

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metallothionein-2 (Human) | BDBM50035179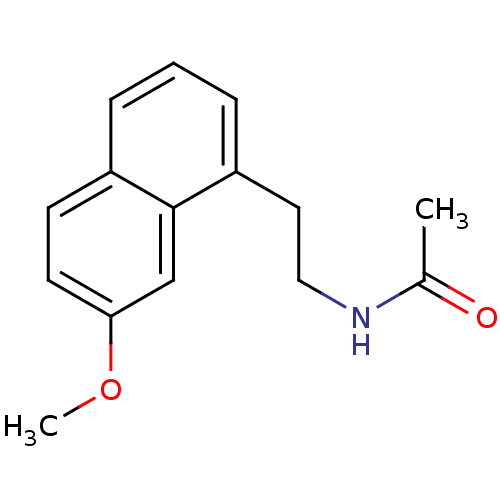 (Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACHÉ LABORATÓRIOS FARMACÊUTICOS S.A. US Patent | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | US Patent US10781182 (2020) BindingDB Entry DOI: 10.7270/Q2JH3Q7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50035179 (Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | Citation and Details BindingDB Entry DOI: 10.7270/Q24Q7Z44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 120 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032698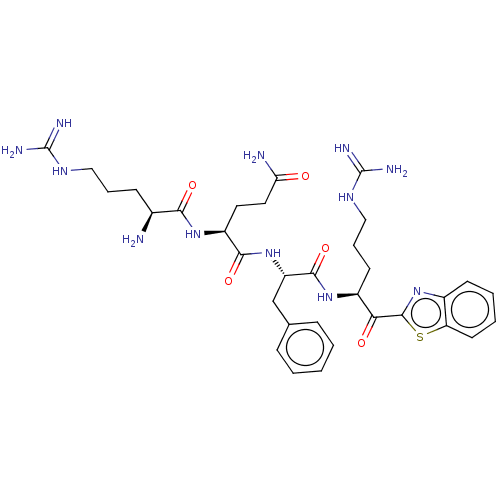 (CHEMBL3354676 | N-0130) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50163152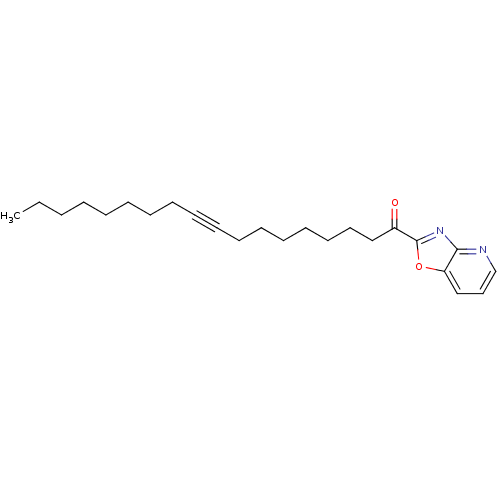 (1-Oxazolo[4,5-b]pyridin-2-yl-octadec-9-yn-1-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50035179 (Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | Citation and Details BindingDB Entry DOI: 10.7270/Q24Q7Z44 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50035179 (Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACHÉ LABORATÓRIOS FARMACÊUTICOS S.A. US Patent | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | US Patent US10781182 (2020) BindingDB Entry DOI: 10.7270/Q2JH3Q7Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202918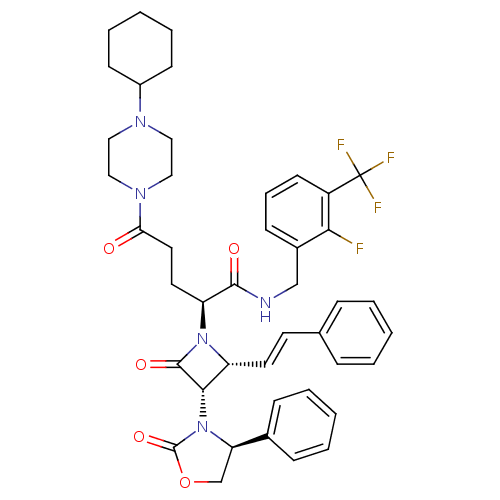 ((S)-N-(2-fluoro-3-(trifluoromethyl)benzyl)-5-(4-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469544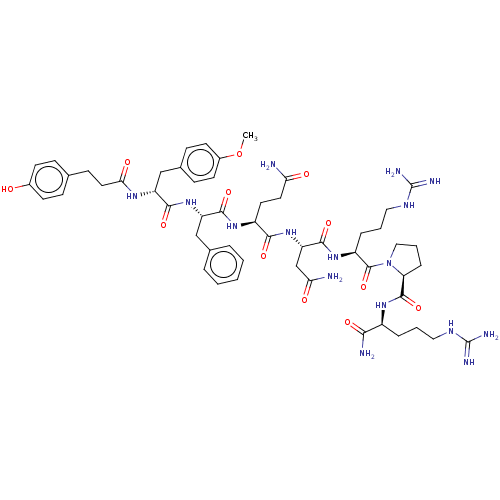 (CHEMBL4281963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Se-Se]-AVP from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054764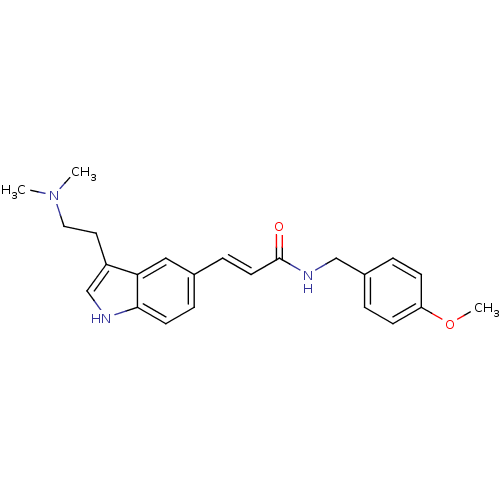 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50575514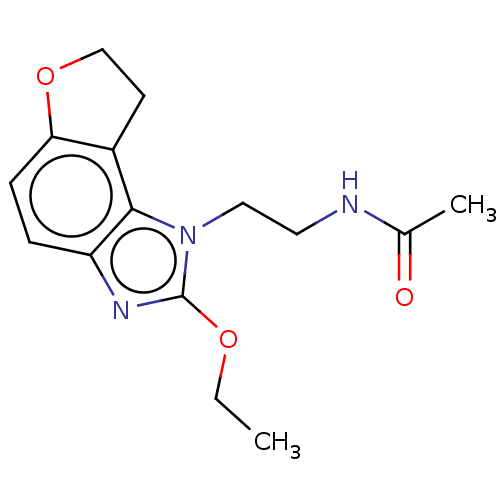 (CHEMBL4852440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 120 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202900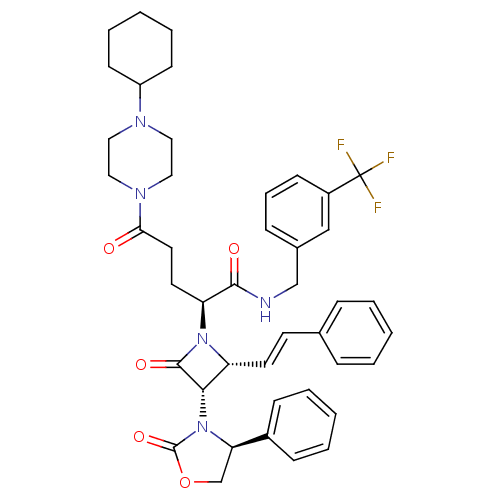 ((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-cyclohexylp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23316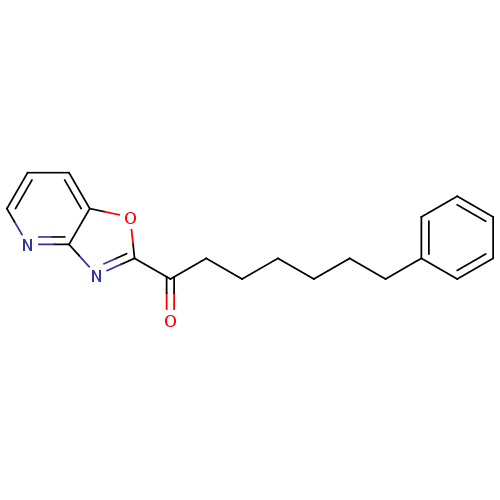 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50575516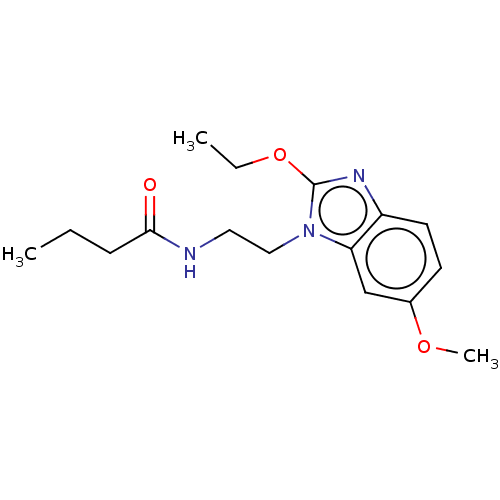 (CHEMBL4874954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 120 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054764 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202920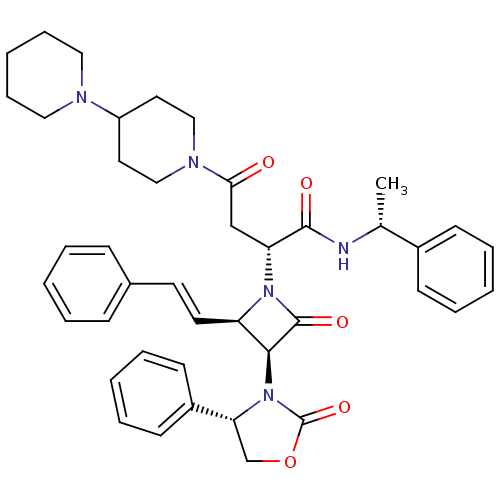 (2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374645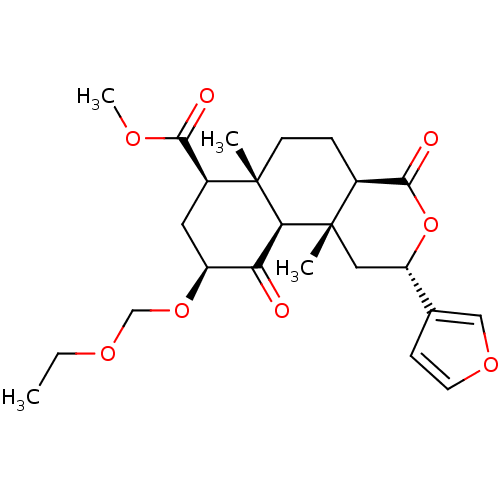 (CHEMBL272939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem 16: 1279-86 (2008) Article DOI: 10.1016/j.bmc.2007.10.067 BindingDB Entry DOI: 10.7270/Q2ZK5HJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575514 (CHEMBL4852440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032698 (CHEMBL3354676 | N-0130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202882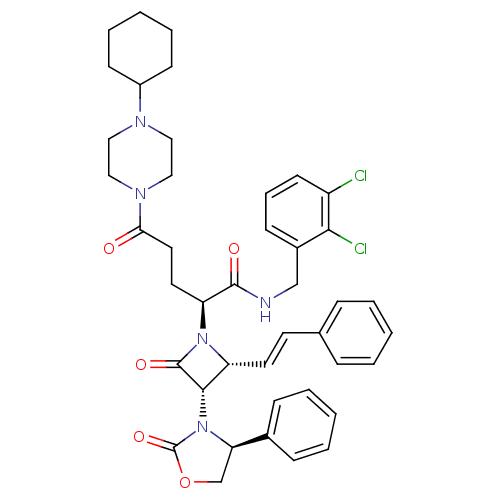 ((S)-N-(2,3-dichlorobenzyl)-5-(4-cyclohexylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50575513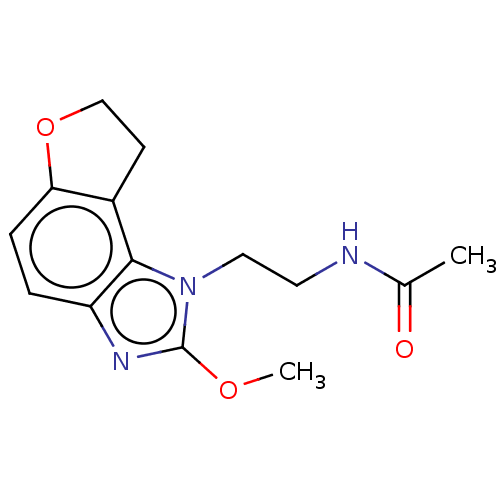 (CHEMBL4873903) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 120 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575516 (CHEMBL4874954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469541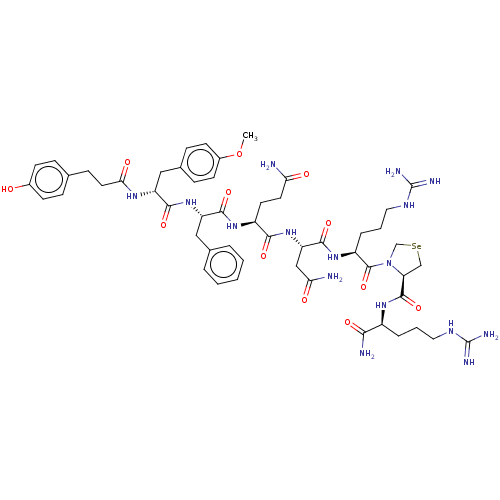 (CHEMBL4289837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Se-Se]-AVP from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50169772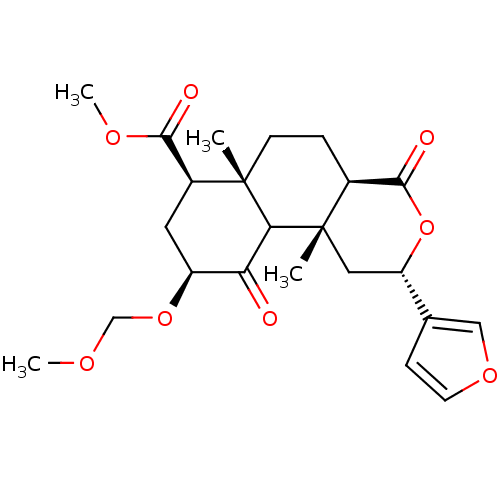 ((3S,4aR,6S,8R,8aR,10aR)-3-Furan-3-yl-6-methoxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonistic activity against human k-opioid receptor using [3H]diprenorphine | Bioorg Med Chem Lett 15: 3744-7 (2005) Article DOI: 10.1016/j.bmcl.2005.05.048 BindingDB Entry DOI: 10.7270/Q2MS3THX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50169772 ((3S,4aR,6S,8R,8aR,10aR)-3-Furan-3-yl-6-methoxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonistic activity against human k-opioid receptor using [3H]diprenorphine | Bioorg Med Chem Lett 15: 3744-7 (2005) Article DOI: 10.1016/j.bmcl.2005.05.048 BindingDB Entry DOI: 10.7270/Q2MS3THX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202902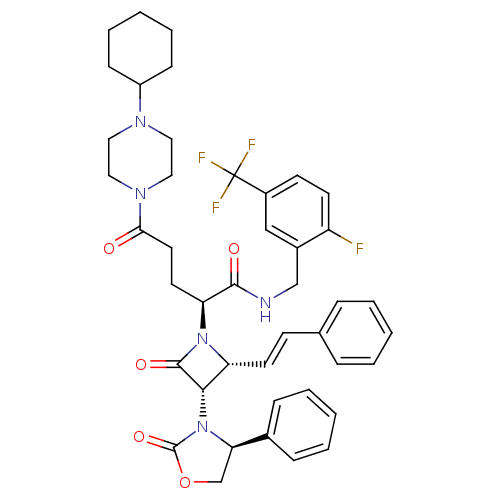 ((S)-N-(2-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575511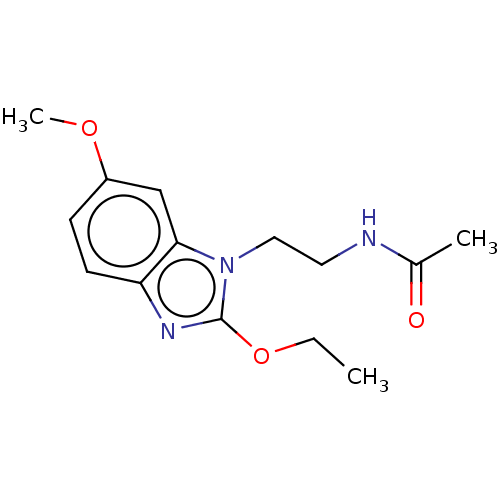 (CHEMBL4859677) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202873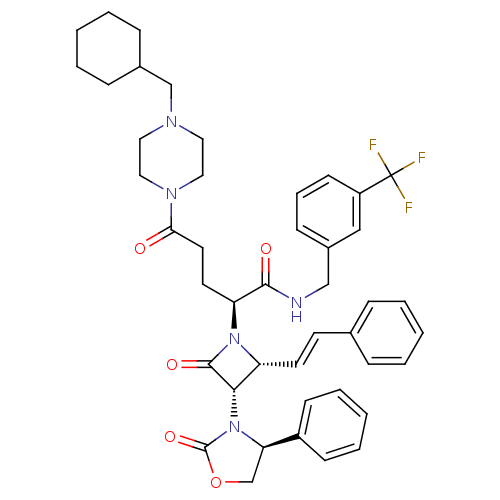 ((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-(cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202916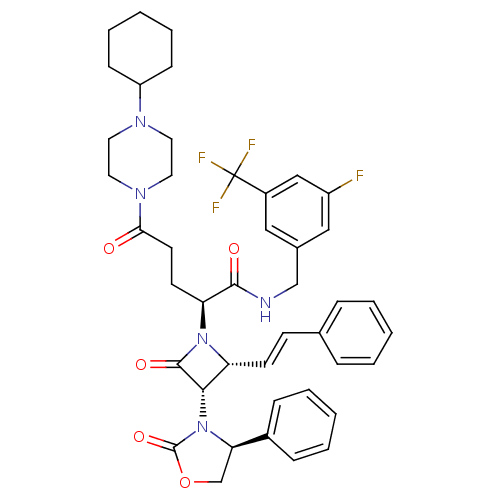 ((S)-N-(3-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525153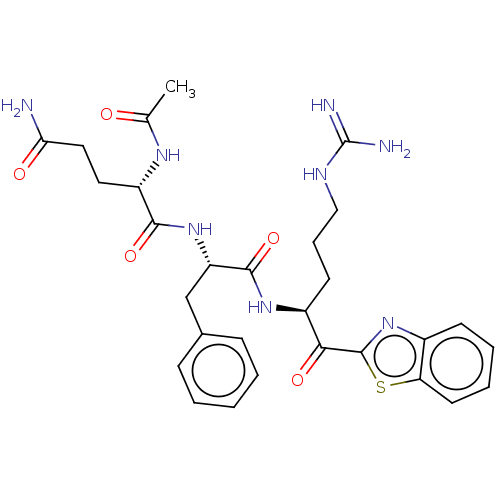 (Ac-QFR-kbt | N-0386 | US10988505, Example 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525256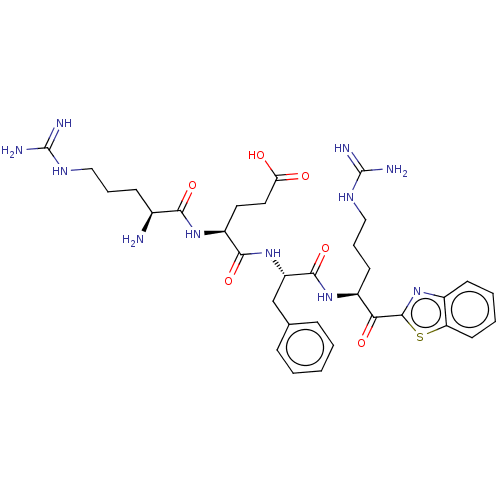 ((H)Arg-Glu-Phe-Arg-kbt | N-0438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114031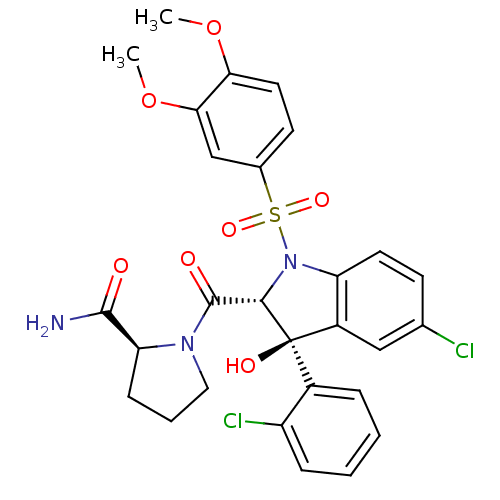 ((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525152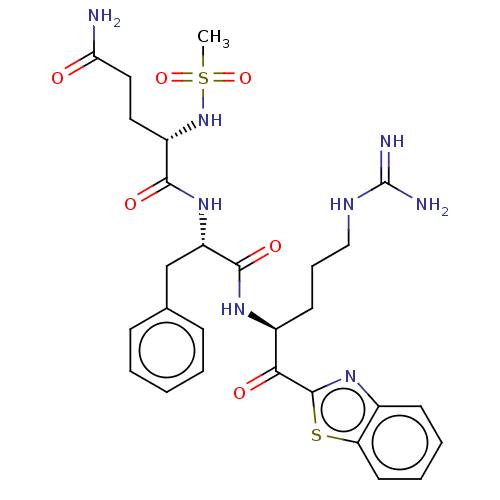 (Ms-QFR-kbt | N-0385 | US10988505, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525153 (Ac-QFR-kbt | N-0386 | US10988505, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374634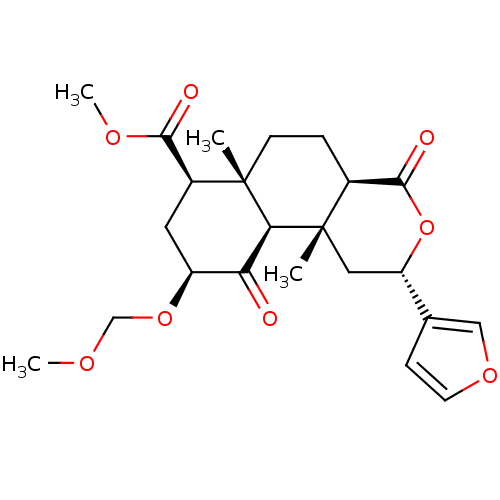 (CHEMBL258098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem 16: 1279-86 (2008) Article DOI: 10.1016/j.bmc.2007.10.067 BindingDB Entry DOI: 10.7270/Q2ZK5HJF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50362873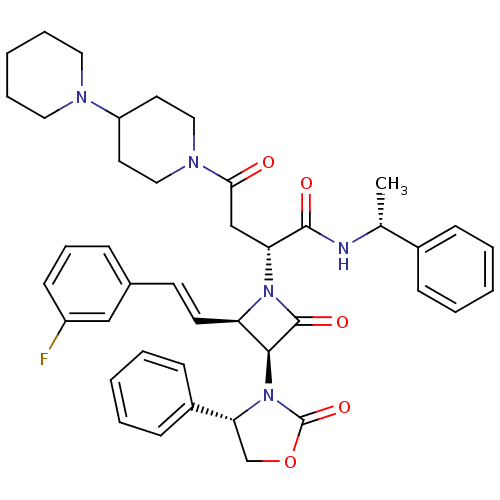 (CHEMBL1940587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Displacement of [3H]arginine-vasopressin from human Vasopressin V1a receptor after 30 mins by liquid scintillation counter | Bioorg Med Chem 20: 1337-45 (2012) Article DOI: 10.1016/j.bmc.2011.12.013 BindingDB Entry DOI: 10.7270/Q2KD1ZCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575513 (CHEMBL4873903) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50362871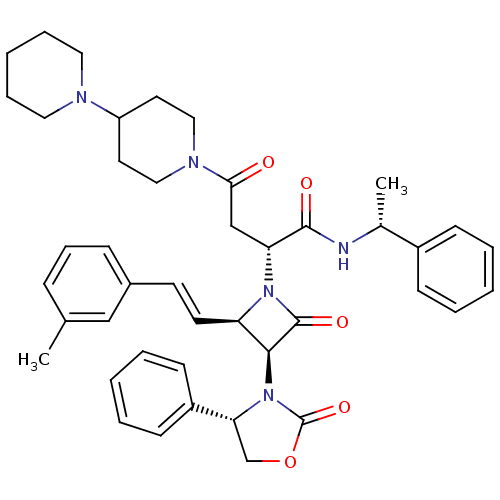 (CHEMBL1940588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Displacement of [3H]arginine-vasopressin from human Vasopressin V1a receptor after 30 mins by liquid scintillation counter | Bioorg Med Chem 20: 1337-45 (2012) Article DOI: 10.1016/j.bmc.2011.12.013 BindingDB Entry DOI: 10.7270/Q2KD1ZCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202860 (2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202871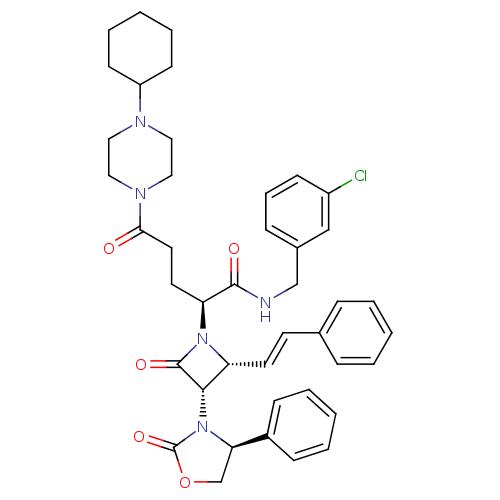 ((S)-N-(3-chlorobenzyl)-5-(4-cyclohexylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469540 (CHEMBL4294901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575517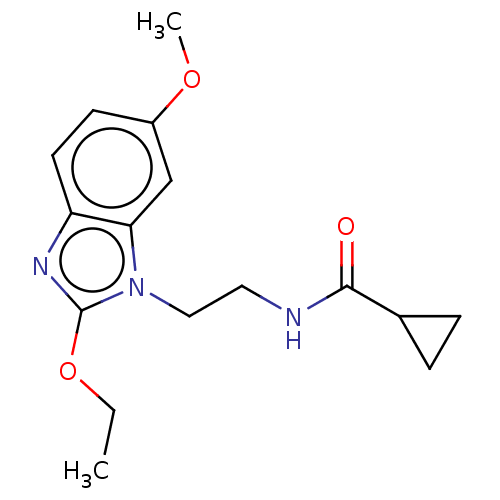 (CHEMBL4869463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469544 (CHEMBL4281963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM463447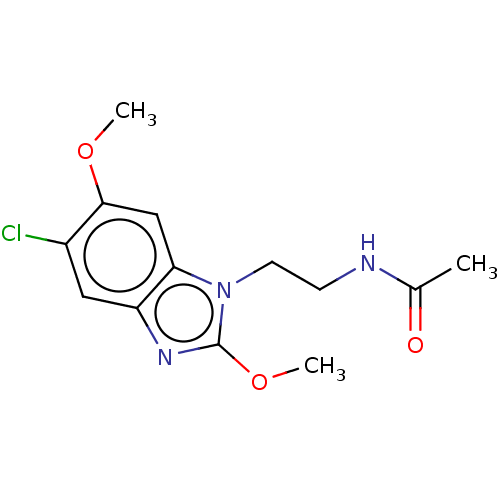 (US10781182, Compound IA2-143 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | Citation and Details BindingDB Entry DOI: 10.7270/Q24Q7Z44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM463447 (US10781182, Compound IA2-143 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 120 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallothionein-2 (Human) | BDBM463447 (US10781182, Compound IA2-143 | US11091445, Compoun...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACHÉ LABORATÓRIOS FARMACÊUTICOS S.A. US Patent | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | US Patent US10781182 (2020) BindingDB Entry DOI: 10.7270/Q2JH3Q7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202877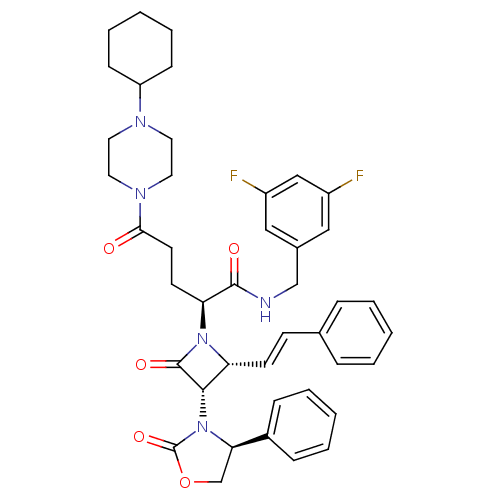 ((S)-N-(3,5-difluorobenzyl)-5-(4-cyclohexylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM463444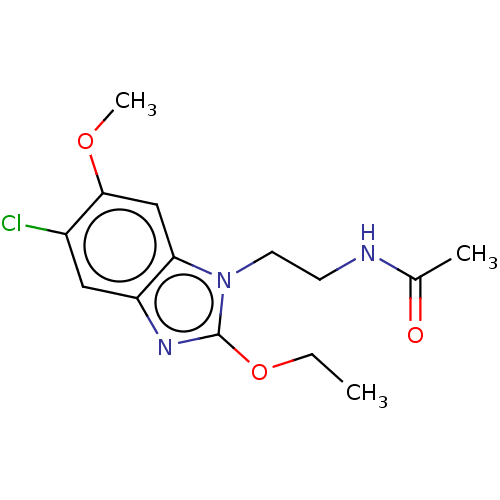 (US10781182, Compound IA2-121 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | Citation and Details BindingDB Entry DOI: 10.7270/Q24Q7Z44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3821 total ) | Next | Last >> |