 Found 980 hits with Last Name = 'groombridge' and Initial = 's'
Found 980 hits with Last Name = 'groombridge' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517560

(CHEMBL4583286)Show SMILES COc1cc(\C=C\C(O)=O)cc(F)c1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H24F3N3O3/c1-13-8-16-15(5-6-19-17(16)11-28-29-19)23(30(13)12-24(2,26)27)22-18(25)9-14(4-7-21(31)32)10-20(22)33-3/h4-7,9-11,13,23H,8,12H2,1-3H3,(H,28,29)(H,31,32)/b7-4+/t13-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM288677

(US10087191, Example 94)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as reduction in ERalpha protein expression after 5 hrs by formaldehyde-staining based a... |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM288677

(US10087191, Example 94)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517555
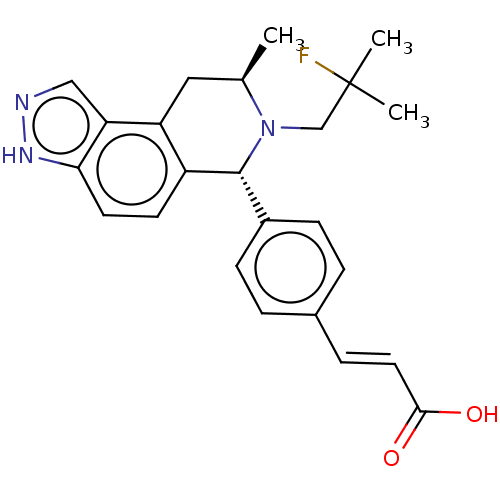
(CHEMBL4515724)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C24H26FN3O2/c1-15-12-19-18(9-10-21-20(19)13-26-27-21)23(28(15)14-24(2,3)25)17-7-4-16(5-8-17)6-11-22(29)30/h4-11,13,15,23H,12,14H2,1-3H3,(H,26,27)(H,29,30)/b11-6+/t15-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517562

(CHEMBL4586022)Show SMILES COc1cc(\C=C\C(O)=O)c(F)cc1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H24F3N3O3/c1-13-8-16-15(5-6-20-18(16)11-28-29-20)23(30(13)12-24(2,26)27)17-10-19(25)14(4-7-22(31)32)9-21(17)33-3/h4-7,9-11,13,23H,8,12H2,1-3H3,(H,28,29)(H,31,32)/b7-4+/t13-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM288677

(US10087191, Example 94)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517560

(CHEMBL4583286)Show SMILES COc1cc(\C=C\C(O)=O)cc(F)c1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H24F3N3O3/c1-13-8-16-15(5-6-19-17(16)11-28-29-19)23(30(13)12-24(2,26)27)22-18(25)9-14(4-7-21(31)32)10-20(22)33-3/h4-7,9-11,13,23H,8,12H2,1-3H3,(H,28,29)(H,31,32)/b7-4+/t13-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517557

(CHEMBL4436390)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@](C)(N1CC(C)(F)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C24H23F4N3O2/c1-13-8-15-16-11-29-30-20(16)6-5-17(15)24(3,31(13)12-23(2,27)28)22-18(25)9-14(10-19(22)26)4-7-21(32)33/h4-7,9-11,13H,8,12H2,1-3H3,(H,29,30)(H,32,33)/b7-4+/t13-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517559
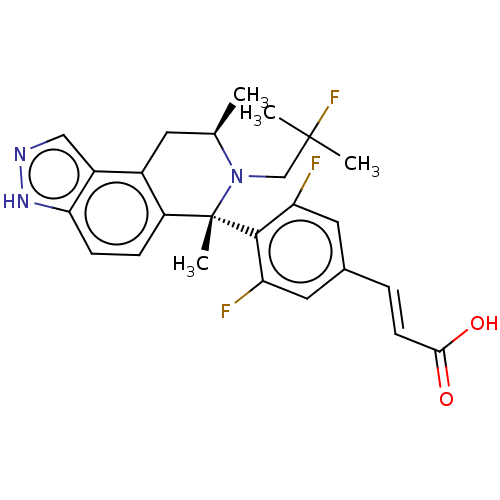
(CHEMBL4584370)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@](C)(N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F3N3O2/c1-14-9-16-17-12-29-30-21(17)7-6-18(16)25(4,31(14)13-24(2,3)28)23-19(26)10-15(11-20(23)27)5-8-22(32)33/h5-8,10-12,14H,9,13H2,1-4H3,(H,29,30)(H,32,33)/b8-5+/t14-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517551

(CHEMBL4436187)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(F)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C23H21F4N3O2/c1-12-7-15-14(4-5-19-16(15)10-28-29-19)22(30(12)11-23(2,26)27)21-17(24)8-13(9-18(21)25)3-6-20(31)32/h3-6,8-10,12,22H,7,11H2,1-2H3,(H,28,29)(H,31,32)/b6-3+/t12-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517551

(CHEMBL4436187)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(F)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C23H21F4N3O2/c1-12-7-15-14(4-5-19-16(15)10-28-29-19)22(30(12)11-23(2,26)27)21-17(24)8-13(9-18(21)25)3-6-20(31)32/h3-6,8-10,12,22H,7,11H2,1-2H3,(H,28,29)(H,31,32)/b6-3+/t12-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448706
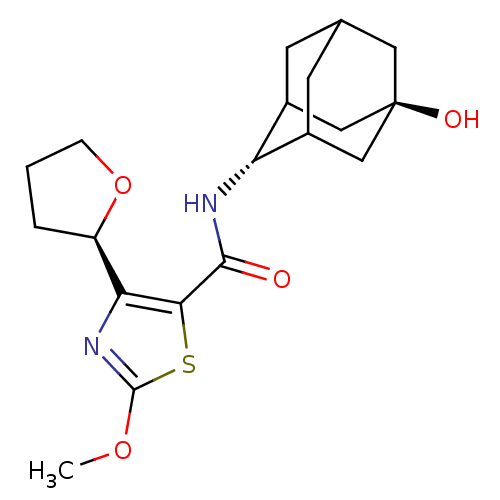
(CHEMBL3127854)Show SMILES COc1nc([C@H]2CCCO2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:22.25,wD:15.16,5.4,TLB:14:15:19:21.22.24,25:22:15.16.17:19,THB:25:16:19:21.22.24,24:22:15:17.18.19,24:18:15:21.25.22,23:22:15:17.18.19,23:22:15.16.17:19,(.87,-30.81,;2.03,-29.8,;3.48,-30.3,;3.94,-31.77,;5.48,-31.79,;6.36,-33.05,;5.87,-34.5,;7.1,-35.43,;8.36,-34.54,;7.91,-33.07,;5.97,-30.33,;4.74,-29.41,;7.31,-29.56,;7.3,-28.02,;8.64,-30.32,;9.97,-29.55,;11.47,-29.16,;11.5,-27.57,;12.56,-26.36,;11.2,-26.81,;11.19,-28.3,;12.5,-28.81,;13.91,-28.49,;15.45,-28.45,;13.95,-26.96,;12.87,-29.75,)| Show InChI InChI=1S/C19H26N2O4S/c1-24-18-21-15(13-3-2-4-25-13)16(26-18)17(22)20-14-11-5-10-6-12(14)9-19(23,7-10)8-11/h10-14,23H,2-9H2,1H3,(H,20,22)/t10?,11?,12?,13-,14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448693
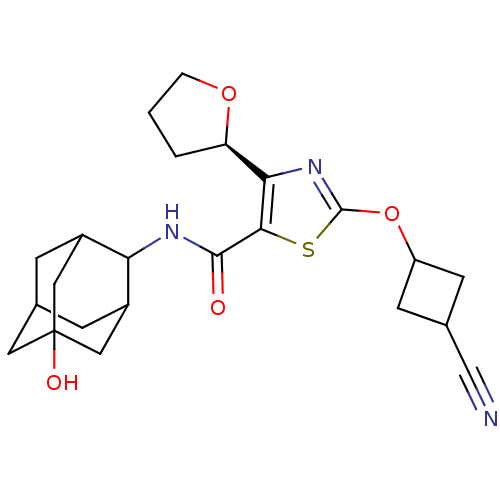
(CHEMBL3127857)Show SMILES OC12CC3CC(C1)C(NC(=O)c1sc(OC4CC(C4)C#N)nc1[C@H]1CCCO1)C(C3)C2 |r,wD:23.25,TLB:8:7:29:30.1.2,6:1:7.5.4:29,THB:6:5:29:30.1.2,2:1:7:4.3.29,2:3:7:30.6.1,0:1:7:4.3.29,0:1:7.5.4:29,(20.14,-39,;18.6,-39.04,;18.64,-37.51,;17.25,-36.9,;16.19,-38.12,;16.16,-39.71,;17.56,-40.3,;14.66,-40.1,;13.33,-40.87,;12,-40.11,;11.99,-38.57,;10.66,-40.88,;9.43,-39.96,;8.17,-40.84,;6.72,-40.34,;5.61,-41.41,;4.08,-41.4,;4.06,-42.94,;5.6,-42.96,;2.95,-44.01,;1.85,-45.09,;8.63,-42.31,;10.17,-42.34,;11.05,-43.6,;10.56,-45.05,;11.79,-45.98,;13.05,-45.09,;12.6,-43.62,;15.88,-38.85,;15.89,-37.35,;17.19,-39.36,)| Show InChI InChI=1S/C23H29N3O4S/c24-11-13-6-16(7-13)30-22-26-19(17-2-1-3-29-17)20(31-22)21(27)25-18-14-4-12-5-15(18)10-23(28,8-12)9-14/h12-18,28H,1-10H2,(H,25,27)/t12?,13?,14?,15?,16?,17-,18?,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448731
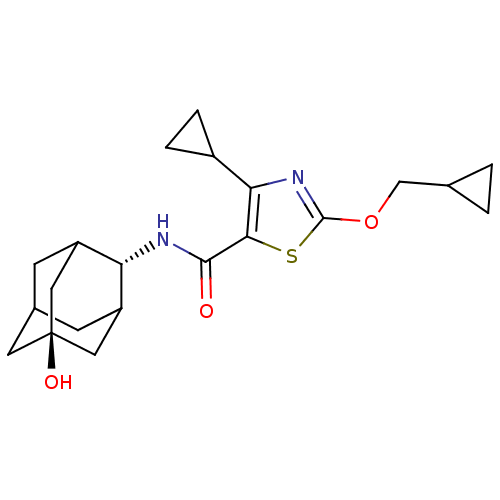
(CHEMBL3127868)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1sc(OCC4CC4)nc1C1CC1)C(C3)C2 |r,wU:1.0,wD:7.8,TLB:8:7:25:26.1.2,6:1:7.5.4:25,THB:6:5:25:26.1.2,2:1:7:4.3.25,2:3:7:26.6.1,0:1:7:4.3.25,0:1:7.5.4:25,(19.29,-27.92,;17.75,-27.95,;17.79,-26.42,;16.4,-25.82,;15.34,-27.03,;15.31,-28.62,;16.72,-29.21,;13.81,-29.01,;12.48,-29.79,;11.15,-29.02,;11.14,-27.48,;9.82,-29.8,;8.59,-28.87,;7.33,-29.76,;5.87,-29.26,;4.71,-30.27,;3.25,-29.77,;2.23,-28.61,;1.73,-30.06,;7.78,-31.23,;9.32,-31.26,;10.21,-32.51,;10.34,-34.04,;11.6,-33.16,;15.03,-27.76,;15.04,-26.27,;16.35,-28.27,)| Show InChI InChI=1S/C21H28N2O3S/c24-19(22-16-14-5-12-6-15(16)9-21(25,7-12)8-14)18-17(13-3-4-13)23-20(27-18)26-10-11-1-2-11/h11-16,25H,1-10H2,(H,22,24)/t12?,14?,15?,16-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448704
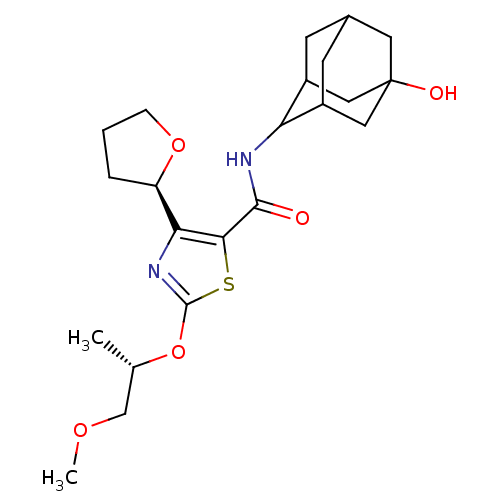
(CHEMBL3127856)Show SMILES COC[C@H](C)Oc1nc([C@H]2CCCO2)c(s1)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wU:3.3,wD:9.8,TLB:18:19:23:25.26.28,29:26:19.20.21:23,THB:29:20:23:25.26.28,28:26:19:21.22.23,28:22:19:25.29.26,27:26:19:21.22.23,27:26:19.20.21:23,(31.36,-30.47,;32.82,-30.97,;33.98,-29.96,;35.44,-30.46,;35.73,-31.97,;36.6,-29.45,;38.06,-29.95,;38.51,-31.42,;40.05,-31.44,;40.93,-32.7,;40.44,-34.15,;41.67,-35.08,;42.93,-34.19,;42.48,-32.72,;40.55,-29.98,;39.32,-29.06,;41.88,-29.21,;41.87,-27.67,;43.21,-29.97,;44.54,-29.2,;46.04,-28.81,;46.07,-27.22,;47.13,-26.01,;45.78,-26.46,;45.76,-27.95,;47.08,-28.46,;48.48,-28.14,;50.02,-28.1,;48.52,-26.61,;47.45,-29.4,)| Show InChI InChI=1S/C22H32N2O5S/c1-12(11-27-2)29-21-24-18(16-4-3-5-28-16)19(30-21)20(25)23-17-14-6-13-7-15(17)10-22(26,8-13)9-14/h12-17,26H,3-11H2,1-2H3,(H,23,25)/t12-,13?,14?,15?,16+,17?,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448705
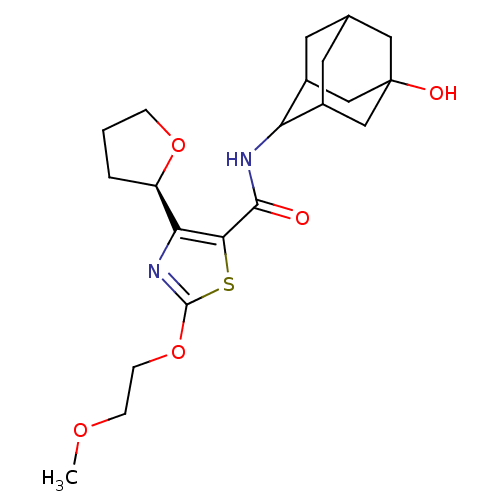
(CHEMBL3127855)Show SMILES COCCOc1nc([C@H]2CCCO2)c(s1)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wD:8.7,TLB:17:18:22:24.25.27,28:25:18.19.20:22,THB:28:19:22:24.25.27,27:25:18:20.21.22,27:21:18:24.28.25,26:25:18:20.21.22,26:25:18.19.20:22,(13.52,-30.59,;14.98,-31.09,;16.14,-30.08,;17.6,-30.58,;18.76,-29.57,;20.21,-30.07,;20.66,-31.54,;22.2,-31.57,;23.09,-32.83,;22.6,-34.28,;23.83,-35.2,;25.09,-34.32,;24.64,-32.85,;22.7,-30.11,;21.47,-29.18,;24.03,-29.33,;24.03,-27.79,;25.37,-30.1,;26.7,-29.32,;28.2,-28.94,;28.22,-27.34,;29.29,-26.13,;27.93,-26.58,;27.92,-28.07,;29.23,-28.58,;30.64,-28.26,;32.18,-28.23,;30.68,-26.73,;29.6,-29.52,)| Show InChI InChI=1S/C21H30N2O5S/c1-26-5-6-28-20-23-17(15-3-2-4-27-15)18(29-20)19(24)22-16-13-7-12-8-14(16)11-21(25,9-12)10-13/h12-16,25H,2-11H2,1H3,(H,22,24)/t12?,13?,14?,15-,16?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517562

(CHEMBL4586022)Show SMILES COc1cc(\C=C\C(O)=O)c(F)cc1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H24F3N3O3/c1-13-8-16-15(5-6-20-18(16)11-28-29-20)23(30(13)12-24(2,26)27)17-10-19(25)14(4-7-22(31)32)9-21(17)33-3/h4-7,9-11,13,23H,8,12H2,1-3H3,(H,28,29)(H,31,32)/b7-4+/t13-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593696
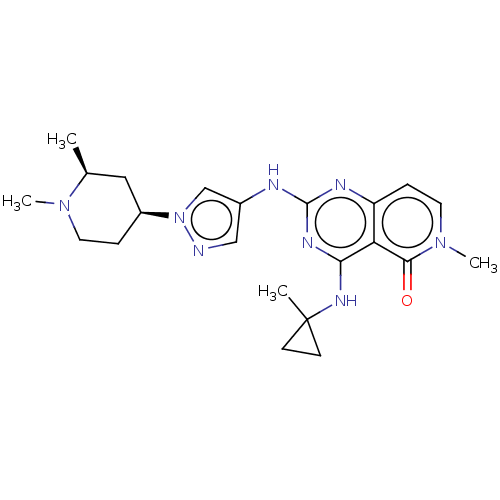
(CHEMBL5200601)Show SMILES C[C@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593694
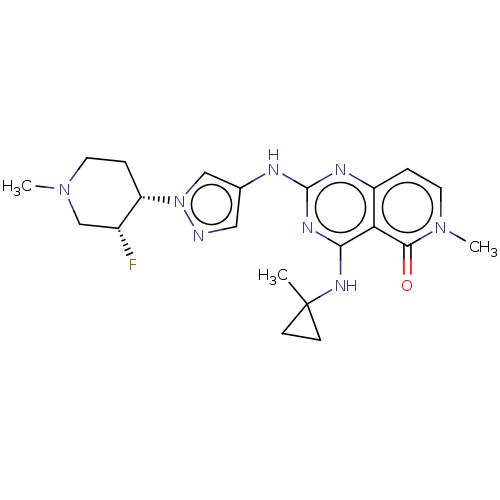
(CHEMBL5193253)Show SMILES CN1CC[C@@H]([C@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517559

(CHEMBL4584370)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@](C)(N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F3N3O2/c1-14-9-16-17-12-29-30-21(17)7-6-18(16)25(4,31(14)13-24(2,3)28)23-19(26)10-15(11-20(23)27)5-8-22(32)33/h5-8,10-12,14H,9,13H2,1-4H3,(H,29,30)(H,32,33)/b8-5+/t14-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517564

(CHEMBL4536148)Show SMILES COc1cc(\C=C\C(O)=O)ccc1[C@H]1N(CC(C)(C)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C25H28FN3O3/c1-15-11-19-17(8-9-21-20(19)13-27-28-21)24(29(15)14-25(2,3)26)18-7-5-16(6-10-23(30)31)12-22(18)32-4/h5-10,12-13,15,24H,11,14H2,1-4H3,(H,27,28)(H,30,31)/b10-6+/t15-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517563

(CHEMBL4541930)Show SMILES CC(C)CN1[C@H](C)Cc2c(ccc3[nH]ncc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-15(2)14-27-16(3)12-20-19(9-10-22-21(20)13-25-26-22)24(27)18-7-4-17(5-8-18)6-11-23(28)29/h4-11,13,15-16,24H,12,14H2,1-3H3,(H,25,26)(H,28,29)/b11-6+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM288677

(US10087191, Example 94)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as reduction in ERalpha protein expression after 5 hrs in absence of 0.25 uM tamoxifen ... |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517557

(CHEMBL4436390)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@](C)(N1CC(C)(F)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C24H23F4N3O2/c1-13-8-15-16-11-29-30-20(16)6-5-17(15)24(3,31(13)12-23(2,27)28)22-18(25)9-14(10-19(22)26)4-7-21(32)33/h4-7,9-11,13H,8,12H2,1-3H3,(H,29,30)(H,32,33)/b7-4+/t13-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517558
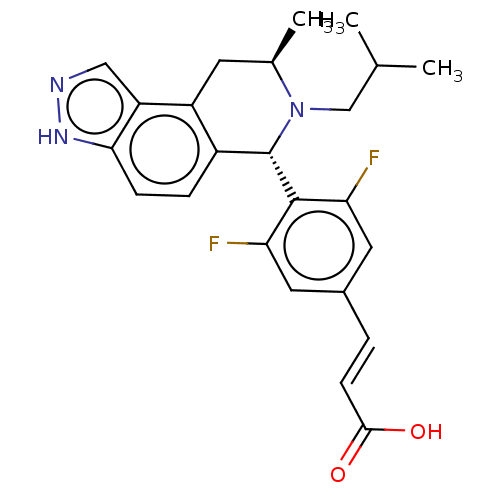
(CHEMBL4462367)Show SMILES CC(C)CN1[C@H](C)Cc2c(ccc3[nH]ncc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C24H25F2N3O2/c1-13(2)12-29-14(3)8-17-16(5-6-21-18(17)11-27-28-21)24(29)23-19(25)9-15(10-20(23)26)4-7-22(30)31/h4-7,9-11,13-14,24H,8,12H2,1-3H3,(H,27,28)(H,30,31)/b7-4+/t14-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593693

(CHEMBL5201376)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593700

(CHEMBL5193024)Show SMILES CN1CC2(CC(C2)n2cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593710
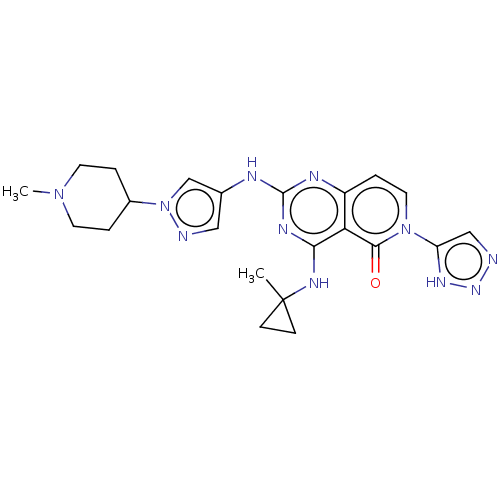
(CHEMBL5193053)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cnn[nH]4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420842

(CHEMBL2086684)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C21H26N6O3/c1-15-13-26(20(28)30-21(2,3)4)7-8-27(15)19-24-11-18(12-25-19)29-14-16-5-6-23-10-17(16)9-22/h5-6,10-12,15H,7-8,13-14H2,1-4H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433856
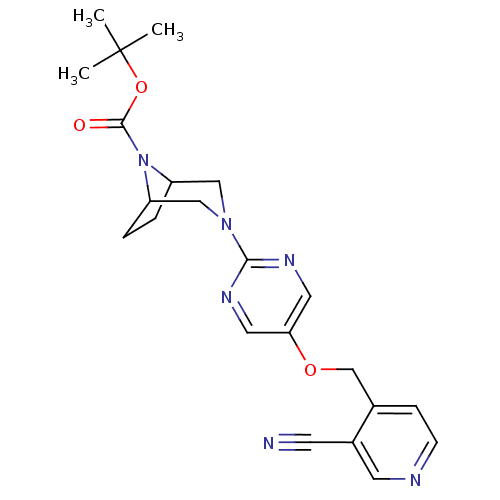
(CHEMBL2382410)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H26N6O3/c1-22(2,3)31-21(29)28-17-4-5-18(28)13-27(12-17)20-25-10-19(11-26-20)30-14-15-6-7-24-9-16(15)8-23/h6-7,9-11,17-18H,4-5,12-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593697

(CHEMBL5196755)Show SMILES C[C@@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593695
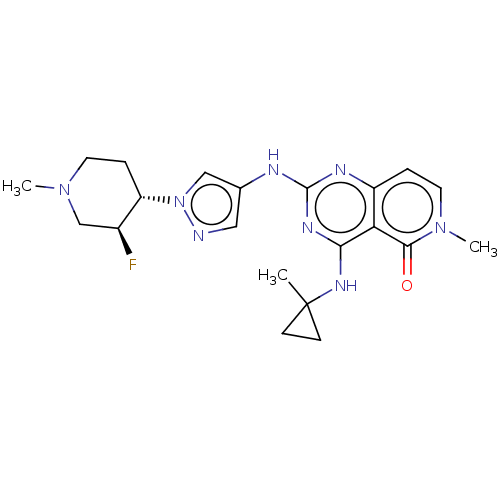
(CHEMBL5209502)Show SMILES CN1CC[C@@H]([C@@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593692

(CHEMBL5200432)Show SMILES CN(C)CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593689
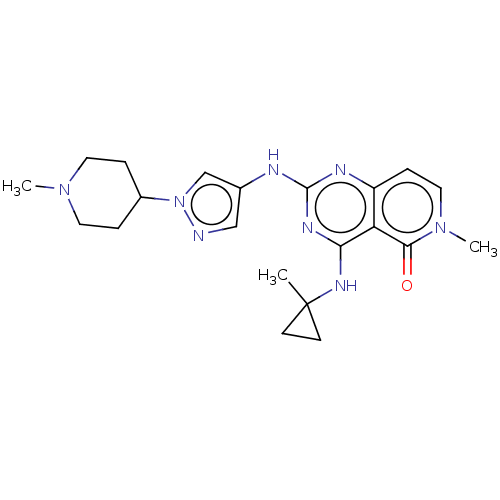
(CHEMBL5174529)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517552

(CHEMBL4517931)Show SMILES COc1cc(\C=C\C(O)=O)ccc1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H25F2N3O3/c1-14-10-18-16(7-8-20-19(18)12-27-28-20)23(29(14)13-24(2,25)26)17-6-4-15(5-9-22(30)31)11-21(17)32-3/h4-9,11-12,14,23H,10,13H2,1-3H3,(H,27,28)(H,30,31)/b9-5+/t14-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM288677

(US10087191, Example 94)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha in human MCF7 cells assessed as inhibition of estradiol-driven PR response by microplate cytometry |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517552

(CHEMBL4517931)Show SMILES COc1cc(\C=C\C(O)=O)ccc1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H25F2N3O3/c1-14-10-18-16(7-8-20-19(18)12-27-28-20)23(29(14)13-24(2,25)26)17-6-4-15(5-9-22(30)31)11-21(17)32-3/h4-9,11-12,14,23H,10,13H2,1-3H3,(H,27,28)(H,30,31)/b9-5+/t14-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517558

(CHEMBL4462367)Show SMILES CC(C)CN1[C@H](C)Cc2c(ccc3[nH]ncc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C24H25F2N3O2/c1-13(2)12-29-14(3)8-17-16(5-6-21-18(17)11-27-28-21)24(29)23-19(25)9-15(10-20(23)26)4-7-22(30)31/h4-7,9-11,13-14,24H,8,12H2,1-3H3,(H,27,28)(H,30,31)/b7-4+/t14-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448734

(CHEMBL3127865)Show SMILES COC[C@H](C)Oc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:24.27,3.3,wD:17.18,TLB:16:17:21:23.24.26,27:24:17.18.19:21,THB:27:18:21:23.24.26,26:24:17:19.20.21,26:20:17:23.27.24,25:24:17:19.20.21,25:24:17.18.19:21,(1.68,-19.21,;3.13,-19.71,;4.29,-18.7,;5.75,-19.2,;6.05,-20.71,;6.91,-18.18,;8.37,-18.68,;8.82,-20.16,;10.36,-20.18,;11.25,-21.43,;11.39,-22.97,;12.65,-22.08,;10.86,-18.72,;9.63,-17.8,;12.19,-17.95,;12.18,-16.41,;13.53,-18.71,;14.86,-17.94,;16.36,-17.55,;16.38,-15.96,;17.44,-14.74,;16.09,-15.2,;16.07,-16.69,;17.39,-17.2,;18.8,-16.88,;20.34,-16.84,;18.84,-15.35,;17.76,-18.14,)| Show InChI InChI=1S/C21H30N2O4S/c1-11(10-26-2)27-20-23-17(13-3-4-13)18(28-20)19(24)22-16-14-5-12-6-15(16)9-21(25,7-12)8-14/h11-16,25H,3-10H2,1-2H3,(H,22,24)/t11-,12?,14?,15?,16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517556
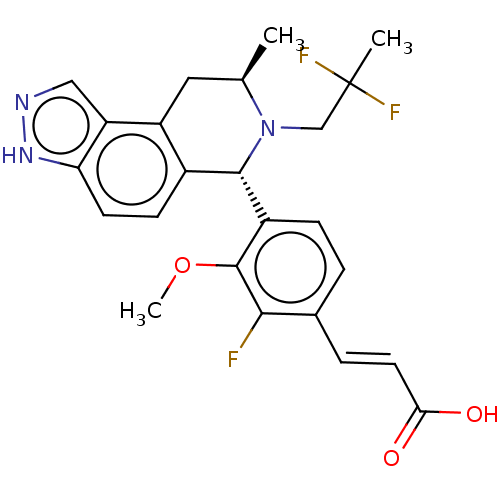
(CHEMBL4518390)Show SMILES COc1c(F)c(\C=C\C(O)=O)ccc1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C24H24F3N3O3/c1-13-10-17-15(7-8-19-18(17)11-28-29-19)22(30(13)12-24(2,26)27)16-6-4-14(5-9-20(31)32)21(25)23(16)33-3/h4-9,11,13,22H,10,12H2,1-3H3,(H,28,29)(H,31,32)/b9-5+/t13-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448733
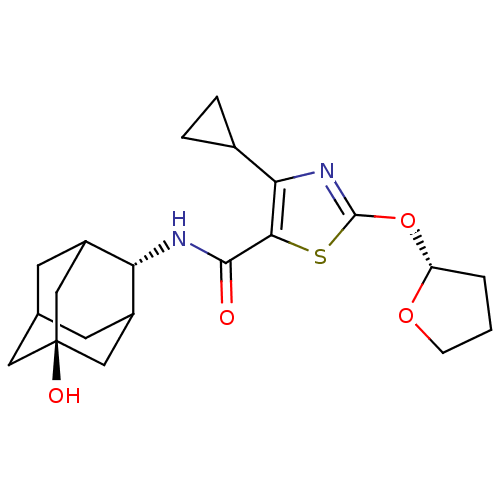
(CHEMBL3127866)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1sc(O[C@@H]4CCCO4)nc1C1CC1)C(C3)C2 |r,wU:1.0,wD:7.8,15.15,TLB:8:7:26:27.1.2,6:1:7.5.4:26,THB:6:5:26:27.1.2,2:1:7:4.3.26,2:3:7:27.6.1,0:1:7:4.3.26,0:1:7.5.4:26,(39.73,-16.8,;38.19,-16.84,;38.23,-15.31,;36.84,-14.7,;35.78,-15.92,;35.75,-17.51,;37.16,-18.1,;34.25,-17.9,;32.92,-18.67,;31.59,-17.91,;31.58,-16.37,;30.26,-18.68,;29.03,-17.76,;27.77,-18.64,;26.31,-18.14,;25.07,-19.06,;25.08,-20.61,;23.62,-21.09,;22.71,-19.85,;23.6,-18.6,;28.22,-20.12,;29.76,-20.14,;30.65,-21.39,;30.78,-22.93,;32.04,-22.04,;35.47,-16.65,;35.49,-15.16,;36.79,-17.16,)| Show InChI InChI=1S/C21H28N2O4S/c24-19(22-16-13-6-11-7-14(16)10-21(25,8-11)9-13)18-17(12-3-4-12)23-20(28-18)27-15-2-1-5-26-15/h11-16,25H,1-10H2,(H,22,24)/t11?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448701
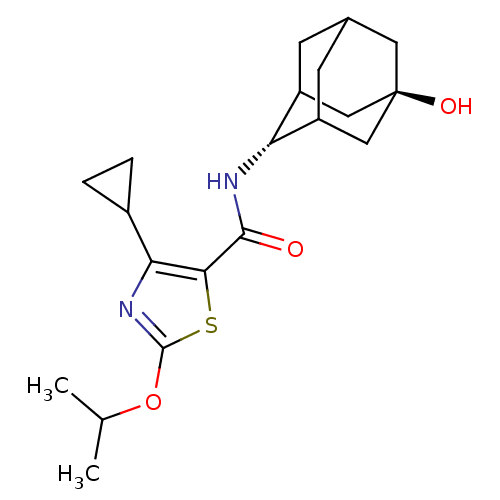
(CHEMBL3127861)Show SMILES CC(C)Oc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:22.25,wD:15.16,TLB:14:15:19:21.22.24,25:22:15.16.17:19,THB:25:16:19:21.22.24,24:22:15:17.18.19,24:18:15:21.25.22,23:22:15:17.18.19,23:22:15.16.17:19,(39.67,-54.36,;41.13,-54.86,;41.43,-56.37,;42.29,-53.85,;43.75,-54.35,;44.2,-55.82,;45.74,-55.85,;46.63,-57.1,;46.77,-58.63,;48.03,-57.75,;46.24,-54.39,;45.01,-53.47,;47.57,-53.61,;47.56,-52.07,;48.91,-54.38,;50.24,-53.6,;51.74,-53.22,;51.76,-51.63,;52.82,-50.41,;51.47,-50.86,;51.45,-52.35,;52.77,-52.87,;54.18,-52.54,;55.71,-52.51,;54.21,-51.01,;53.14,-53.8,)| Show InChI InChI=1S/C20H28N2O3S/c1-10(2)25-19-22-16(12-3-4-12)17(26-19)18(23)21-15-13-5-11-6-14(15)9-20(24,7-11)8-13/h10-15,24H,3-9H2,1-2H3,(H,21,23)/t11?,13?,14?,15-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517564

(CHEMBL4536148)Show SMILES COc1cc(\C=C\C(O)=O)ccc1[C@H]1N(CC(C)(C)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| Show InChI InChI=1S/C25H28FN3O3/c1-15-11-19-17(8-9-21-20(19)13-27-28-21)24(29(15)14-25(2,3)26)18-7-5-16(6-10-23(30)31)12-22(18)32-4/h5-10,12-13,15,24H,11,14H2,1-4H3,(H,27,28)(H,30,31)/b10-6+/t15-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400147
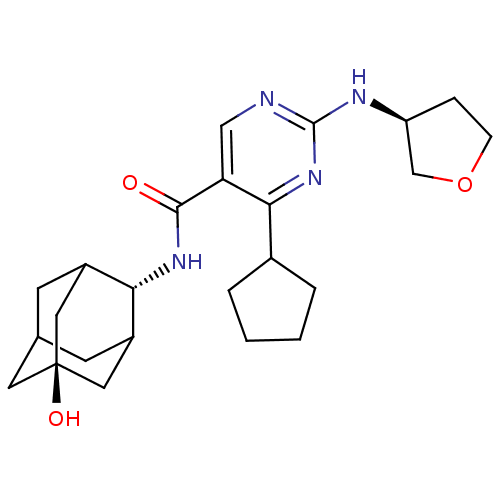
(CHEMBL2179014)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(N[C@H]4CCOC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,16.16,wD:1.0,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(33.95,-38.8,;32.42,-38.79,;31.38,-37.67,;29.96,-38.21,;29.31,-39.49,;30.34,-40.56,;31.64,-40.01,;30.35,-42.31,;29.02,-43.08,;27.68,-42.32,;27.68,-40.78,;26.35,-43.09,;25.02,-42.33,;23.69,-43.1,;23.69,-44.64,;22.35,-45.41,;21.02,-44.64,;19.61,-45.27,;18.58,-44.13,;19.35,-42.79,;20.86,-43.11,;25.02,-45.41,;26.36,-44.64,;27.69,-45.41,;27.86,-46.94,;29.37,-47.26,;30.13,-45.92,;29.1,-44.78,;31.15,-40.98,;29.98,-39.66,;32.56,-40.42,)| Show InChI InChI=1S/C24H34N4O3/c29-22(27-20-16-7-14-8-17(20)11-24(30,9-14)10-16)19-12-25-23(26-18-5-6-31-13-18)28-21(19)15-3-1-2-4-15/h12,14-18,20,30H,1-11,13H2,(H,27,29)(H,25,26,28)/t14?,16?,17?,18-,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HPLC assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517561

(CHEMBL4586630)Show SMILES CC(C)CN1[C@H](c2ccc(\C=C\C(O)=O)cc2)c2ccc3[nH]ncc3c2CC1(C)C |r| Show InChI InChI=1S/C25H29N3O2/c1-16(2)15-28-24(18-8-5-17(6-9-18)7-12-23(29)30)19-10-11-22-21(14-26-27-22)20(19)13-25(28,3)4/h5-12,14,16,24H,13,15H2,1-4H3,(H,26,27)(H,29,30)/b12-7+/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by in multiplexed cell assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50517555

(CHEMBL4515724)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C24H26FN3O2/c1-15-12-19-18(9-10-21-20(19)13-26-27-21)23(28(15)14-24(2,3)25)17-7-4-16(5-8-17)6-11-22(29)30/h4-11,13,15,23H,12,14H2,1-3H3,(H,26,27)(H,29,30)/b11-6+/t15-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged ERalpha LBD expressed in baculovirus-infected insect cells by lantha screen assay |
J Med Chem 62: 1593-1608 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01837
BindingDB Entry DOI: 10.7270/Q2NZ8C16 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448697

(CHEMBL3127858)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1sc(OC4CCOCC4)nc1[C@H]1CCCO1)C(C3)C2 |r,wU:1.0,wD:7.8,23.25,TLB:8:7:29:30.1.2,6:1:7.5.4:29,THB:6:5:29:30.1.2,2:1:7:4.3.29,2:3:7:30.6.1,0:1:7:4.3.29,0:1:7.5.4:29,(41.98,-39.31,;40.44,-39.34,;40.47,-37.81,;39.08,-37.21,;38.02,-38.42,;38,-40.01,;39.4,-40.6,;36.5,-40.4,;35.17,-41.18,;33.83,-40.41,;33.82,-38.87,;32.5,-41.19,;31.27,-40.26,;30.01,-41.15,;28.55,-40.65,;27.22,-41.43,;25.89,-40.66,;24.57,-41.43,;24.57,-42.97,;25.91,-43.74,;27.24,-42.97,;30.46,-42.62,;32,-42.64,;32.88,-43.9,;32.39,-45.36,;33.62,-46.28,;34.88,-45.4,;34.43,-43.92,;37.71,-39.15,;37.73,-37.66,;39.03,-39.66,)| Show InChI InChI=1S/C23H32N2O5S/c26-21(24-18-14-8-13-9-15(18)12-23(27,10-13)11-14)20-19(17-2-1-5-29-17)25-22(31-20)30-16-3-6-28-7-4-16/h13-18,27H,1-12H2,(H,24,26)/t13?,14?,15?,17-,18-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448702
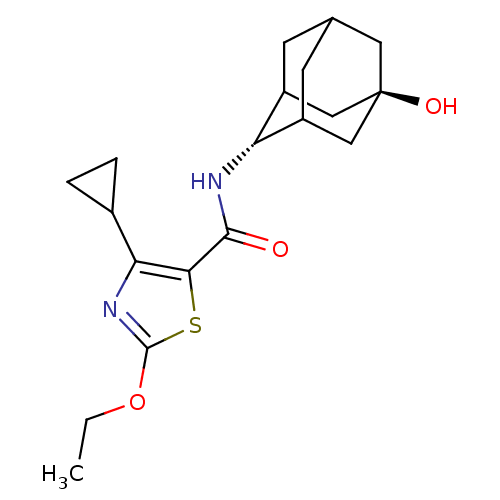
(CHEMBL3127860)Show SMILES CCOc1nc(C2CC2)c(s1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.24,wD:14.15,TLB:13:14:18:20.21.23,24:21:14.15.16:18,THB:24:15:18:20.21.23,23:21:14:16.17.18,23:17:14:20.24.21,22:21:14:16.17.18,22:21:14.15.16:18,(18.99,-53.98,;20.45,-54.48,;21.61,-53.47,;23.07,-53.97,;23.52,-55.44,;25.06,-55.47,;25.95,-56.72,;26.09,-58.25,;27.35,-57.37,;25.56,-54.01,;24.33,-53.09,;26.89,-53.23,;26.88,-51.69,;28.23,-54,;29.56,-53.22,;31.06,-52.84,;31.08,-51.25,;32.14,-50.03,;30.79,-50.48,;30.77,-51.97,;32.09,-52.49,;33.5,-52.16,;35.04,-52.13,;33.53,-50.63,;32.46,-53.42,)| Show InChI InChI=1S/C19H26N2O3S/c1-2-24-18-21-15(11-3-4-11)16(25-18)17(22)20-14-12-5-10-6-13(14)9-19(23,7-10)8-12/h10-14,23H,2-9H2,1H3,(H,20,22)/t10?,12?,13?,14-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis |
J Med Chem 57: 970-86 (2014)
Article DOI: 10.1021/jm4016729
BindingDB Entry DOI: 10.7270/Q2Z32149 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593704
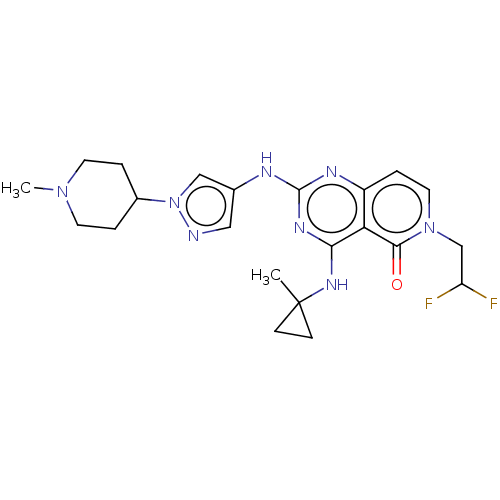
(CHEMBL5186874)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(CC(F)F)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593706

(CHEMBL5190750)Show SMILES C[C@H](F)Cn1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data