 Found 104 hits with Last Name = 'guha' and Initial = 'm'
Found 104 hits with Last Name = 'guha' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405407

(CHEMBL5285361)Show SMILES COc1ccc(CCNC[C@H](O)COc2ccc(Cc3nc(c[nH]3)C(C)=O)cc2)cc1OC Show InChI InChI=1S/C25H31N3O5/c1-17(29)22-15-27-25(28-22)13-18-4-7-21(8-5-18)33-16-20(30)14-26-11-10-19-6-9-23(31-2)24(12-19)32-3/h4-9,12,15,20,26,30H,10-11,13-14,16H2,1-3H3,(H,27,28)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405402
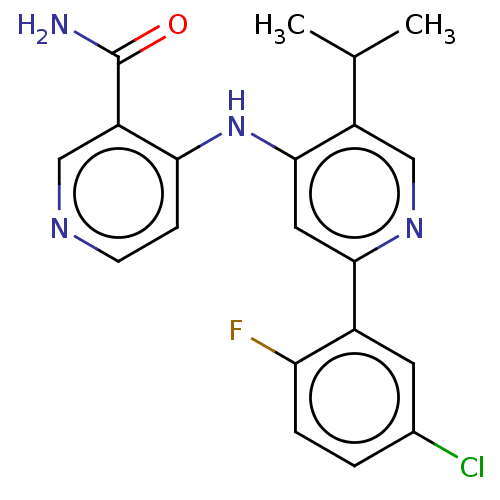
(CHEMBL5267945)Show SMILES COc1ccc(CCNCC(O)COc2ccc(COCc3ncc[nH]3)cc2)cc1OC Show InChI InChI=1S/C24H31N3O5/c1-29-22-8-5-18(13-23(22)30-2)9-10-25-14-20(28)16-32-21-6-3-19(4-7-21)15-31-17-24-26-11-12-27-24/h3-8,11-13,20,25,28H,9-10,14-17H2,1-2H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405385
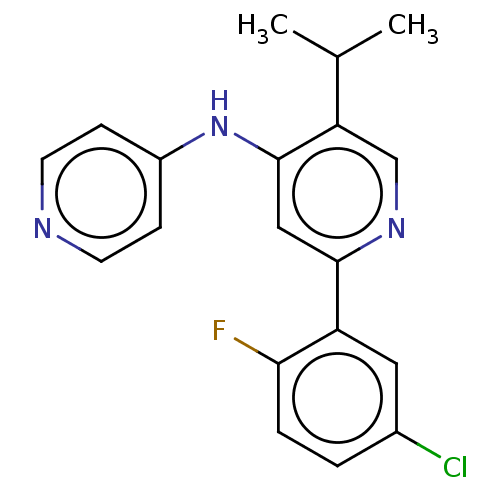
(CHEMBL5285746)Show SMILES CC(CCc1ccccc1)NCC(O)c1cc(C(N)=O)c2[nH]ccc2c1 Show InChI InChI=1S/C21H25N3O2/c1-14(7-8-15-5-3-2-4-6-15)24-13-19(25)17-11-16-9-10-23-20(16)18(12-17)21(22)26/h2-6,9-12,14,19,23-25H,7-8,13H2,1H3,(H2,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405403

(CHEMBL5274165)Show InChI InChI=1S/C16H22BrN3O2/c1-11(2)18-8-13(21)10-22-14-5-3-12(4-6-14)7-16-19-9-15(17)20-16/h3-6,9,11,13,18,21H,7-8,10H2,1-2H3,(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
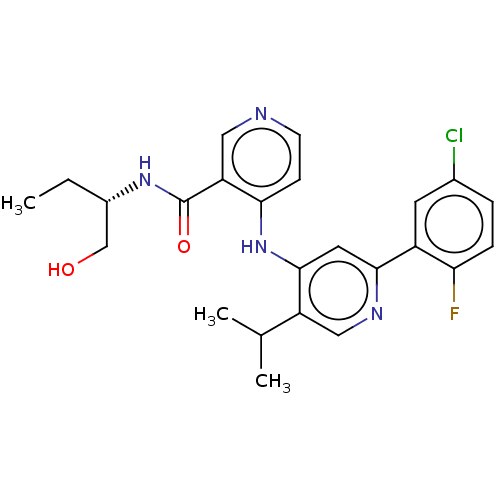
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM514529
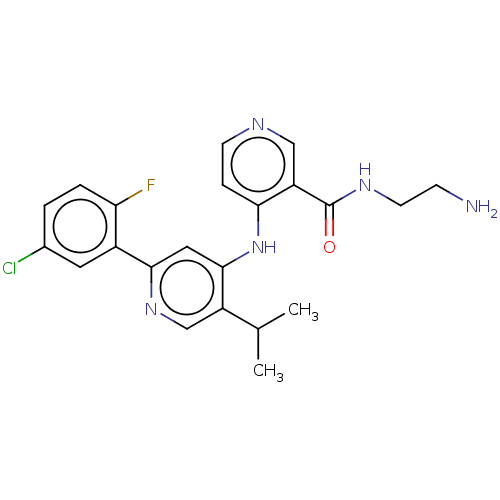
(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCN)-c1cc(Cl)ccc1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405404
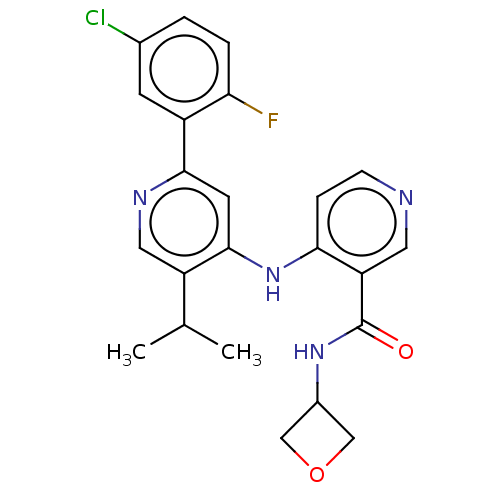
(CHEMBL5268400)Show InChI InChI=1S/C15H21N3O2/c1-11(2)18-9-13(19)10-20-14-5-3-12(4-6-14)15-16-7-8-17-15/h3-8,11,13,18-19H,9-10H2,1-2H3,(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405409
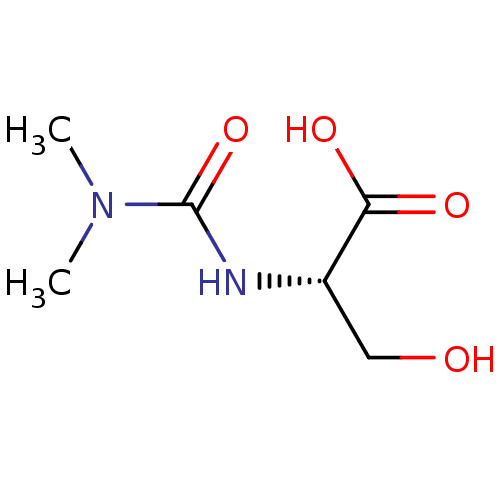
(CHEMBL5267264)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2Cl)-c2ncc(Br)[nH]2)cc1OC Show InChI InChI=1S/C22H25BrClN3O4/c1-29-19-5-3-14(9-20(19)30-2)7-8-25-11-16(28)13-31-18-6-4-15(10-17(18)24)22-26-12-21(23)27-22/h3-6,9-10,12,16,25,28H,7-8,11,13H2,1-2H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280348
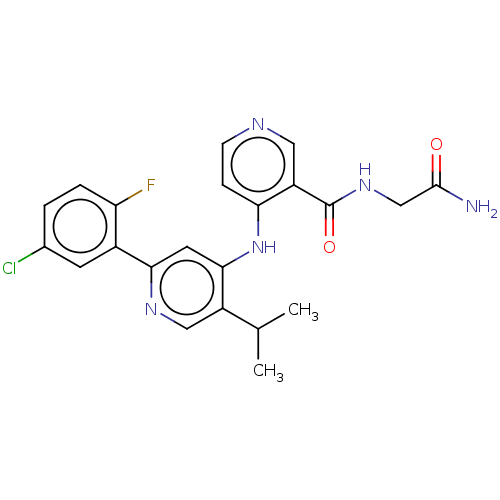
(N-(2-amino-2-oxo-ethyl)-4-[[2-(5-chloro-2-fluoro-p...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCC(N)=O)-c1cc(Cl)ccc1F Show InChI InChI=1S/C22H21ClFN5O2/c1-12(2)15-10-27-19(14-7-13(23)3-4-17(14)24)8-20(15)29-18-5-6-26-9-16(18)22(31)28-11-21(25)30/h3-10,12H,11H2,1-2H3,(H2,25,30)(H,28,31)(H,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280340

((S)-4-(2-(5-chloro-2-fluorophenyl)-5-isopropylpyri...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)N[C@@H](C)CO)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-11-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-10-18(20)23(31)28-14(3)12-30/h4-11,13-14,30H,12H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405408

(CHEMBL5290233)Show SMILES COCc1cnc([nH]1)-c1ccc(OC[C@@H](O)CNCCc2ccc(OC)c(OC)c2)cc1 |r| Show InChI InChI=1S/C24H31N3O5/c1-29-15-19-13-26-24(27-19)18-5-7-21(8-6-18)32-16-20(28)14-25-11-10-17-4-9-22(30-2)23(12-17)31-3/h4-9,12-13,20,25,28H,10-11,14-16H2,1-3H3,(H,26,27)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280355
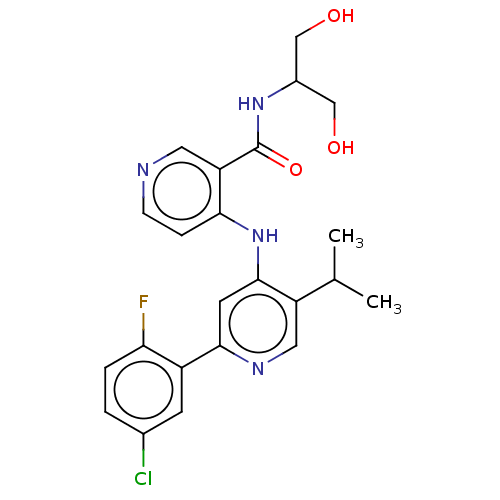
(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4-pyr...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NC(CO)CO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C23H24ClFN4O3/c1-13(2)17-10-27-21(16-7-14(24)3-4-19(16)25)8-22(17)29-20-5-6-26-9-18(20)23(32)28-15(11-30)12-31/h3-10,13,15,30-31H,11-12H2,1-2H3,(H,28,32)(H,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280337
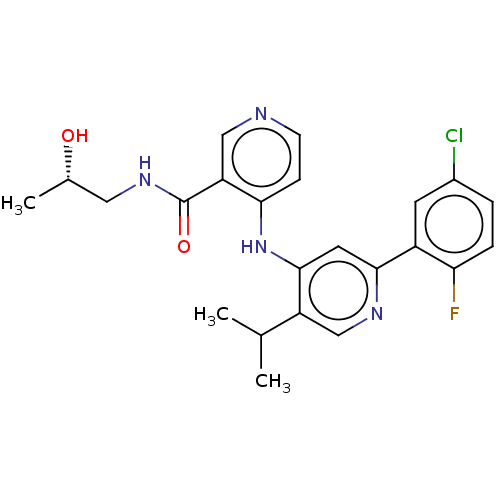
((S)-4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4...)Show SMILES C[C@H](O)CNC(=O)c1cnccc1Nc1cc(ncc1C(C)C)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-12-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-11-18(20)23(31)28-10-14(3)30/h4-9,11-14,30H,10H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280347
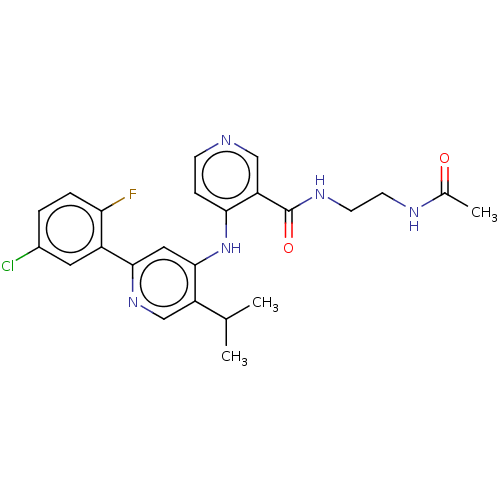
(N-(2-acetamidoethyl)-4-[[2-(5-chloro-2-fluoro-phen...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNC(C)=O)-c1cc(Cl)ccc1F Show InChI InChI=1S/C24H25ClFN5O2/c1-14(2)18-13-30-22(17-10-16(25)4-5-20(17)26)11-23(18)31-21-6-7-27-12-19(21)24(33)29-9-8-28-15(3)32/h4-7,10-14H,8-9H2,1-3H3,(H,28,32)(H,29,33)(H,27,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405410

(CHEMBL5266024)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cn2)-c2nc(c[nH]2)-c2cccs2)cc1OC Show InChI InChI=1S/C25H28N4O4S/c1-31-21-7-5-17(12-22(21)32-2)9-10-26-14-19(30)16-33-24-8-6-18(13-27-24)25-28-15-20(29-25)23-4-3-11-34-23/h3-8,11-13,15,19,26,30H,9-10,14,16H2,1-2H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280351

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4-pyr...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNS(C)(=O)=O)-c1cc(Cl)ccc1F Show InChI InChI=1S/C23H25ClFN5O3S/c1-14(2)17-13-28-21(16-10-15(24)4-5-19(16)25)11-22(17)30-20-6-7-26-12-18(20)23(31)27-8-9-29-34(3,32)33/h4-7,10-14,29H,8-9H2,1-3H3,(H,27,31)(H,26,28,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405405
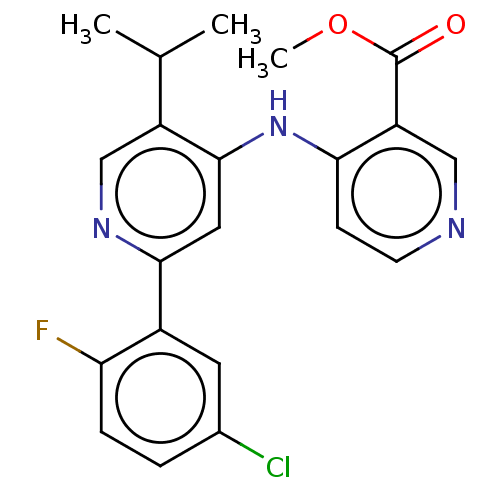
(CHEMBL5287924)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2)-c2nc(c[nH]2)C(C)(C)C)cc1OC Show InChI InChI=1S/C26H35N3O4/c1-26(2,3)24-16-28-25(29-24)19-7-9-21(10-8-19)33-17-20(30)15-27-13-12-18-6-11-22(31-4)23(14-18)32-5/h6-11,14,16,20,27,30H,12-13,15,17H2,1-5H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405406
(CHEMBL5285227)Show InChI InChI=1S/C19H23N3O2S/c1-2-9-20-11-15(23)13-24-16-7-5-14(6-8-16)19-21-12-17(22-19)18-4-3-10-25-18/h3-8,10,12,15,20,23H,2,9,11,13H2,1H3,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327527
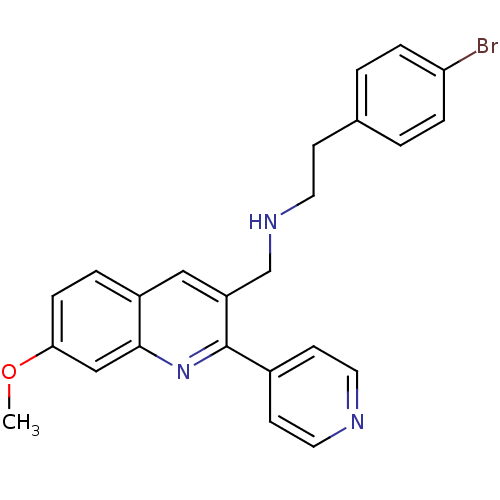
(2-(4-bromophenyl)-N-((7-methoxy-2-(pyridin-4-yl)qu...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccncc1 Show InChI InChI=1S/C24H22BrN3O/c1-29-22-7-4-19-14-20(16-27-11-8-17-2-5-21(25)6-3-17)24(28-23(19)15-22)18-9-12-26-13-10-18/h2-7,9-10,12-15,27H,8,11,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280355

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4-pyr...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NC(CO)CO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C23H24ClFN4O3/c1-13(2)17-10-27-21(16-7-14(24)3-4-19(16)25)8-22(17)29-20-5-6-26-9-18(20)23(32)28-15(11-30)12-31/h3-10,13,15,30-31H,11-12H2,1-2H3,(H,28,32)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327528
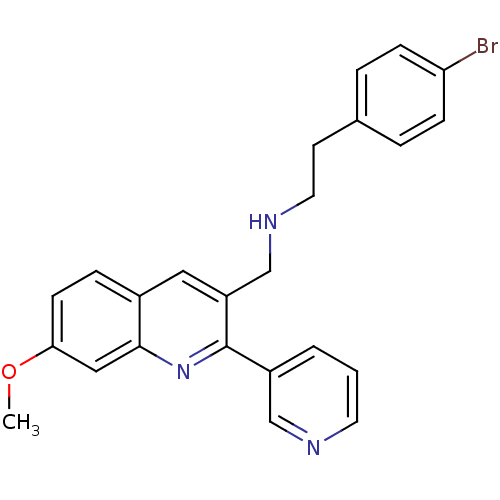
(2-(4-bromophenyl)-N-((7-methoxy-2-(pyridin-3-yl)qu...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1cccnc1 Show InChI InChI=1S/C24H22BrN3O/c1-29-22-9-6-18-13-20(16-27-12-10-17-4-7-21(25)8-5-17)24(28-23(18)14-22)19-3-2-11-26-15-19/h2-9,11,13-15,27H,10,12,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327516
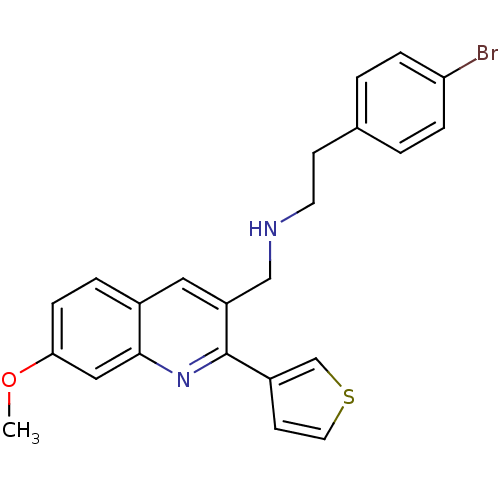
(2-(4-bromophenyl)-N-((7-methoxy-2-(thiophen-3-yl)q...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C23H21BrN2OS/c1-27-21-7-4-17-12-19(14-25-10-8-16-2-5-20(24)6-3-16)23(26-22(17)13-21)18-9-11-28-15-18/h2-7,9,11-13,15,25H,8,10,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405413

(CHEMBL5273556)Show SMILES COc1ccc(CCNCC(O)COc2ccc(CCc3ncc(Br)[nH]3)cc2)cc1OC Show InChI InChI=1S/C24H30BrN3O4/c1-30-21-9-5-18(13-22(21)31-2)11-12-26-14-19(29)16-32-20-7-3-17(4-8-20)6-10-24-27-15-23(25)28-24/h3-5,7-9,13,15,19,26,29H,6,10-12,14,16H2,1-2H3,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against angiotensin converting enzyme (ACE) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405412

(CHEMBL5276114)Show InChI InChI=1S/C22H26N2O5/c1-26-20-8-3-16(13-21(20)27-2)9-10-23-14-18(25)15-29-19-6-4-17(5-7-19)22-24-11-12-28-22/h3-8,11-13,18,23,25H,9-10,14-15H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against neutral endopeptidase (NEP) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405411
(CHEMBL5277114)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2Cl)-c2nc(c[nH]2)-c2cccs2)cc1OC Show InChI InChI=1S/C26H28ClN3O4S/c1-32-23-7-5-17(12-24(23)33-2)9-10-28-14-19(31)16-34-22-8-6-18(13-20(22)27)26-29-15-21(30-26)25-4-3-11-35-25/h3-8,11-13,15,19,28,31H,9-10,14,16H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327540
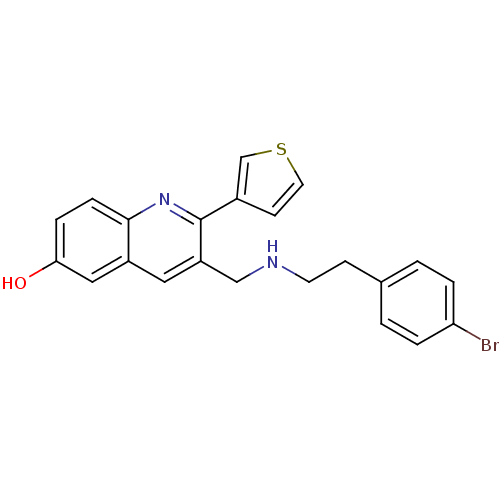
(3-((4-bromophenethylamino)methyl)-2-(thiophen-3-yl...)Show SMILES Oc1ccc2nc(-c3ccsc3)c(CNCCc3ccc(Br)cc3)cc2c1 Show InChI InChI=1S/C22H19BrN2OS/c23-19-3-1-15(2-4-19)7-9-24-13-18-11-17-12-20(26)5-6-21(17)25-22(18)16-8-10-27-14-16/h1-6,8,10-12,14,24,26H,7,9,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327521
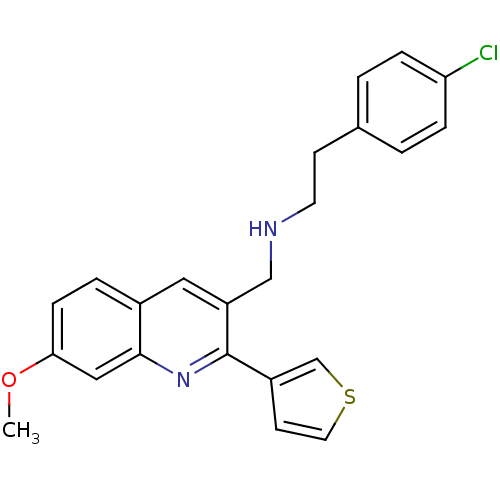
(2-(4-chlorophenyl)-N-((7-methoxy-2-(thiophen-3-yl)...)Show SMILES COc1ccc2cc(CNCCc3ccc(Cl)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C23H21ClN2OS/c1-27-21-7-4-17-12-19(14-25-10-8-16-2-5-20(24)6-3-16)23(26-22(17)13-21)18-9-11-28-15-18/h2-7,9,11-13,15,25H,8,10,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM21679
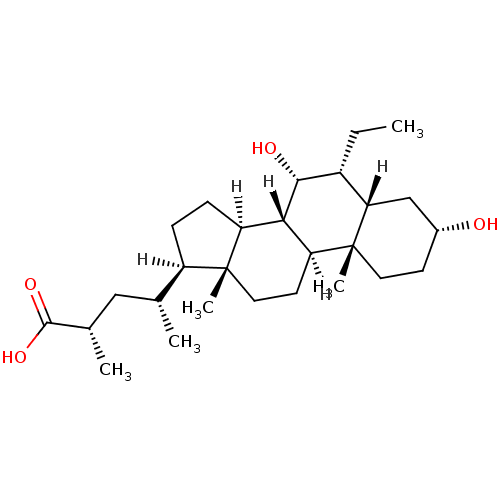
((2S,4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-e...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)C[C@H](C)C(O)=O Show InChI InChI=1S/C27H46O4/c1-6-18-22-14-17(28)9-11-27(22,5)21-10-12-26(4)19(15(2)13-16(3)25(30)31)7-8-20(26)23(21)24(18)29/h15-24,28-29H,6-14H2,1-5H3,(H,30,31)/t15-,16+,17-,18-,19-,20+,21+,22+,23+,24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM21679

((2S,4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-e...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)C[C@H](C)C(O)=O Show InChI InChI=1S/C27H46O4/c1-6-18-22-14-17(28)9-11-27(22,5)21-10-12-26(4)19(15(2)13-16(3)25(30)31)7-8-20(26)23(21)24(18)29/h15-24,28-29H,6-14H2,1-5H3,(H,30,31)/t15-,16+,17-,18-,19-,20+,21+,22+,23+,24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327538
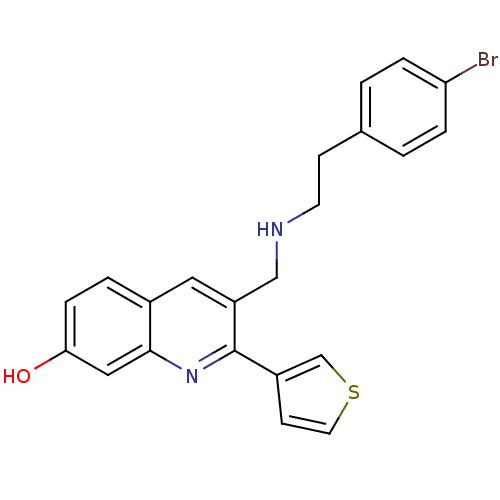
(3-((4-bromophenethylamino)methyl)-2-(thiophen-3-yl...)Show SMILES Oc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C22H19BrN2OS/c23-19-4-1-15(2-5-19)7-9-24-13-18-11-16-3-6-20(26)12-21(16)25-22(18)17-8-10-27-14-17/h1-6,8,10-12,14,24,26H,7,9,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327541
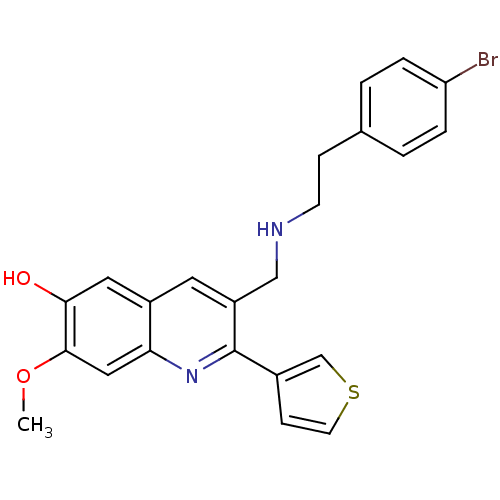
(3-((4-bromophenethylamino)methyl)-7-methoxy-2-(thi...)Show SMILES COc1cc2nc(-c3ccsc3)c(CNCCc3ccc(Br)cc3)cc2cc1O Show InChI InChI=1S/C23H21BrN2O2S/c1-28-22-12-20-17(11-21(22)27)10-18(23(26-20)16-7-9-29-14-16)13-25-8-6-15-2-4-19(24)5-3-15/h2-5,7,9-12,14,25,27H,6,8,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405415
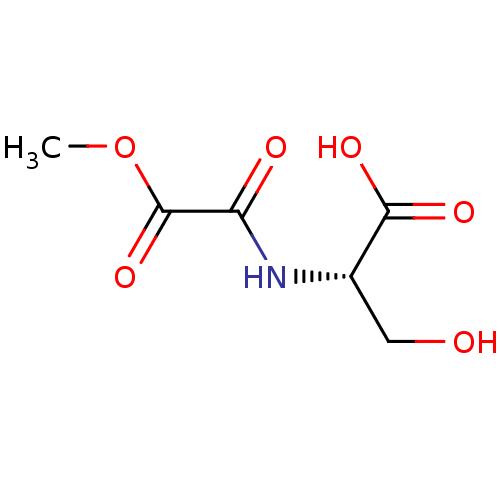
(CHEMBL5278370)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2)-c2ncc(CN3CCOCC3)[nH]2)cc1OC Show InChI InChI=1S/C27H36N4O5/c1-33-25-8-3-20(15-26(25)34-2)9-10-28-17-23(32)19-36-24-6-4-21(5-7-24)27-29-16-22(30-27)18-31-11-13-35-14-12-31/h3-8,15-16,23,28,32H,9-14,17-19H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against neutral endopeptidase (NEP) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327541

(3-((4-bromophenethylamino)methyl)-7-methoxy-2-(thi...)Show SMILES COc1cc2nc(-c3ccsc3)c(CNCCc3ccc(Br)cc3)cc2cc1O Show InChI InChI=1S/C23H21BrN2O2S/c1-28-22-12-20-17(11-21(22)27)10-18(23(26-20)16-7-9-29-14-16)13-25-8-6-15-2-4-19(24)5-3-15/h2-5,7,9-12,14,25,27H,6,8,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327526
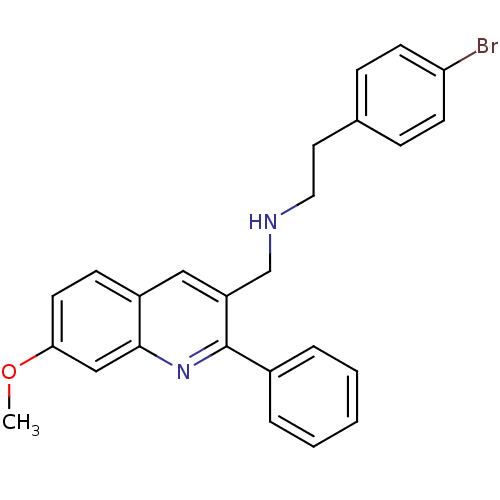
(2-(4-bromophenyl)-N-((7-methoxy-2-phenylquinolin-3...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccccc1 Show InChI InChI=1S/C25H23BrN2O/c1-29-23-12-9-20-15-21(17-27-14-13-18-7-10-22(26)11-8-18)25(28-24(20)16-23)19-5-3-2-4-6-19/h2-12,15-16,27H,13-14,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327525
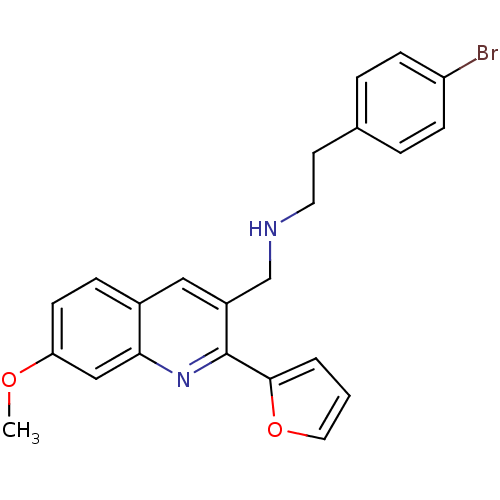
(2-(4-bromophenyl)-N-((2-(furan-2-yl)-7-methoxyquin...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccco1 Show InChI InChI=1S/C23H21BrN2O2/c1-27-20-9-6-17-13-18(15-25-11-10-16-4-7-19(24)8-5-16)23(26-21(17)14-20)22-3-2-12-28-22/h2-9,12-14,25H,10-11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327523
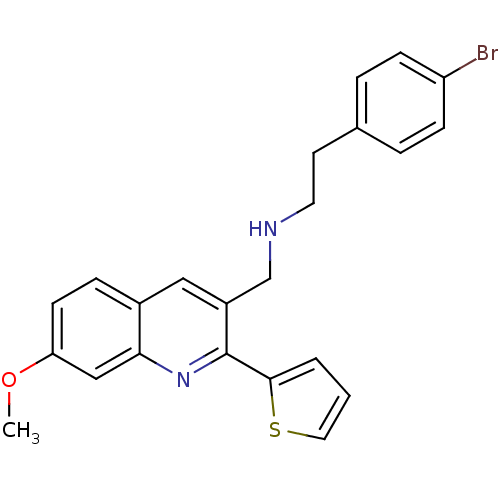
(2-(4-bromophenyl)-N-((7-methoxy-2-(thiophen-2-yl)q...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1cccs1 Show InChI InChI=1S/C23H21BrN2OS/c1-27-20-9-6-17-13-18(15-25-11-10-16-4-7-19(24)8-5-16)23(26-21(17)14-20)22-3-2-12-28-22/h2-9,12-14,25H,10-11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327520
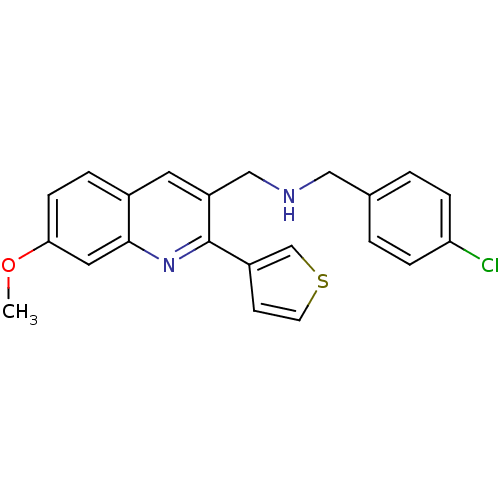
(CHEMBL1258310 | N-(4-chlorobenzyl)-1-(7-methoxy-2-...)Show SMILES COc1ccc2cc(CNCc3ccc(Cl)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C22H19ClN2OS/c1-26-20-7-4-16-10-18(13-24-12-15-2-5-19(23)6-3-15)22(25-21(16)11-20)17-8-9-27-14-17/h2-11,14,24H,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405414
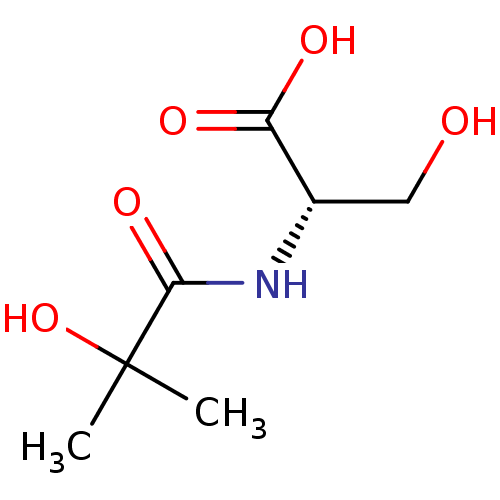
(CHEMBL5276307)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2OC)-c2nc(C)c[nH]2)cc1OC Show InChI InChI=1S/C24H31N3O5/c1-16-13-26-24(27-16)18-6-8-21(23(12-18)31-4)32-15-19(28)14-25-10-9-17-5-7-20(29-2)22(11-17)30-3/h5-8,11-13,19,25,28H,9-10,14-15H2,1-4H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 605 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against neutral endopeptidase (NEP) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327524
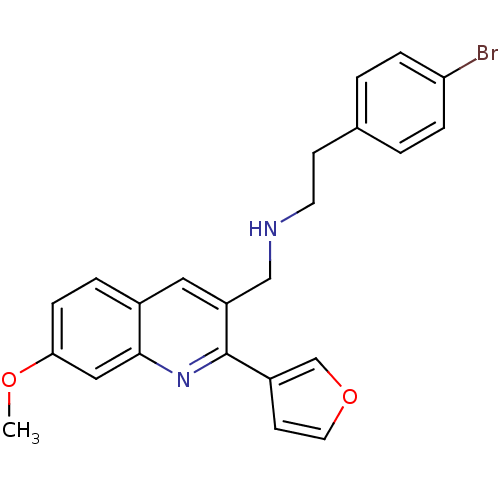
(2-(4-bromophenyl)-N-((2-(furan-3-yl)-7-methoxyquin...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccoc1 Show InChI InChI=1S/C23H21BrN2O2/c1-27-21-7-4-17-12-19(14-25-10-8-16-2-5-20(24)6-3-16)23(26-22(17)13-21)18-9-11-28-15-18/h2-7,9,11-13,15,25H,8,10,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327518
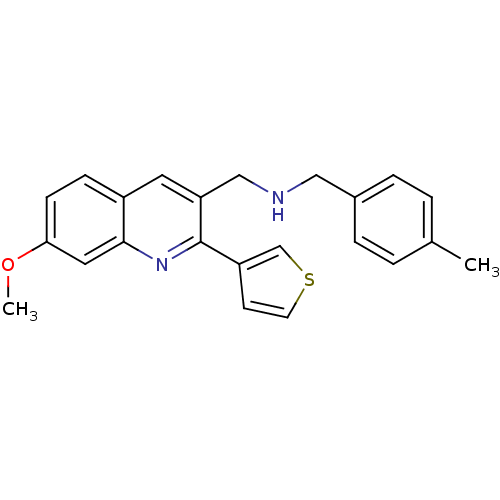
(1-(7-methoxy-2-(thiophen-3-yl)quinolin-3-yl)-N-(4-...)Show InChI InChI=1S/C23H22N2OS/c1-16-3-5-17(6-4-16)13-24-14-20-11-18-7-8-21(26-2)12-22(18)25-23(20)19-9-10-27-15-19/h3-12,15,24H,13-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327519
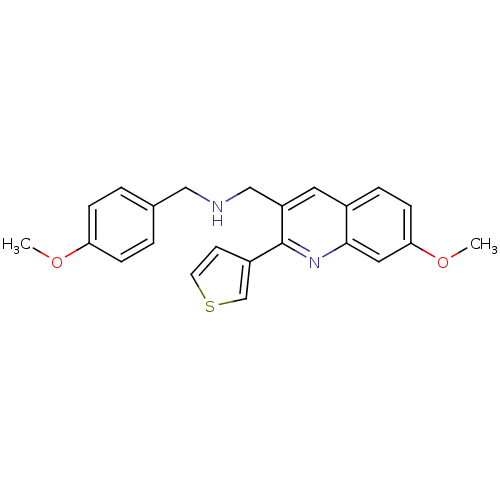
(1-(7-methoxy-2-(thiophen-3-yl)quinolin-3-yl)-N-(4-...)Show InChI InChI=1S/C23H22N2O2S/c1-26-20-6-3-16(4-7-20)13-24-14-19-11-17-5-8-21(27-2)12-22(17)25-23(19)18-9-10-28-15-18/h3-12,15,24H,13-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327540

(3-((4-bromophenethylamino)methyl)-2-(thiophen-3-yl...)Show SMILES Oc1ccc2nc(-c3ccsc3)c(CNCCc3ccc(Br)cc3)cc2c1 Show InChI InChI=1S/C22H19BrN2OS/c23-19-3-1-15(2-4-19)7-9-24-13-18-11-17-12-20(26)5-6-21(17)25-22(18)16-8-10-27-14-16/h1-6,8,10-12,14,24,26H,7,9,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327531
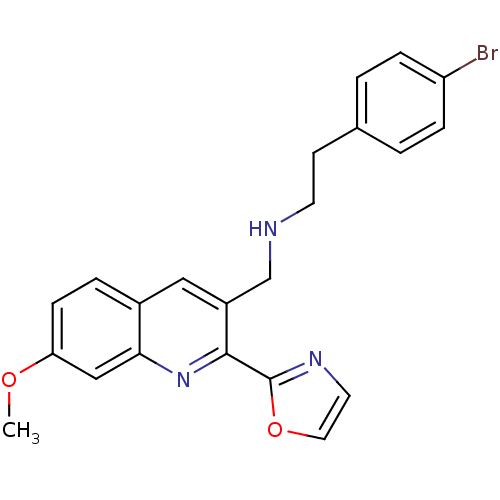
(2-(4-bromophenyl)-N-((7-methoxy-2-(oxazol-2-yl)qui...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ncco1 Show InChI InChI=1S/C22H20BrN3O2/c1-27-19-7-4-16-12-17(14-24-9-8-15-2-5-18(23)6-3-15)21(26-20(16)13-19)22-25-10-11-28-22/h2-7,10-13,24H,8-9,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327522
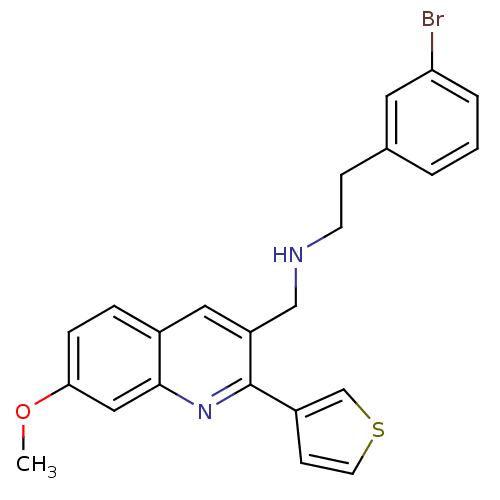
(2-(3-bromophenyl)-N-((7-methoxy-2-(thiophen-3-yl)q...)Show SMILES COc1ccc2cc(CNCCc3cccc(Br)c3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C23H21BrN2OS/c1-27-21-6-5-17-12-19(14-25-9-7-16-3-2-4-20(24)11-16)23(26-22(17)13-21)18-8-10-28-15-18/h2-6,8,10-13,15,25H,7,9,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327516

(2-(4-bromophenyl)-N-((7-methoxy-2-(thiophen-3-yl)q...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C23H21BrN2OS/c1-27-21-7-4-17-12-19(14-25-10-8-16-2-5-20(24)6-3-16)23(26-22(17)13-21)18-9-11-28-15-18/h2-7,9,11-13,15,25H,8,10,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327521

(2-(4-chlorophenyl)-N-((7-methoxy-2-(thiophen-3-yl)...)Show SMILES COc1ccc2cc(CNCCc3ccc(Cl)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C23H21ClN2OS/c1-27-21-7-4-17-12-19(14-25-10-8-16-2-5-20(24)6-3-16)23(26-22(17)13-21)18-9-11-28-15-18/h2-7,9,11-13,15,25H,8,10,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327527

(2-(4-bromophenyl)-N-((7-methoxy-2-(pyridin-4-yl)qu...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccncc1 Show InChI InChI=1S/C24H22BrN3O/c1-29-22-7-4-19-14-20(16-27-11-8-17-2-5-21(25)6-3-17)24(28-23(19)15-22)18-9-12-26-13-10-18/h2-7,9-10,12-15,27H,8,11,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327518

(1-(7-methoxy-2-(thiophen-3-yl)quinolin-3-yl)-N-(4-...)Show InChI InChI=1S/C23H22N2OS/c1-16-3-5-17(6-4-16)13-24-14-20-11-18-7-8-21(26-2)12-22(18)25-23(20)19-9-10-27-15-19/h3-12,15,24H,13-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327525

(2-(4-bromophenyl)-N-((2-(furan-2-yl)-7-methoxyquin...)Show SMILES COc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccco1 Show InChI InChI=1S/C23H21BrN2O2/c1-27-20-9-6-17-13-18(15-25-11-10-16-4-7-19(24)8-5-16)23(26-21(17)14-20)22-3-2-12-28-22/h2-9,12-14,25H,10-11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50327520

(CHEMBL1258310 | N-(4-chlorobenzyl)-1-(7-methoxy-2-...)Show SMILES COc1ccc2cc(CNCc3ccc(Cl)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C22H19ClN2OS/c1-26-20-7-4-16-10-18(13-24-12-15-2-5-19(23)6-3-15)22(25-21(16)11-20)17-8-9-27-14-17/h2-11,14,24H,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50327534
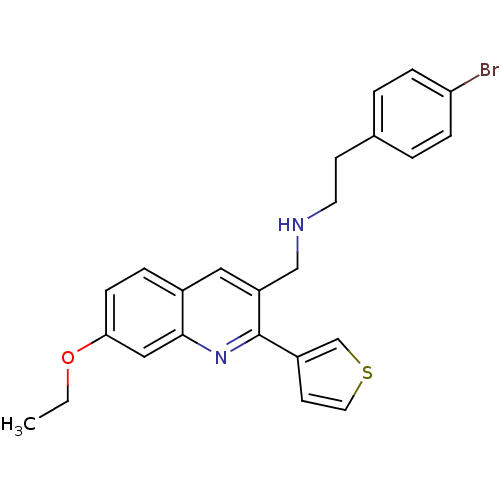
(2-(4-bromophenyl)-N-((7-ethoxy-2-(thiophen-3-yl)qu...)Show SMILES CCOc1ccc2cc(CNCCc3ccc(Br)cc3)c(nc2c1)-c1ccsc1 Show InChI InChI=1S/C24H23BrN2OS/c1-2-28-22-8-5-18-13-20(15-26-11-9-17-3-6-21(25)7-4-17)24(27-23(18)14-22)19-10-12-29-16-19/h3-8,10,12-14,16,26H,2,9,11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 receptor expressed in HEK293 cells assessed as intracellular cAMP level |
Bioorg Med Chem Lett 20: 5718-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.014
BindingDB Entry DOI: 10.7270/Q2N87B1H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data