

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM50481568 (CHEMBL592712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis RmlC expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 18: 896-908 (2010) Article DOI: 10.1016/j.bmc.2009.11.033 BindingDB Entry DOI: 10.7270/Q2N300SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM51599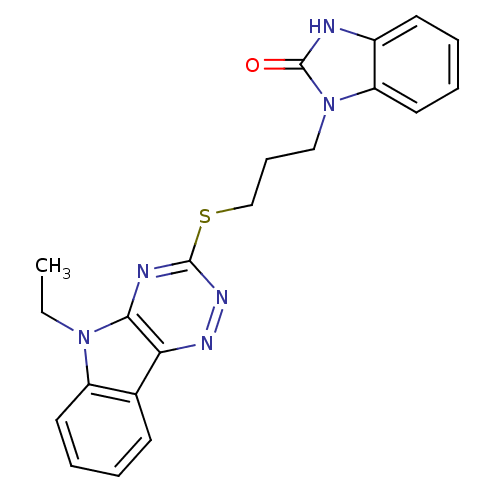 (3-[3-[(5-ethyl-[1,2,4]triazin[5,6-b]indol-3-yl)thi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis RmlC expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 18: 896-908 (2010) Article DOI: 10.1016/j.bmc.2009.11.033 BindingDB Entry DOI: 10.7270/Q2N300SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50194500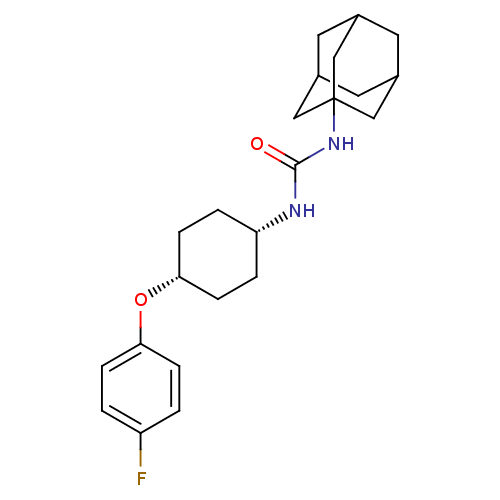 (1-adamantan-1-yl-3-[4-(4-fluoro-phenoxy)-cyclohexy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351248 (CHEMBL1818384) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217458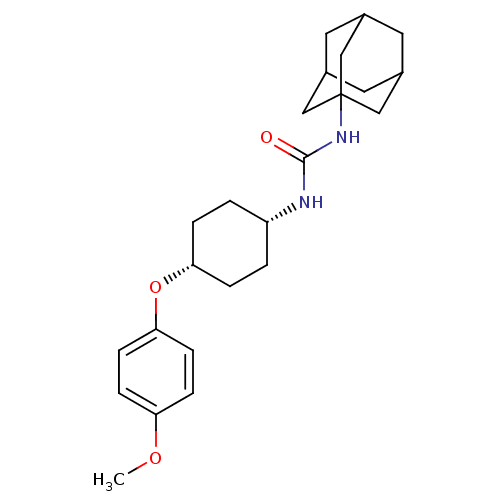 (CHEMBL244192 | cis-1-adamantan-1-yl-3-[4-(4-methox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383462 (CHEMBL2031797) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217459 (CHEMBL243335 | trans-1-adamantan-1-yl-3-[4-(4-fluo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383471 (CHEMBL2031812) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383517 (CHEMBL2032045) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196661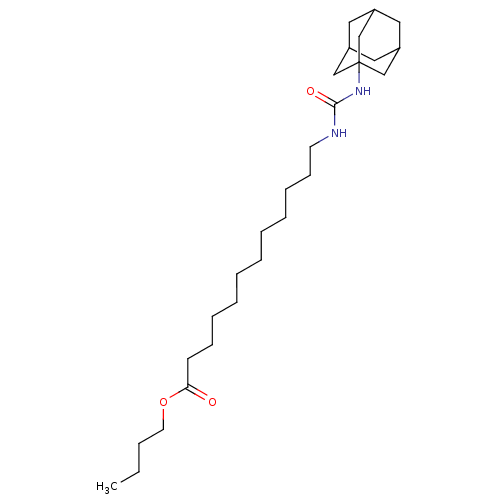 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217451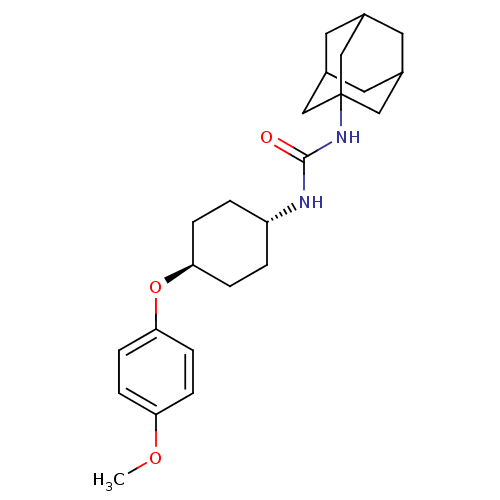 (CHEMBL427695 | trans-1-adamantan-1-yl-3-[4-(4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383484 (CHEMBL2031931 | US8815951, 343) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383482 (CHEMBL2031930) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383470 (CHEMBL2031808) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383473 (CHEMBL2031816) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383476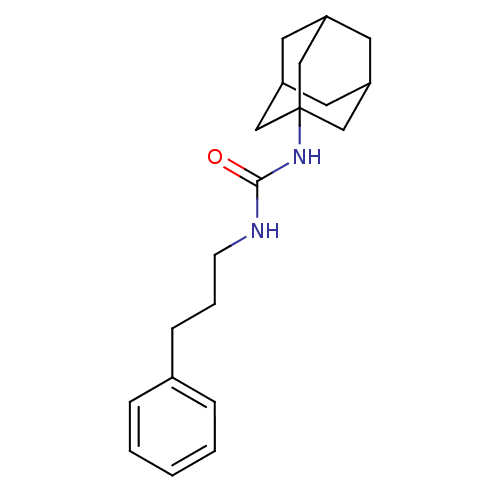 (CHEMBL2031923 | US8815951, 438) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383503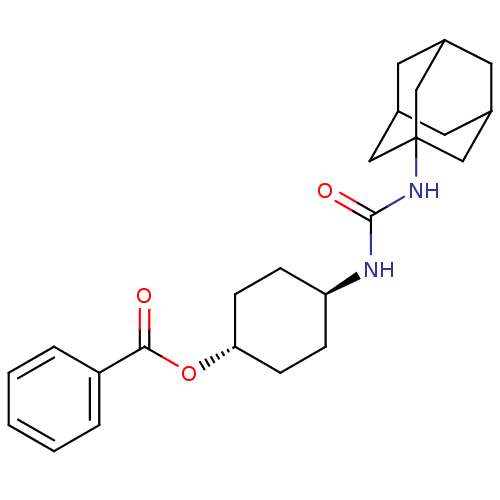 (CHEMBL2031950) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383479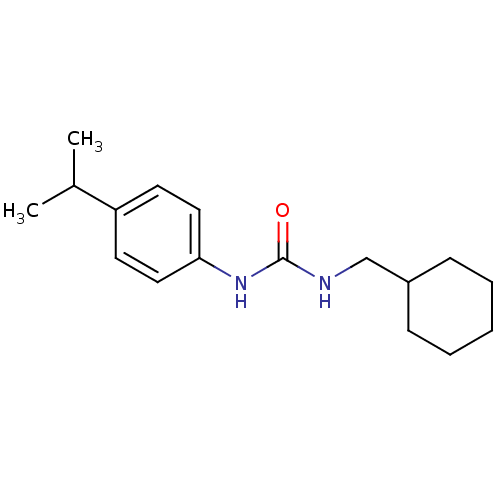 (CHEMBL2031927 | US8815951, 180) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217470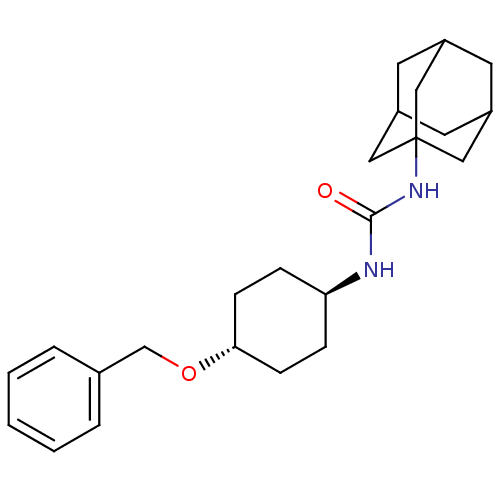 (CHEMBL395988 | trans-1-adamantan-1-yl-3-(4-benzylo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217460 (CHEMBL243125 | trans-1-adamantan-1-yl-3-[4-(4-brom...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223391 (1-Adamantan-1-yl-3-{3-[2-(2-ethoxy-ethoxy)-ethoxy]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383478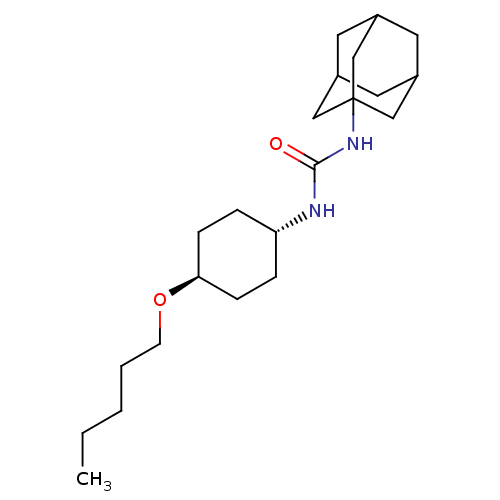 (CHEMBL2031926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383494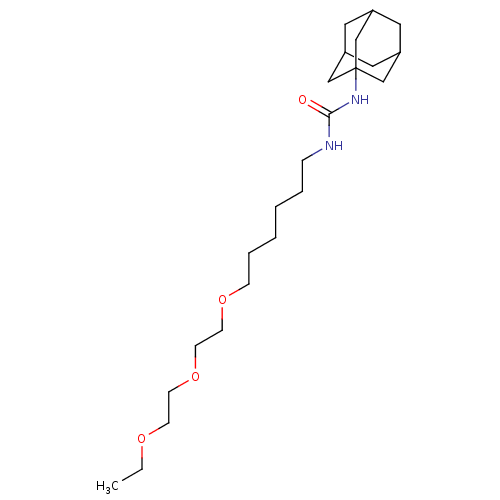 (CHEMBL2031941) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383469 (CHEMBL2031807) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383510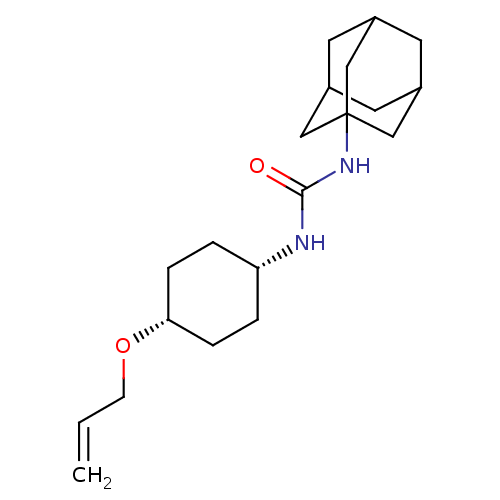 (CHEMBL2031951) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217454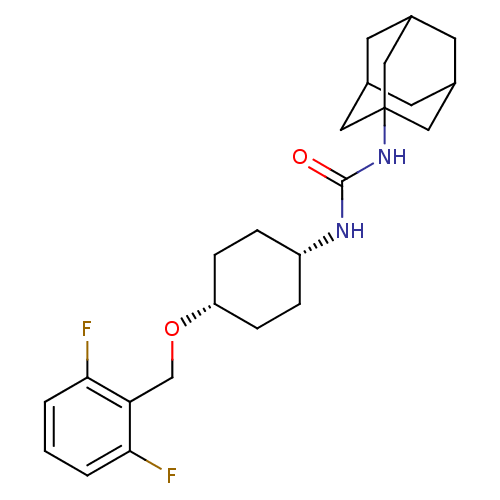 (CHEMBL395738 | cis-1-adamantan-1-yl-3-[4-(2,6-difl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383504 (CHEMBL2031802) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217448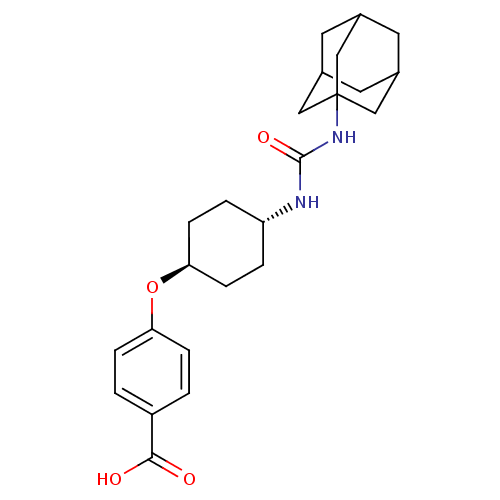 (CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383477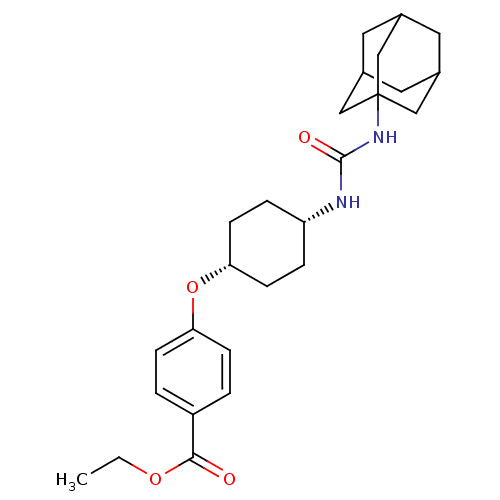 (CHEMBL2031924) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383508 (CHEMBL2031789) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383472 (CHEMBL2031814) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383467 (CHEMBL2031803) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM41910 (1-cyclohexyl-3-(4-propan-2-ylphenyl)urea | 1-cyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223375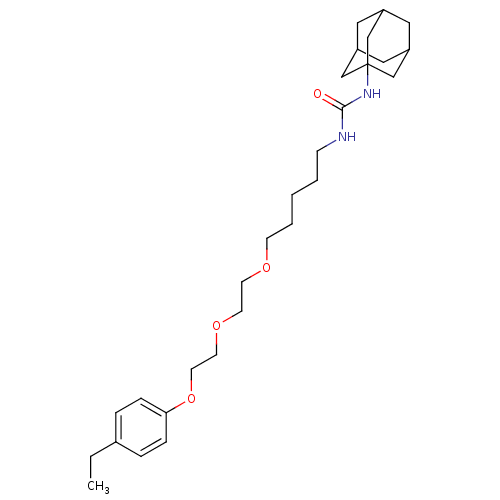 (1-adamantan-1-yl-3-(5-{2-[2-(4-ethylphenoxy)ethoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383465 (CHEMBL2031800) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217468 (1-adamantan-1-yl-3-[4-(4-fluorophenoxy)phenyl]urea...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383502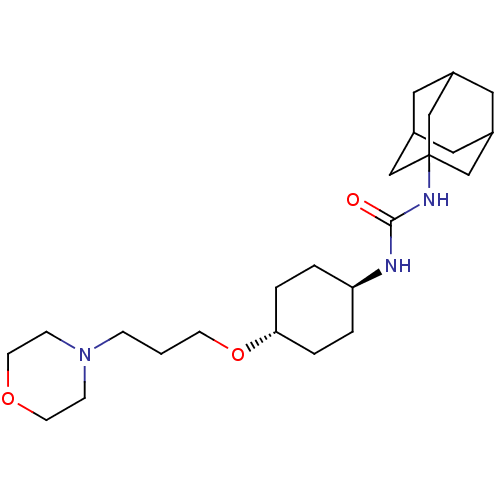 (CHEMBL2031949) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383487 (CHEMBL2031934) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383505 (CHEMBL2031932 | US8815951, 501) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223398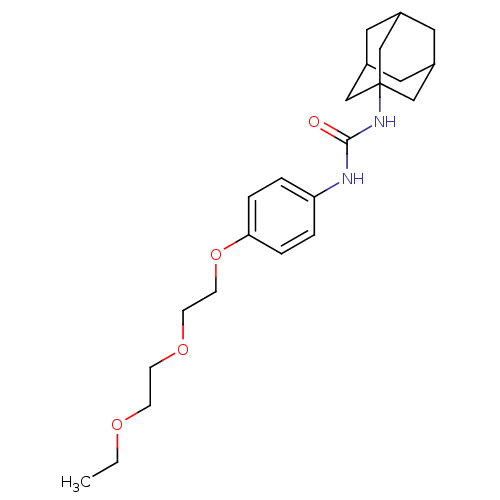 (1-Adamantan-1-yl-3-{4-[2-(2-ethoxy-ethoxy)-ethoxy]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50267210 (3-[4-(3-Adamantan-1-yl-ureido)-phenyl]-propionic a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383516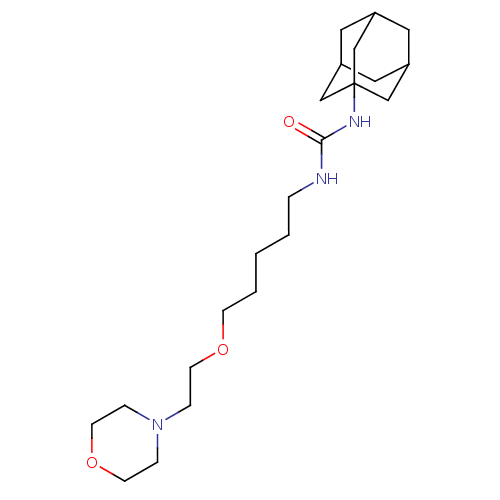 (CHEMBL2032043) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383464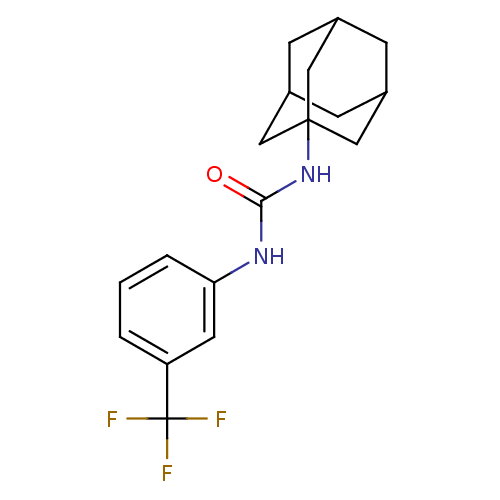 (CHEMBL2031799) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383518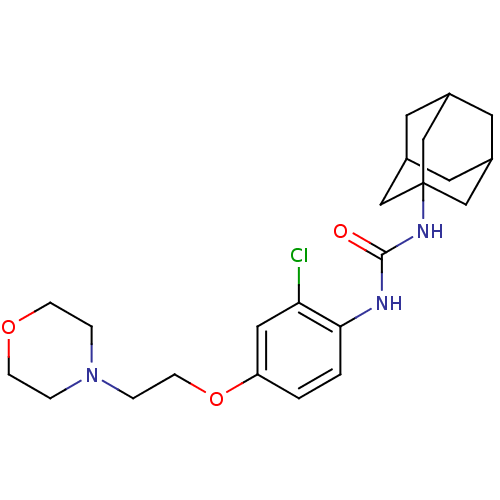 (CHEMBL2032046) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383511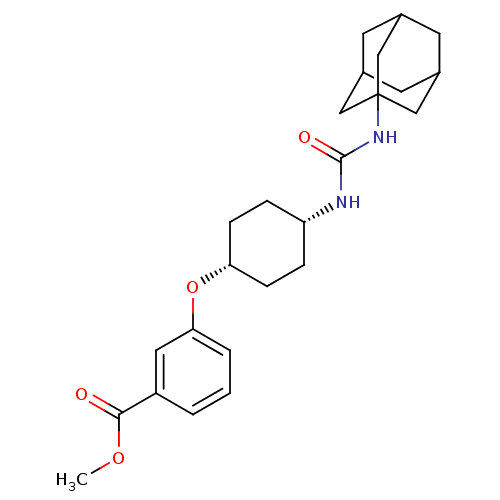 (CHEMBL2031795) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383463 (CHEMBL2031798) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383480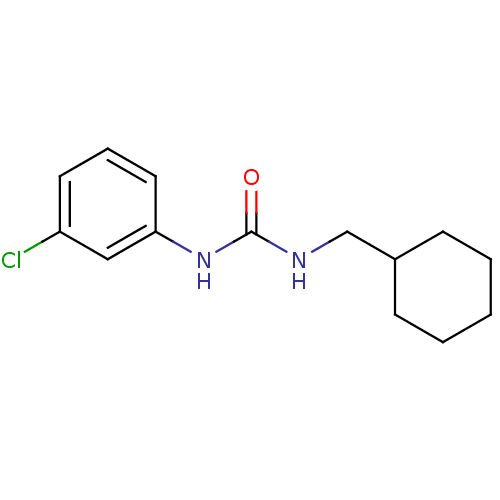 (CHEMBL2031928 | US8815951, 179) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217479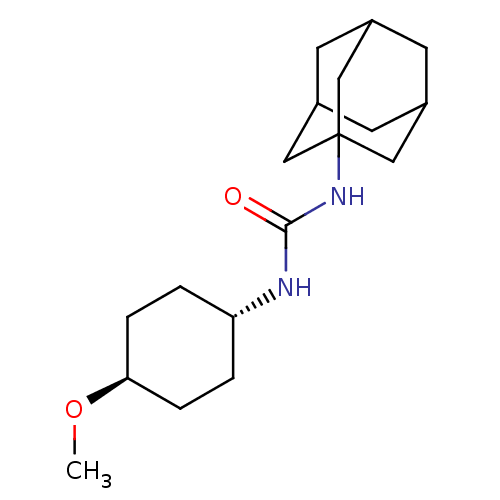 (CHEMBL242256 | trans-1-adamantan-1-yl-3-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50383474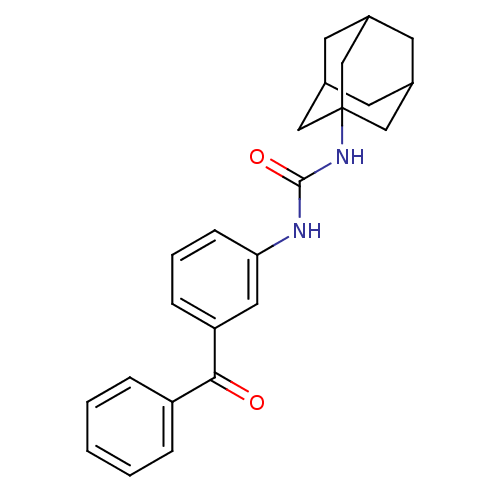 (CHEMBL2031817) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25733 (CHEMBL476614 | Urea-based compound, 14 | methyl 4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using CMNPC as substrate assessed as appearance of 6-methoxy-2-naphthaldehyde after 10 mins... | Bioorg Med Chem 20: 3255-62 (2012) Article DOI: 10.1016/j.bmc.2012.03.058 BindingDB Entry DOI: 10.7270/Q28W3FB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |