

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436357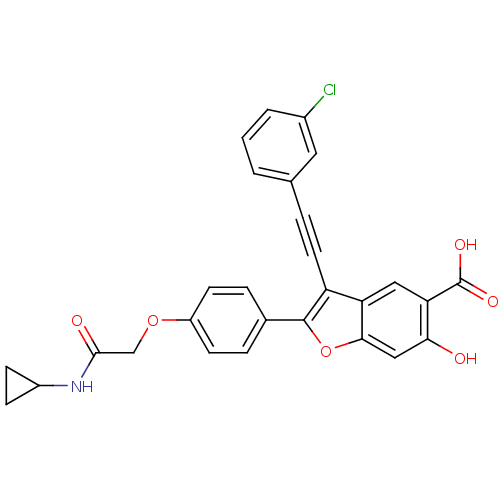 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50112356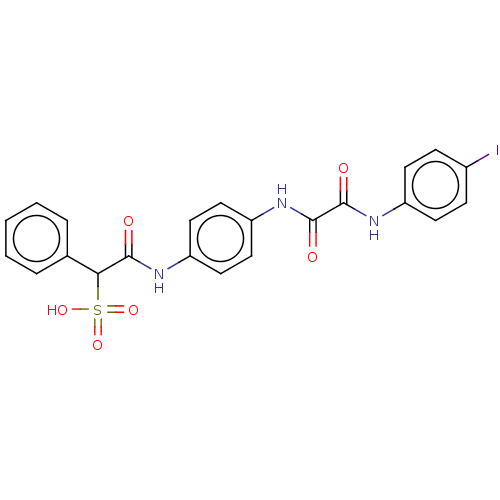 (CHEMBL3609373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-like domain-containing CTD phosphatase 1 (Homo sapiens (Human)) | BDBM50087856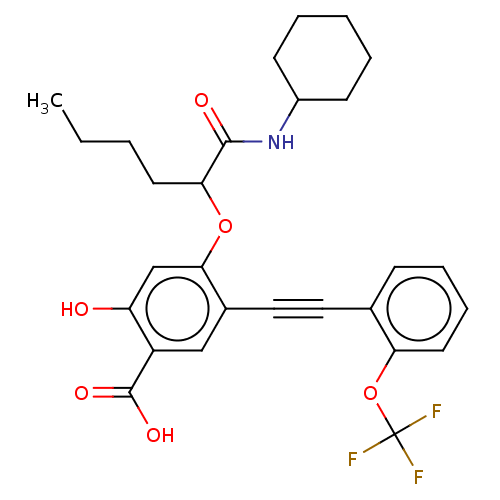 (CHEMBL3426913) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Competitive inhibition of His6-tagged UBLCP1 (unknown origin) expressed in Escherichia coli BL21 cells by Lineweaver-Burk plot analysis in presence o... | Bioorg Med Chem 23: 2798-809 (2015) Article DOI: 10.1016/j.bmc.2015.03.066 BindingDB Entry DOI: 10.7270/Q2VD7168 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50335895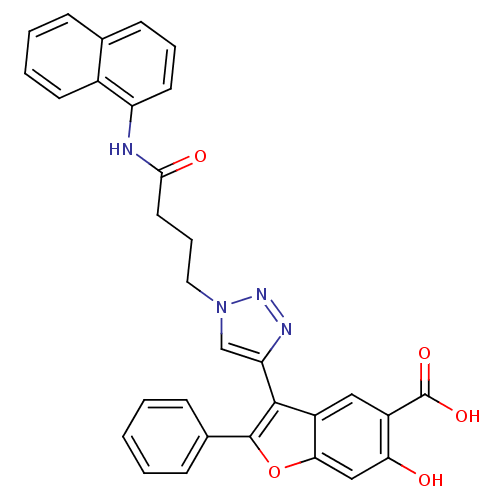 (6-hydroxy-3-(1-(4-(naphthalen-1-ylamino)-4-oxobuty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50308158 (3-(1-(3-(Biphenyl-4-ylamino)-3-oxopropyl)-1H-1,2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 Src homology-2 domain expressed in Escherichia coli BL21 (DE3) assessed as inhibition of p-nitrophenyl phosphate hydrolysis by Lin... | J Med Chem 53: 2482-93 (2010) Article DOI: 10.1021/jm901645u BindingDB Entry DOI: 10.7270/Q2639PVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50420258 (CEFSULODIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436358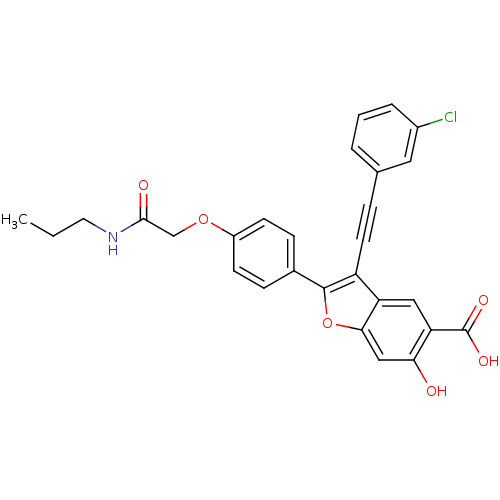 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054344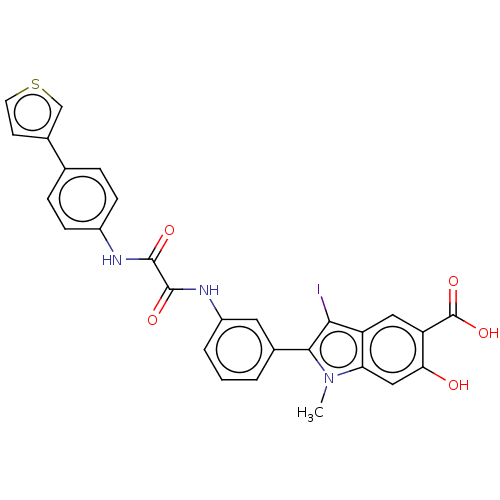 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054257 (CHEMBL3319376 | US9522881, 11a-21 L97L08 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436357 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Reversible inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP ... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436357 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 294) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436357 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436360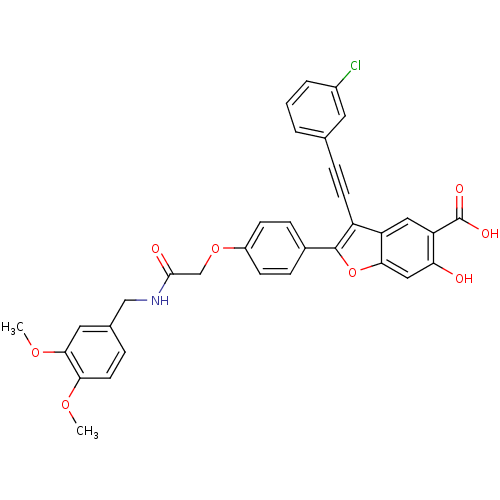 (CHEMBL2396723) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436357 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Reversible inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP ... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436363 (CHEMBL2396720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436364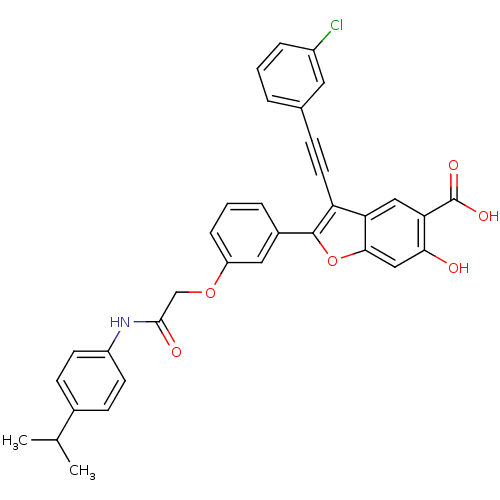 (CHEMBL2396717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436364 (CHEMBL2396717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 294) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436362 (CHEMBL2396721) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054258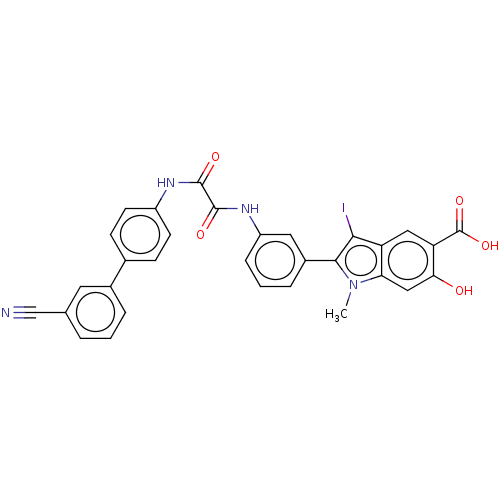 (CHEMBL3319377 | US9522881, 11a-22 L97L07 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight phosphotyrosine protein phosphatase (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant LMWPTP (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054259 (CHEMBL3319378 | US9522881, 11a-23 L97L03) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054260 (CHEMBL3319379 | US9522881, 11a-24 L97L05 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 13 (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant FAP-1 (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054261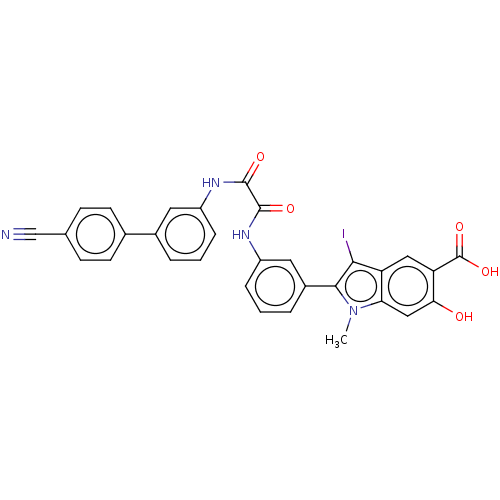 (CHEMBL3319380 | US9522881, 11a-25 L97L06 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTPbeta (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-like domain-containing CTD phosphatase 1 (Homo sapiens (Human)) | BDBM50087856 (CHEMBL3426913) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of His6-tagged UBLCP1 (unknown origin) expressed in Escherichia coli BL21 cells using pNPP as substrate at pH 6 at 25 degC by spectrophoto... | Bioorg Med Chem 23: 2798-809 (2015) Article DOI: 10.1016/j.bmc.2015.03.066 BindingDB Entry DOI: 10.7270/Q2VD7168 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436361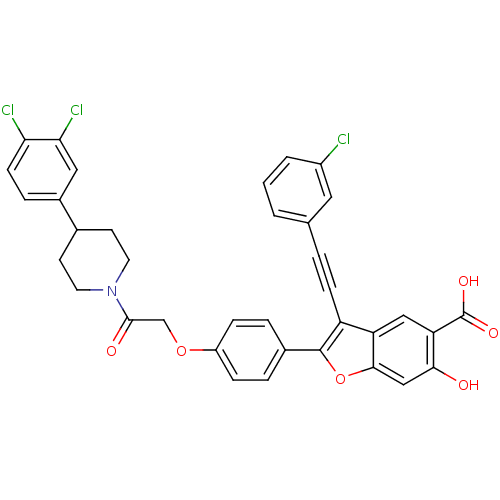 (CHEMBL2396722) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant SHP-2 (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTP-MEG2 (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436367 (CHEMBL2396714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054406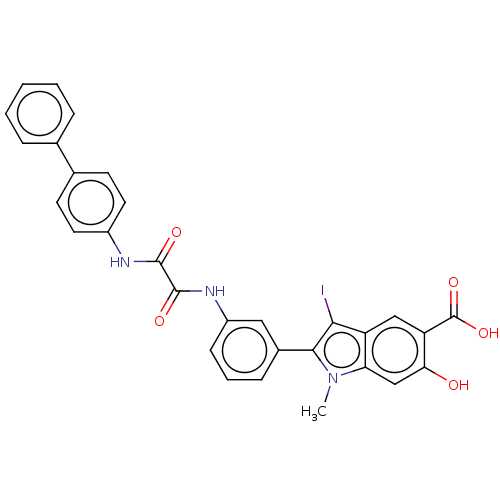 (CHEMBL3319357 | US9522881, 11a-2 (L97N08) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of LYP (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054262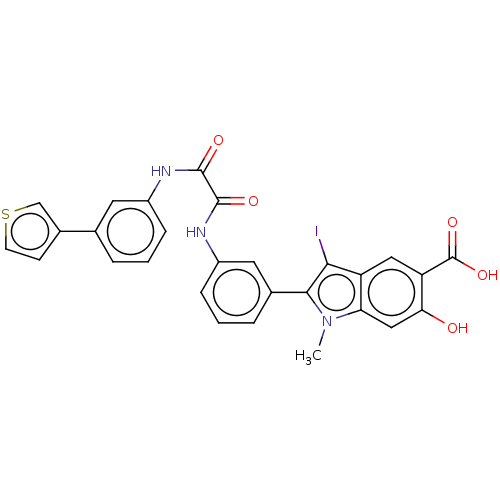 (CHEMBL3319381 | US9522881, 11a-26 L97L02 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054405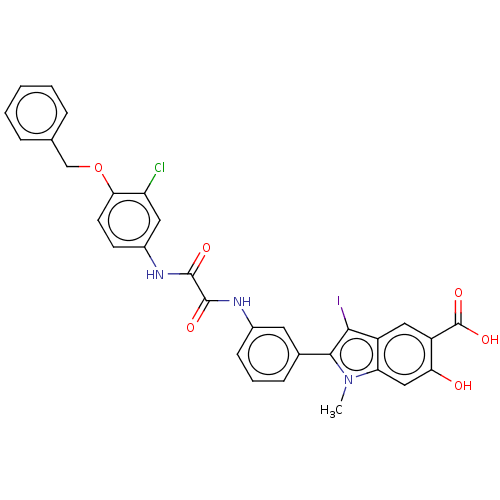 (CHEMBL3319358 | US9522881, 11a-3 (L97M50) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436359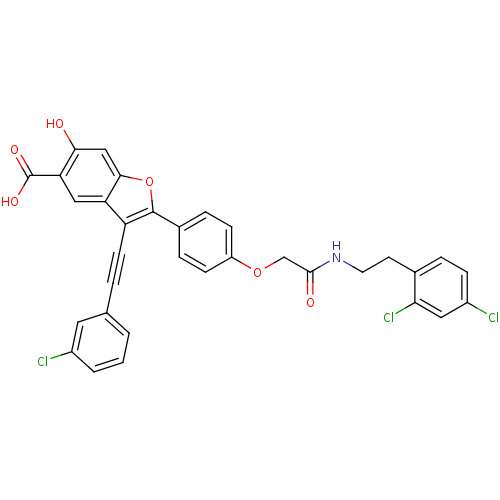 (CHEMBL2396724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436368 (CHEMBL2396713) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50436368 (CHEMBL2396713) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 294) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP1 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054404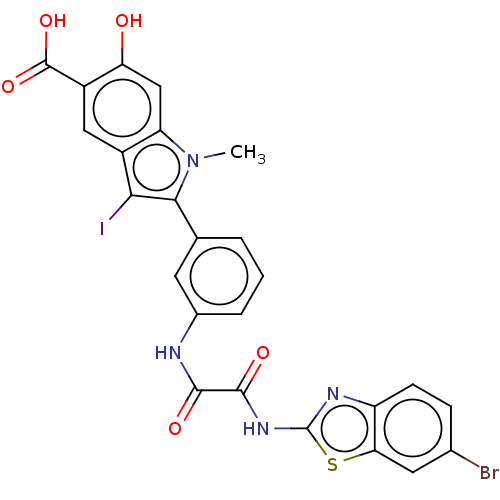 (CHEMBL3319359 | US9522881, 11a-4 (L97M61) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054403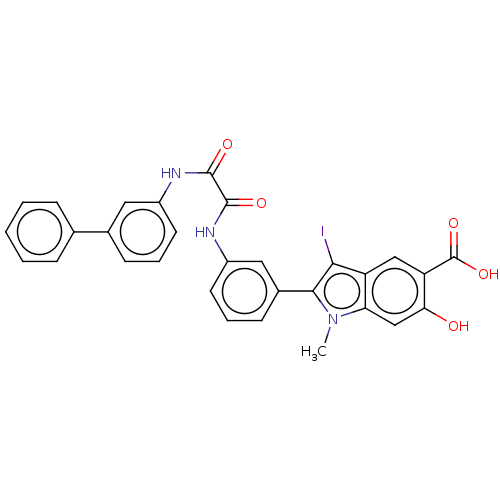 (CHEMBL3319360 | US9522881, 11a-5 (L97M48) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 12 (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTP-PEST (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of VHR (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054402 (CHEMBL3319361 | US9522881, 11a-6 (L97M52) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CD45 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 (Homo sapiens (Human)) | BDBM50436358 (CHEMBL2396718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant SHP-1 (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50425818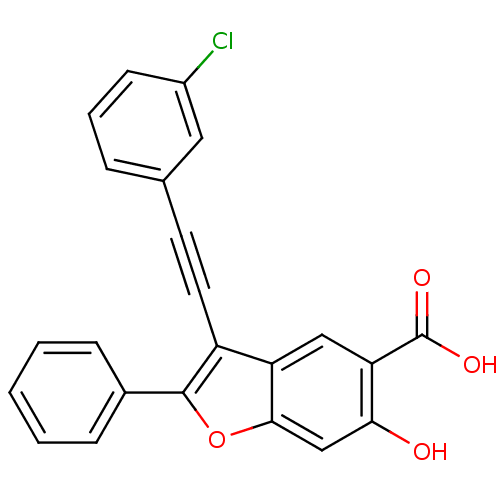 (CHEMBL2316905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of LYP (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50425818 (CHEMBL2316905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50425818 (CHEMBL2316905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 294) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat... | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 429 total ) | Next | Last >> |