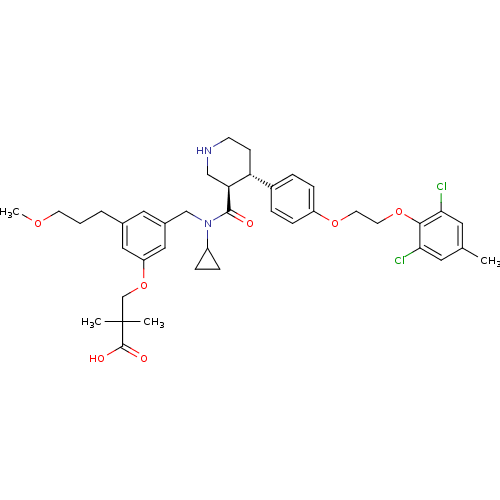
(3-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C40H50Cl2N2O7/c1-26-18-35(41)37(36(42)19-26)50-17-16-49-31-11-7-29(8-12-31)33-13-14-43-23-34(33)38(45)44(30-9-10-30)24-28-20-27(6-5-15-48-4)21-32(22-28)51-25-40(2,3)39(46)47/h7-8,11-12,18-22,30,33-34,43H,5-6,9-10,13-17,23-25H2,1-4H3,(H,46,47)/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340414
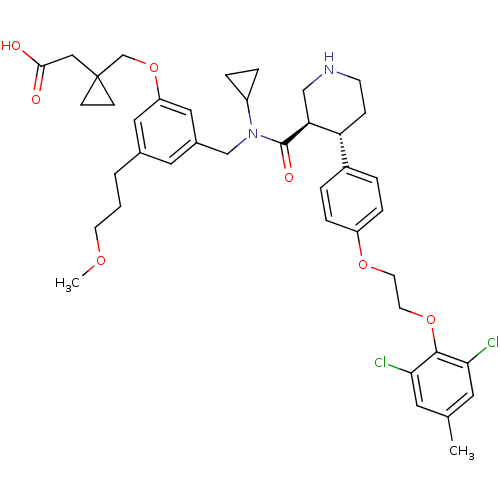
(2-(1-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(CC(O)=O)CC2)c1 |r| Show InChI InChI=1S/C41H50Cl2N2O7/c1-27-18-36(42)39(37(43)19-27)51-17-16-50-32-9-5-30(6-10-32)34-11-14-44-24-35(34)40(48)45(31-7-8-31)25-29-20-28(4-3-15-49-2)21-33(22-29)52-26-41(12-13-41)23-38(46)47/h5-6,9-10,18-22,31,34-35,44H,3-4,7-8,11-17,23-26H2,1-2H3,(H,46,47)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340413
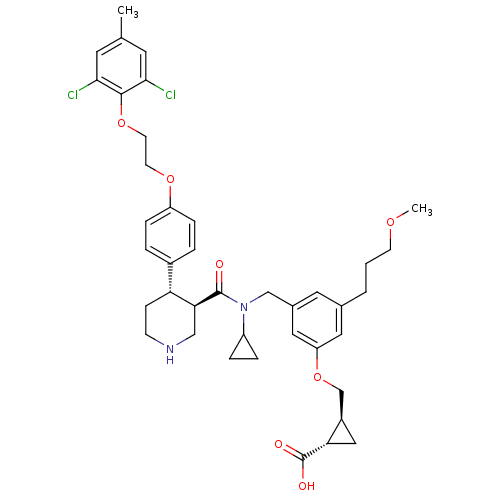
((1S,2S)-2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(O)=O)c1 |r| Show InChI InChI=1S/C40H48Cl2N2O7/c1-25-16-36(41)38(37(42)17-25)50-15-14-49-31-9-5-28(6-10-31)33-11-12-43-22-35(33)39(45)44(30-7-8-30)23-27-18-26(4-3-13-48-2)19-32(20-27)51-24-29-21-34(29)40(46)47/h5-6,9-10,16-20,29-30,33-35,43H,3-4,7-8,11-15,21-24H2,1-2H3,(H,46,47)/t29-,33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340420
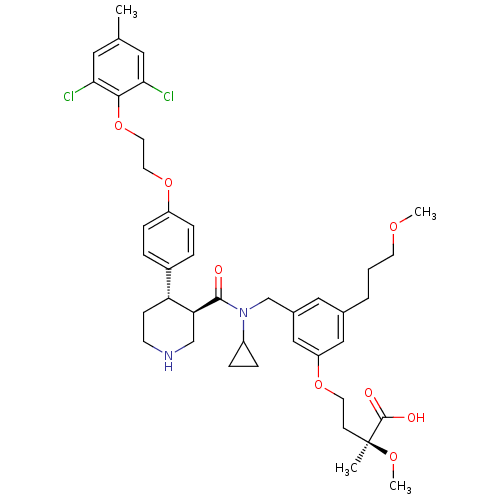
((S)-4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC[C@](C)(OC)C(O)=O)c1 |r| Show InChI InChI=1S/C41H52Cl2N2O8/c1-27-20-36(42)38(37(43)21-27)53-19-18-52-32-11-7-30(8-12-32)34-13-15-44-25-35(34)39(46)45(31-9-10-31)26-29-22-28(6-5-16-49-3)23-33(24-29)51-17-14-41(2,50-4)40(47)48/h7-8,11-12,20-24,31,34-35,44H,5-6,9-10,13-19,25-26H2,1-4H3,(H,47,48)/t34-,35+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340425
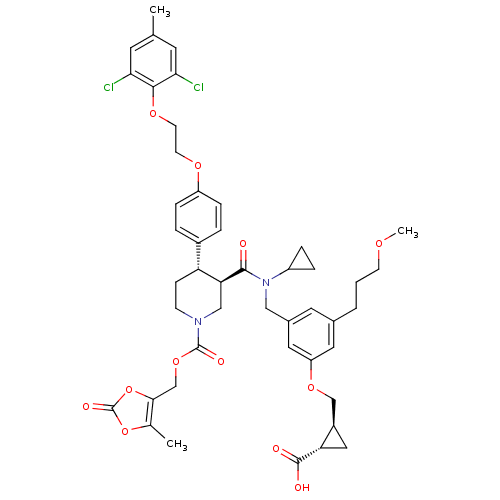
((1S,2S)-2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CN(CC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)C(=O)OCc2oc(=O)oc2C)cc(OC[C@H]2C[C@@H]2C(O)=O)c1 |r| Show InChI InChI=1S/C46H52Cl2N2O12/c1-27-17-39(47)42(40(48)18-27)58-16-15-57-34-10-6-31(7-11-34)36-12-13-49(45(54)60-26-41-28(2)61-46(55)62-41)24-38(36)43(51)50(33-8-9-33)23-30-19-29(5-4-14-56-3)20-35(21-30)59-25-32-22-37(32)44(52)53/h6-7,10-11,17-21,32-33,36-38H,4-5,8-9,12-16,22-26H2,1-3H3,(H,52,53)/t32-,36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340421

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C41H51Cl2N3O8S/c1-26-17-37(42)39(38(43)18-26)53-16-15-52-32-10-6-29(7-11-32)34-12-13-44-23-36(34)41(48)46(31-8-9-31)24-28-19-27(5-4-14-51-2)20-33(21-28)54-25-30-22-35(30)40(47)45-55(3,49)50/h6-7,10-11,17-21,30-31,34-36,44H,4-5,8-9,12-16,22-25H2,1-3H3,(H,45,47)/t30-,34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340419

(1-(2-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichl...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCC2(CCCC2)C(O)=O)c1 |r| Show InChI InChI=1S/C43H54Cl2N2O7/c1-29-22-38(44)40(39(45)23-29)54-21-20-53-34-11-7-32(8-12-34)36-13-17-46-27-37(36)41(48)47(33-9-10-33)28-31-24-30(6-5-18-51-2)25-35(26-31)52-19-16-43(42(49)50)14-3-4-15-43/h7-8,11-12,22-26,33,36-37,46H,3-6,9-10,13-21,27-28H2,1-2H3,(H,49,50)/t36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340417

(5-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C42H54Cl2N2O7/c1-28-21-37(43)39(38(44)22-28)53-20-19-52-33-12-8-31(9-13-33)35-14-16-45-26-36(35)40(47)46(32-10-11-32)27-30-23-29(7-5-17-50-4)24-34(25-30)51-18-6-15-42(2,3)41(48)49/h8-9,12-13,21-25,32,35-36,45H,5-7,10-11,14-20,26-27H2,1-4H3,(H,48,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340416

(4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C41H52Cl2N2O7/c1-27-20-36(42)38(37(43)21-27)52-19-18-51-32-11-7-30(8-12-32)34-13-15-44-25-35(34)39(46)45(31-9-10-31)26-29-22-28(6-5-16-49-4)23-33(24-29)50-17-14-41(2,3)40(47)48/h7-8,11-12,20-24,31,34-35,44H,5-6,9-10,13-19,25-26H2,1-4H3,(H,47,48)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340418

(4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES CCC(CC)(CCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1)C(O)=O |r| Show InChI InChI=1S/C43H56Cl2N2O7/c1-5-43(6-2,42(49)50)16-19-52-35-25-30(8-7-18-51-4)24-31(26-35)28-47(33-11-12-33)41(48)37-27-46-17-15-36(37)32-9-13-34(14-10-32)53-20-21-54-40-38(44)22-29(3)23-39(40)45/h9-10,13-14,22-26,33,36-37,46H,5-8,11-12,15-21,27-28H2,1-4H3,(H,49,50)/t36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340407

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCOC)c1 |r| Show InChI InChI=1S/C38H48Cl2N2O6/c1-26-19-35(39)37(36(40)20-26)48-18-17-46-31-10-6-29(7-11-31)33-12-13-41-24-34(33)38(43)42(30-8-9-30)25-28-21-27(5-4-14-44-2)22-32(23-28)47-16-15-45-3/h6-7,10-11,19-23,30,33-34,41H,4-5,8-9,12-18,24-25H2,1-3H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340410

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES CCNC(=O)NCCCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C41H54Cl2N4O6/c1-4-45-41(49)46-15-6-18-51-34-24-29(7-5-17-50-3)23-30(25-34)27-47(32-10-11-32)40(48)36-26-44-16-14-35(36)31-8-12-33(13-9-31)52-19-20-53-39-37(42)21-28(2)22-38(39)43/h8-9,12-13,21-25,32,35-36,44H,4-7,10-11,14-20,26-27H2,1-3H3,(H2,45,46,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340413

((1S,2S)-2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(O)=O)c1 |r| Show InChI InChI=1S/C40H48Cl2N2O7/c1-25-16-36(41)38(37(42)17-25)50-15-14-49-31-9-5-28(6-10-31)33-11-12-43-22-35(33)39(45)44(30-7-8-30)23-27-18-26(4-3-13-48-2)19-32(20-27)51-24-29-21-34(29)40(46)47/h5-6,9-10,16-20,29-30,33-35,43H,3-4,7-8,11-15,21-24H2,1-2H3,(H,46,47)/t29-,33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340409

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCNS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O7S/c1-27-20-36(40)38(37(41)21-27)51-19-18-50-32-11-7-30(8-12-32)34-13-15-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-4-16-48-2)23-33(24-29)49-17-5-14-43-52(3,46)47/h7-8,11-12,20-24,31,34-35,42-43H,4-6,9-10,13-19,25-26H2,1-3H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340406

((3R,4S)-N-(3-((1-(cyanomethyl)cyclopropyl)methoxy)...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(CC#N)CC2)c1 |r| Show InChI InChI=1S/C41H49Cl2N3O5/c1-28-20-37(42)39(38(43)21-28)50-19-18-49-33-9-5-31(6-10-33)35-11-16-45-25-36(35)40(47)46(32-7-8-32)26-30-22-29(4-3-17-48-2)23-34(24-30)51-27-41(12-13-41)14-15-44/h5-6,9-10,20-24,32,35-36,45H,3-4,7-8,11-14,16-19,25-27H2,1-2H3/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340422

((3R,4S)-N-(3-((1-((2H-tetrazol-5-yl)methyl)cyclopr...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(Cc3nnn[nH]3)CC2)c1 |r| Show InChI InChI=1S/C41H50Cl2N6O5/c1-27-18-36(42)39(37(43)19-27)53-17-16-52-32-9-5-30(6-10-32)34-11-14-44-24-35(34)40(50)49(31-7-8-31)25-29-20-28(4-3-15-51-2)21-33(22-29)54-26-41(12-13-41)23-38-45-47-48-46-38/h5-6,9-10,18-22,31,34-35,44H,3-4,7-8,11-17,23-26H2,1-2H3,(H,45,46,47,48)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340414

(2-(1-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(CC(O)=O)CC2)c1 |r| Show InChI InChI=1S/C41H50Cl2N2O7/c1-27-18-36(42)39(37(43)19-27)51-17-16-50-32-9-5-30(6-10-32)34-11-14-44-24-35(34)40(48)45(31-7-8-31)25-29-20-28(4-3-15-49-2)21-33(22-29)52-26-41(12-13-41)23-38(46)47/h5-6,9-10,18-22,31,34-35,44H,3-4,7-8,11-17,23-26H2,1-2H3,(H,46,47)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340408

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCN(C)C)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O5/c1-27-20-36(40)38(37(41)21-27)49-19-18-48-32-11-7-30(8-12-32)34-13-14-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-5-16-46-4)23-33(24-29)47-17-15-43(2)3/h7-8,11-12,20-24,31,34-35,42H,5-6,9-10,13-19,25-26H2,1-4H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340415

(3-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C40H50Cl2N2O7/c1-26-18-35(41)37(36(42)19-26)50-17-16-49-31-11-7-29(8-12-31)33-13-14-43-23-34(33)38(45)44(30-9-10-30)24-28-20-27(6-5-15-48-4)21-32(22-28)51-25-40(2,3)39(46)47/h7-8,11-12,18-22,30,33-34,43H,5-6,9-10,13-17,23-25H2,1-4H3,(H,46,47)/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340420

((S)-4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC[C@](C)(OC)C(O)=O)c1 |r| Show InChI InChI=1S/C41H52Cl2N2O8/c1-27-20-36(42)38(37(43)21-27)53-19-18-52-32-11-7-30(8-12-32)34-13-15-44-25-35(34)39(46)45(31-9-10-31)26-29-22-28(6-5-16-49-3)23-33(24-29)51-17-14-41(2,50-4)40(47)48/h7-8,11-12,20-24,31,34-35,44H,5-6,9-10,13-19,25-26H2,1-4H3,(H,47,48)/t34-,35+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340412

((1S,2S)-ethyl 2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(...)Show SMILES CCOC(=O)[C@H]1C[C@@H]1COc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C42H52Cl2N2O7/c1-4-50-42(48)36-23-31(36)26-53-34-21-28(6-5-15-49-3)20-29(22-34)25-46(32-9-10-32)41(47)37-24-45-14-13-35(37)30-7-11-33(12-8-30)51-16-17-52-40-38(43)18-27(2)19-39(40)44/h7-8,11-12,18-22,31-32,35-37,45H,4-6,9-10,13-17,23-26H2,1-3H3/t31-,35-,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340411

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)O)c1 |r| Show InChI InChI=1S/C39H50Cl2N2O6/c1-26-18-35(40)37(36(41)19-26)48-17-16-47-31-11-7-29(8-12-31)33-13-14-42-23-34(33)38(44)43(30-9-10-30)24-28-20-27(6-5-15-46-4)21-32(22-28)49-25-39(2,3)45/h7-8,11-12,18-22,30,33-34,42,45H,5-6,9-10,13-17,23-25H2,1-4H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182306
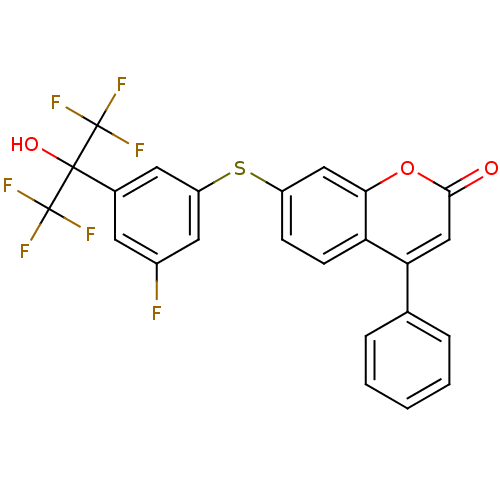
(7-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3c(cc(=O)oc3c2)-c2ccccc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H13F7O3S/c25-15-8-14(22(33,23(26,27)28)24(29,30)31)9-17(10-15)35-16-6-7-18-19(13-4-2-1-3-5-13)12-21(32)34-20(18)11-16/h1-12,33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182303
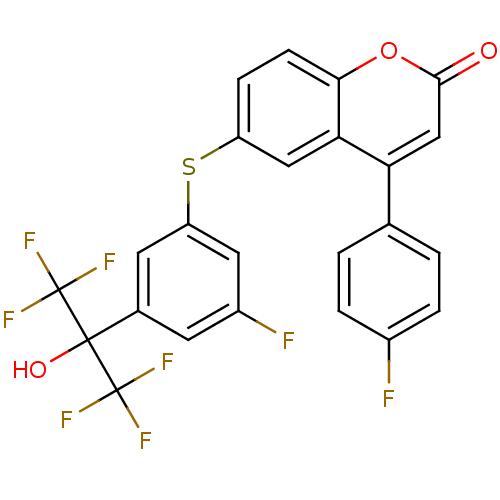
(6-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3oc(=O)cc(-c4ccc(F)cc4)c3c2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H12F8O3S/c25-14-3-1-12(2-4-14)18-11-21(33)35-20-6-5-16(10-19(18)20)36-17-8-13(7-15(26)9-17)22(34,23(27,28)29)24(30,31)32/h1-11,34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182308

(7-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C22H11F7O4S/c23-13-5-12(20(31,21(24,25)26)22(27,28)29)6-15(7-13)34-14-1-2-16-17(11-3-4-32-10-11)9-19(30)33-18(16)8-14/h1-10,31H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182304
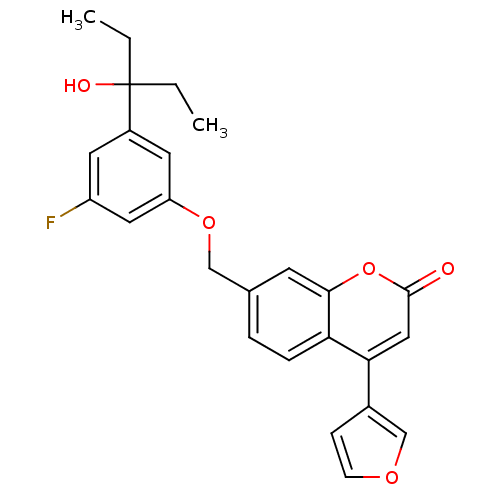
(7-((3-fluoro-5-(3-hydroxypentan-3-yl)phenoxy)methy...)Show SMILES CCC(O)(CC)c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1 Show InChI InChI=1S/C25H23FO5/c1-3-25(28,4-2)18-10-19(26)12-20(11-18)30-14-16-5-6-21-22(17-7-8-29-15-17)13-24(27)31-23(21)9-16/h5-13,15,28H,3-4,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340413

((1S,2S)-2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(O)=O)c1 |r| Show InChI InChI=1S/C40H48Cl2N2O7/c1-25-16-36(41)38(37(42)17-25)50-15-14-49-31-9-5-28(6-10-31)33-11-12-43-22-35(33)39(45)44(30-7-8-30)23-27-18-26(4-3-13-48-2)19-32(20-27)51-24-29-21-34(29)40(46)47/h5-6,9-10,16-20,29-30,33-35,43H,3-4,7-8,11-15,21-24H2,1-2H3,(H,46,47)/t29-,33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in human citrated-plasma |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM10675
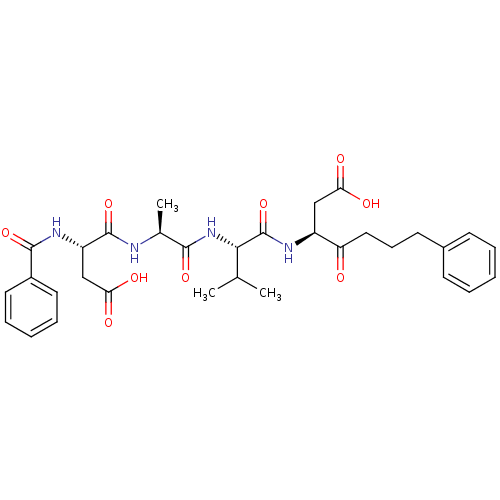
((3S)-3-[(2S)-2-[(2S)-2-[(3S)-3-formamido-3-(phenyl...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)c1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)CCCc1ccccc1 |r| Show InChI InChI=1S/C32H40N4O9/c1-19(2)28(32(45)34-23(17-26(38)39)25(37)16-10-13-21-11-6-4-7-12-21)36-29(42)20(3)33-31(44)24(18-27(40)41)35-30(43)22-14-8-5-9-15-22/h4-9,11-12,14-15,19-20,23-24,28H,10,13,16-18H2,1-3H3,(H,33,44)(H,34,45)(H,35,43)(H,36,42)(H,38,39)(H,40,41)/t20-,23-,24-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Merck Frosst Canada & Co.
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem Lett 14: 805-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.10.064
BindingDB Entry DOI: 10.7270/Q20P0X88 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182302

(7-((3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypr...)Show SMILES OC(c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccc(F)cc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C25H14F8O4/c26-16-4-2-14(3-5-16)20-11-22(34)37-21-7-13(1-6-19(20)21)12-36-18-9-15(8-17(27)10-18)23(35,24(28,29)30)25(31,32)33/h1-11,35H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 13: 1195-8 (2003)
BindingDB Entry DOI: 10.7270/Q2GT5MJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50340421

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C41H51Cl2N3O8S/c1-26-17-37(42)39(38(43)18-26)53-16-15-52-32-10-6-29(7-11-32)34-12-13-44-23-36(34)41(48)46(31-8-9-31)24-28-19-27(5-4-14-51-2)20-33(21-28)54-25-30-22-35(30)40(47)45-55(3,49)50/h6-7,10-11,17-21,30-31,34-36,44H,4-5,8-9,12-16,22-25H2,1-3H3,(H,45,47)/t30-,34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in human citrated-plasma |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340420

((S)-4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC[C@](C)(OC)C(O)=O)c1 |r| Show InChI InChI=1S/C41H52Cl2N2O8/c1-27-20-36(42)38(37(43)21-27)53-19-18-52-32-11-7-30(8-12-32)34-13-15-44-25-35(34)39(46)45(31-9-10-31)26-29-22-28(6-5-16-49-3)23-33(24-29)51-17-14-41(2,50-4)40(47)48/h7-8,11-12,20-24,31,34-35,44H,5-6,9-10,13-19,25-26H2,1-4H3,(H,47,48)/t34-,35+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in human citrated-plasma |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182300

(7-((3-fluoro-5-((1S,3S,5R)-3-hydroxy-6,8-dioxa-bic...)Show SMILES O[C@]1(C[C@H]2CO[C@@H](C1)O2)c1cc(F)cc(OCc2ccc3c(cc(nc3c2)C#N)-c2ccoc2)c1 Show InChI InChI=1S/C27H21FN2O5/c28-19-6-18(27(31)10-22-15-34-26(11-27)35-22)7-21(8-19)33-13-16-1-2-23-24(17-3-4-32-14-17)9-20(12-29)30-25(23)5-16/h1-9,14,22,26,31H,10-11,13,15H2/t22?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
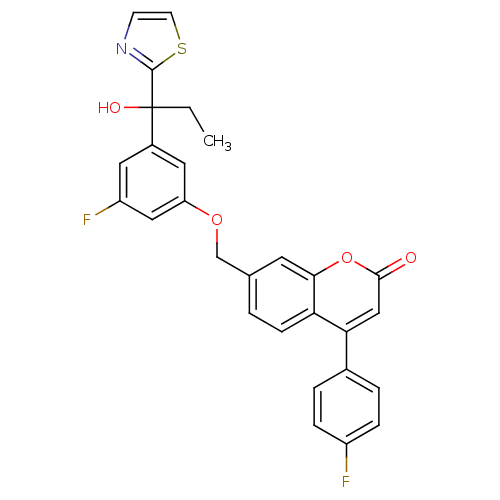
(Homo sapiens (Human)) | BDBM50182305
(7-((3-fluoro-5-(1-hydroxy-1-(thiazol-2-yl)propyl)p...)Show SMILES CCC(O)(c1nccs1)c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C28H21F2NO4S/c1-2-28(33,27-31-9-10-36-27)19-12-21(30)14-22(13-19)34-16-17-3-8-23-24(15-26(32)35-25(23)11-17)18-4-6-20(29)7-5-18/h3-15,33H,2,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50340409

((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCNS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O7S/c1-27-20-36(40)38(37(41)21-27)51-19-18-50-32-11-7-30(8-12-32)34-13-15-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-4-16-48-2)23-33(24-29)49-17-5-14-43-52(3,46)47/h7-8,11-12,20-24,31,34-35,42-43H,4-6,9-10,13-19,25-26H2,1-3H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin in human citrated-plasma |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data