

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM282825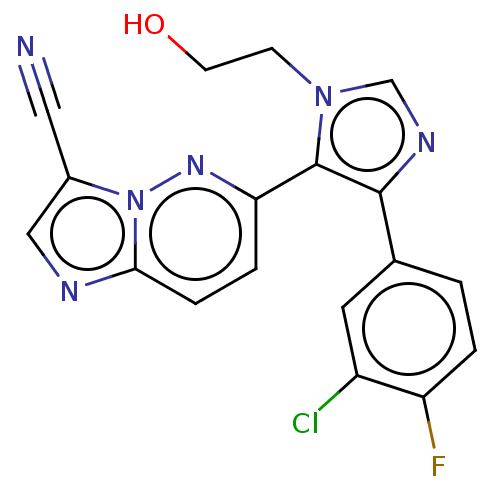 (6-(4-(3-chloro-4-fluorophenyl)-1-(2-hydroxyethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of TGFBR1 in human whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylation | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Mus musculus) | BDBM282825 (6-(4-(3-chloro-4-fluorophenyl)-1-(2-hydroxyethyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of TGFBR1 in mouse whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylation | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272095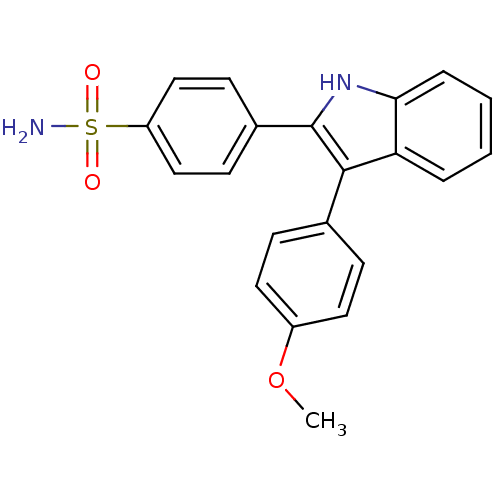 (4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272129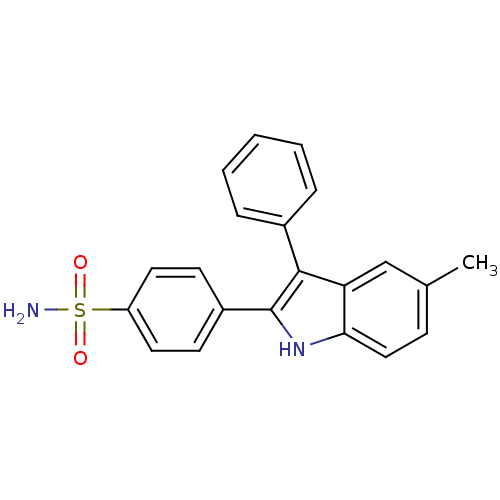 (4-(5-methyl-3-phenyl-1H-indol-2-yl)benzenesulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272096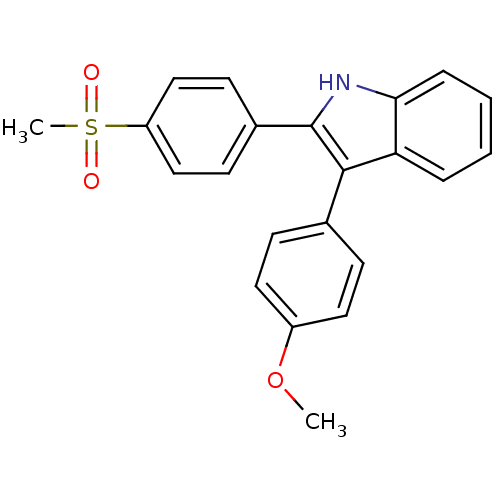 (3-(4-methoxyphenyl)-2-(4-(methylsulfonyl)phenyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272131 (3-(4-fluorophenyl)-2-(4-(methylsulfonyl)phenyl)-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272125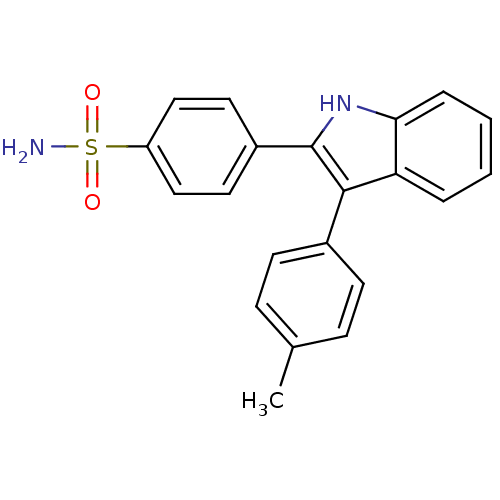 (4-(3-p-tolyl-1H-indol-2-yl)benzenesulfonamide | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272126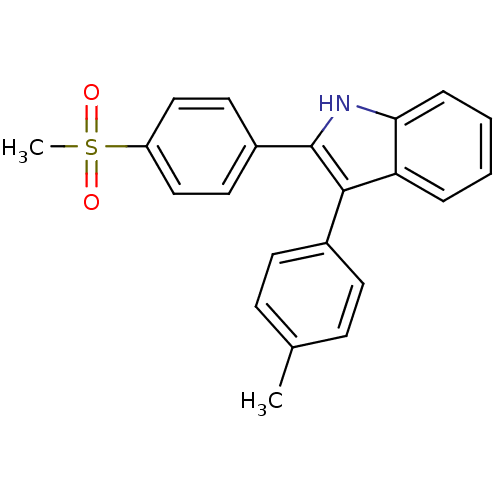 (2-(4-(methylsulfonyl)phenyl)-3-p-tolyl-1H-indole |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272105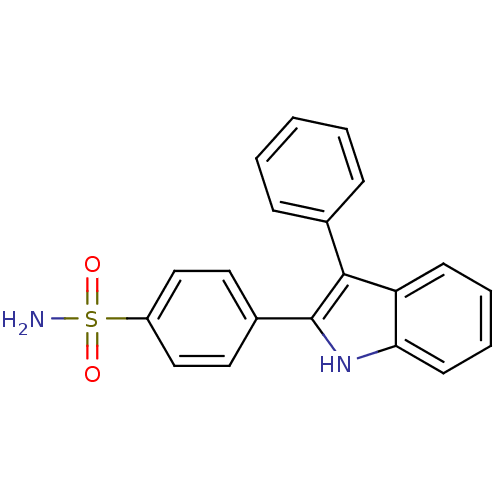 (4-(3-phenyl-1H-indol-2-yl)benzenesulfonamide | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272110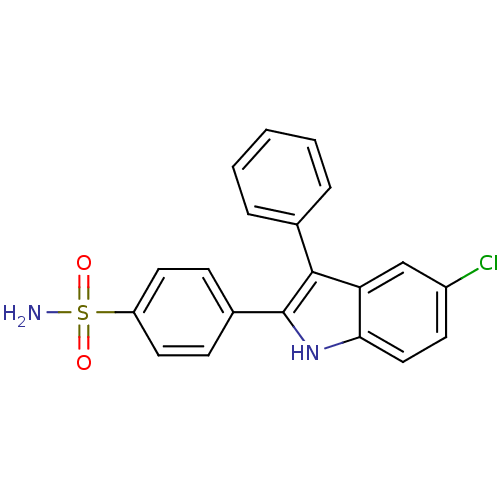 (4-(5-chloro-3-phenyl-1H-indol-2-yl)benzenesulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272097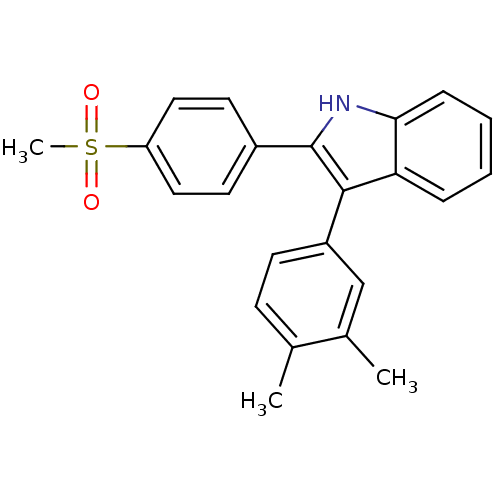 (3-(3,4-dimethylphenyl)-2-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272128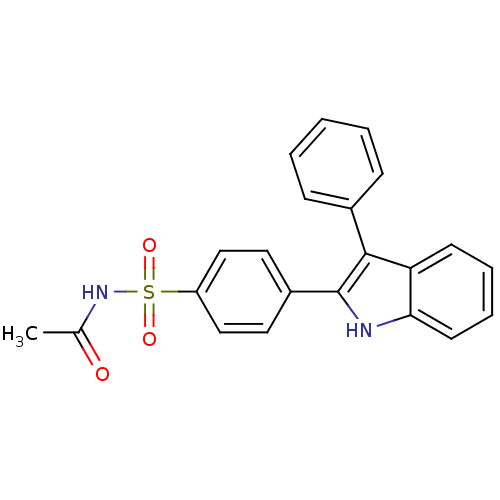 (CHEMBL500943 | N-(4-(3-phenyl-1H-indol-2-yl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245763 (US9428511, 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272130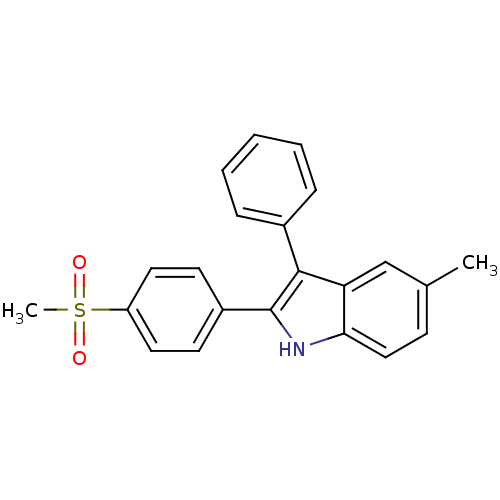 (5-methyl-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245770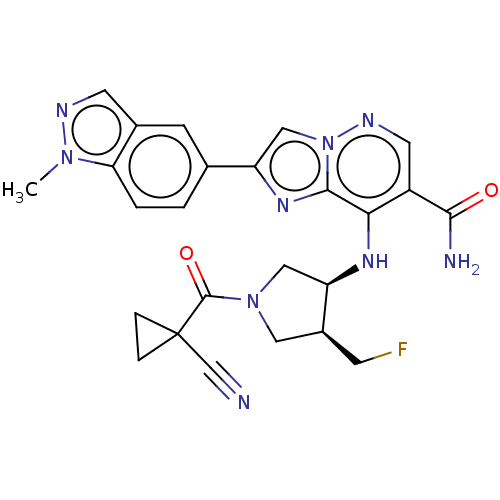 (US9428511, 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245761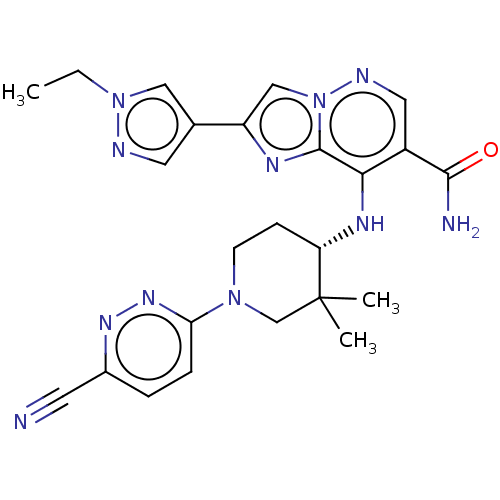 (US9428511, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272111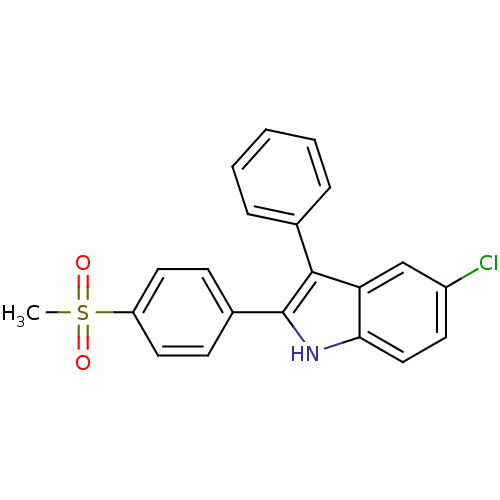 (5-chloro-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272124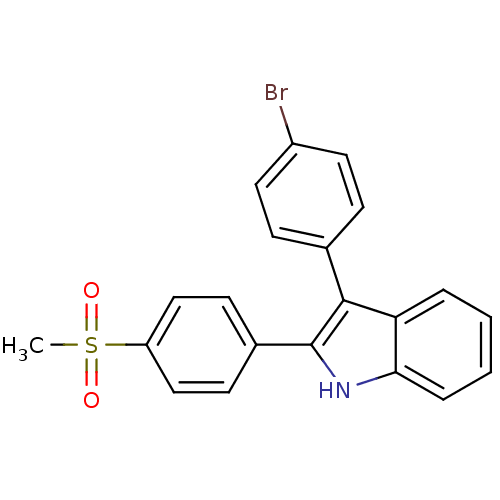 (3-(4-bromophenyl)-2-(4-(methylsulfonyl)phenyl)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245762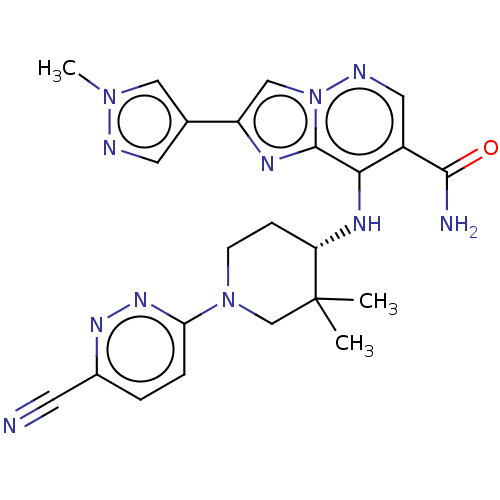 (US9428511, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245772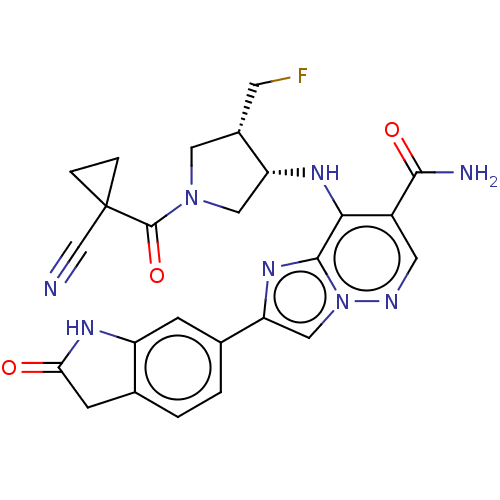 (US9428511, 64) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245779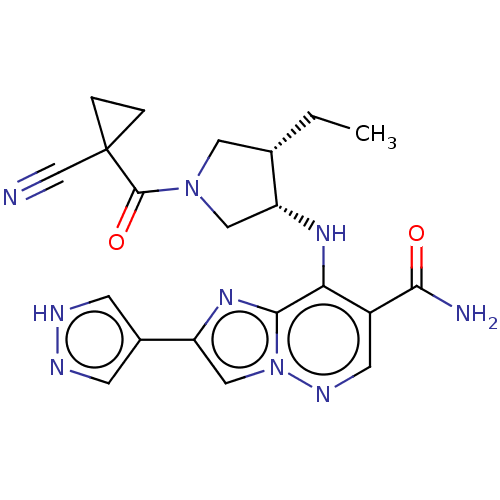 (US9428511, 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245760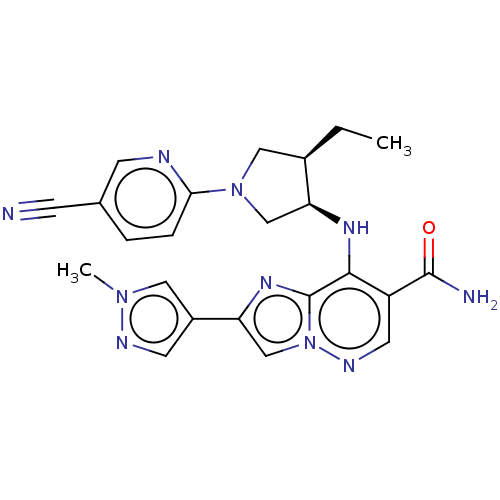 (US9428511, 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245763 (US9428511, 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245762 (US9428511, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272090 (4-(5-chloro-3-(4-chlorophenyl)-1H-indol-2-yl)benze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245761 (US9428511, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245747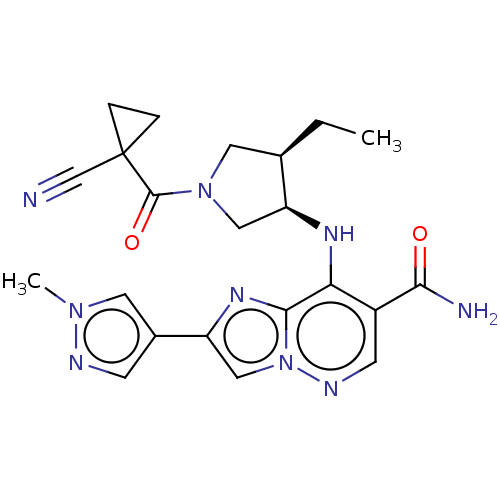 (US9428511, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245751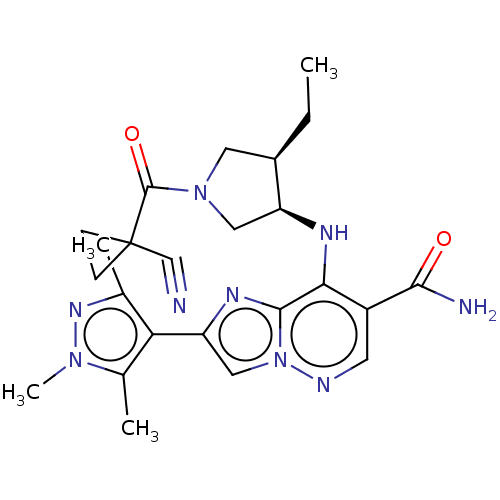 (US9428511, 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245734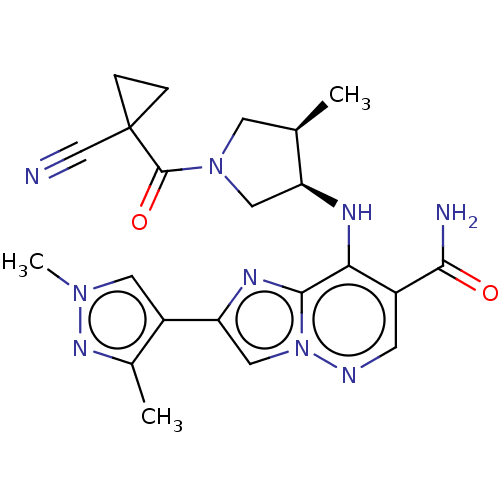 (US9428511, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245722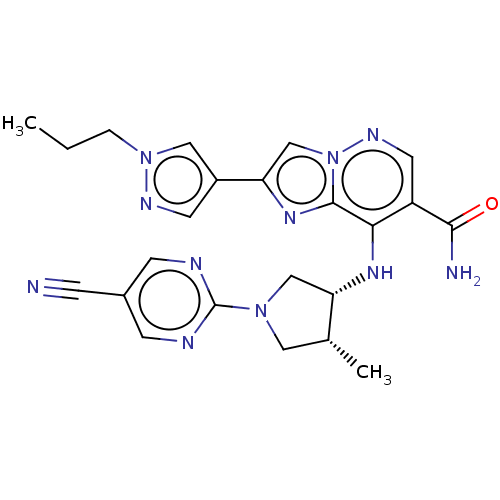 (US9428511, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272106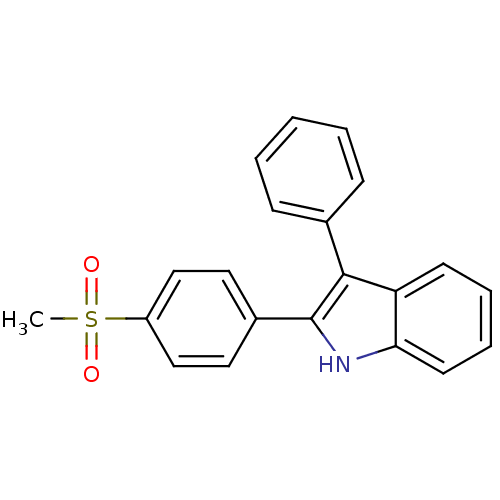 (2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-indole | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245780 (US9428511, 73 | US9428511, 74 | US9428511, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245780 (US9428511, 73 | US9428511, 74 | US9428511, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245780 (US9428511, 73 | US9428511, 74 | US9428511, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245756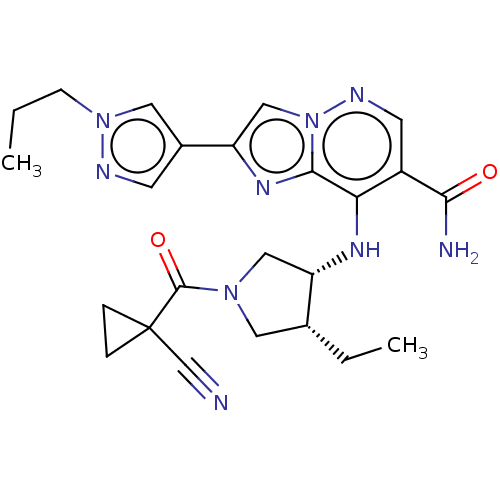 (US9428511, 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50509622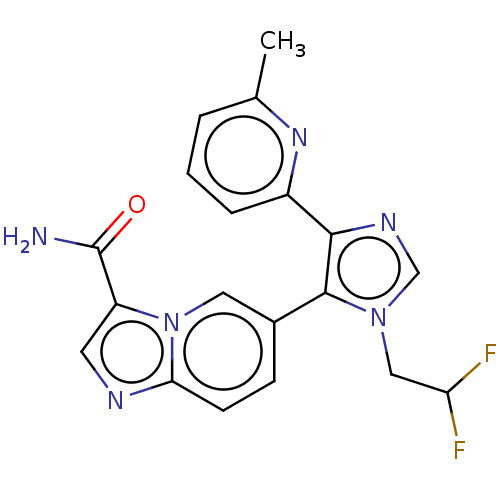 (CHEMBL4455462) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of His-tagged TGFBR1 kinase domain T204D mutant (unknown origin) incubated for 1 hr by HTRF analysis | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245753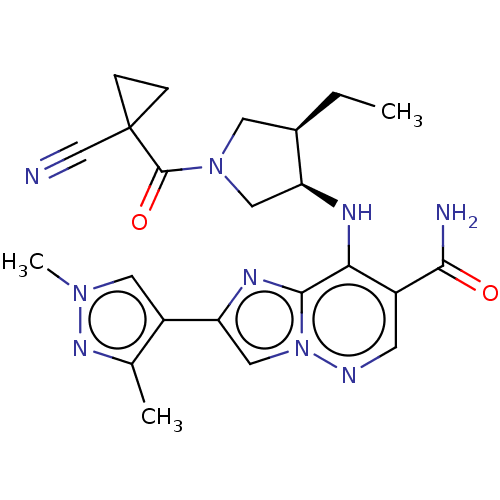 (US9428511, 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245736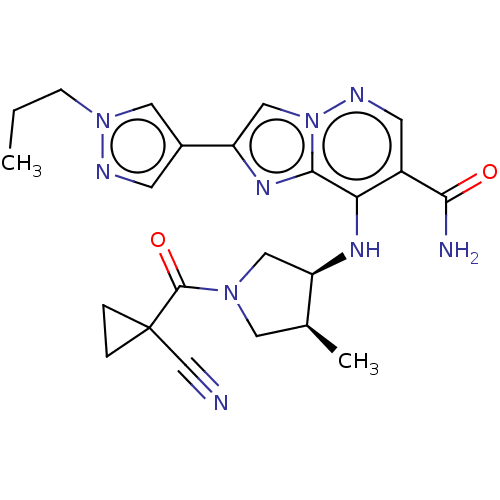 (US9428511, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245784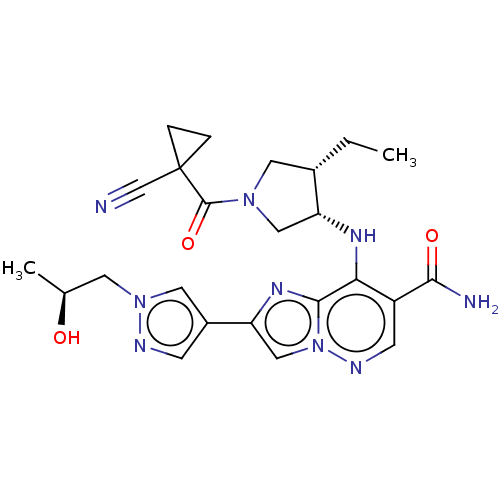 (US9428511, 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272092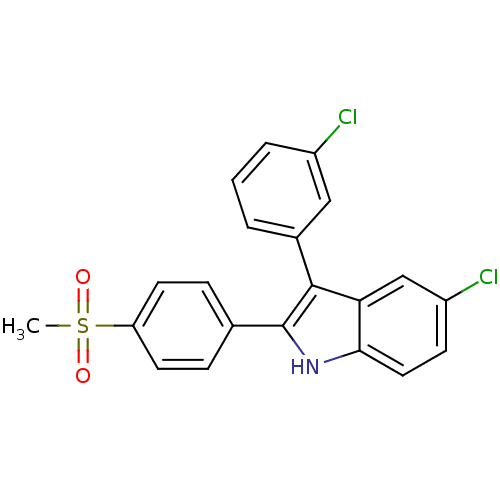 (5-chloro-3-(3-chlorophenyl)-2-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245734 (US9428511, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245776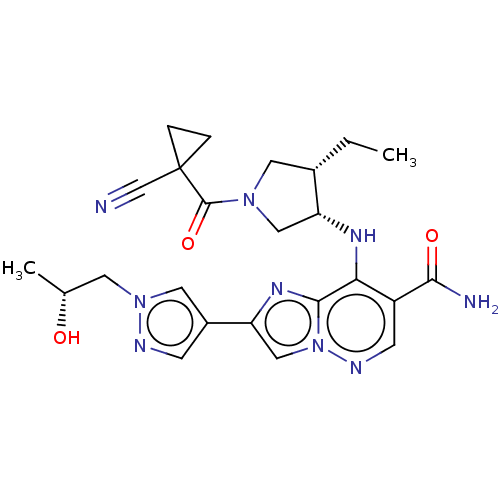 (US9428511, 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245738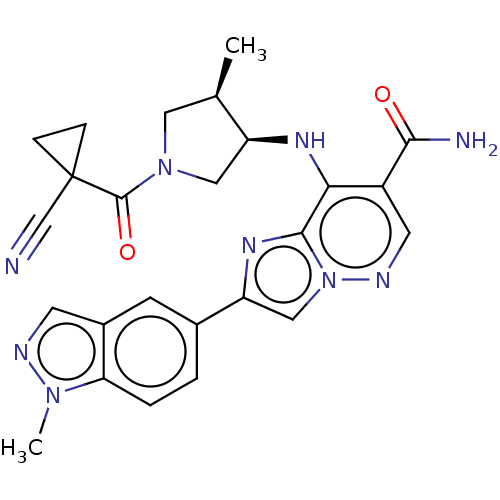 (US9428511, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245755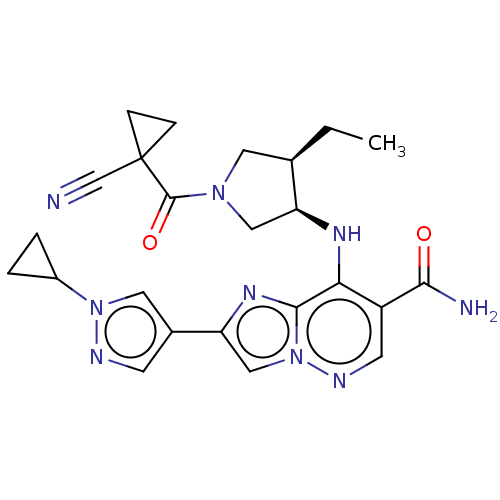 (US9428511, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50509619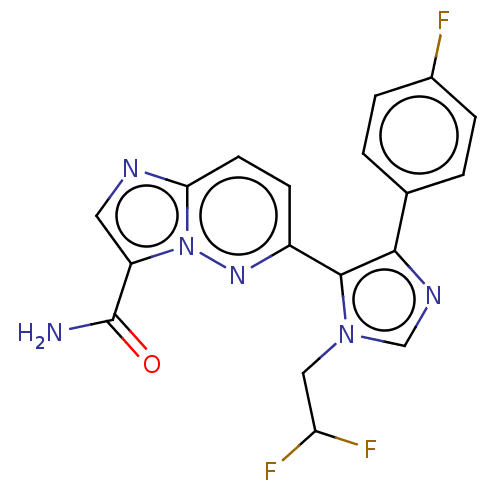 (CHEMBL4440988) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of His-tagged TGFBR1 kinase domain T204D mutant (unknown origin) incubated for 1 hr by HTRF analysis | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245743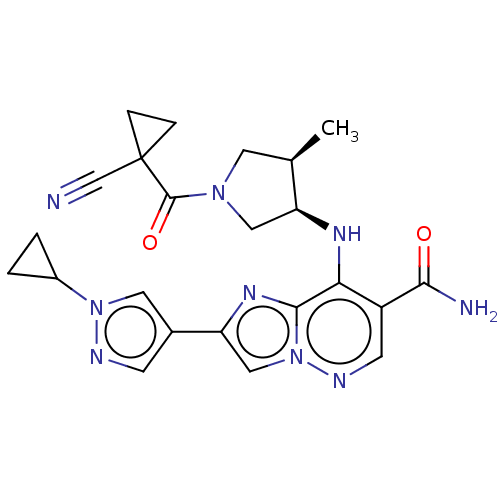 (US9428511, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245719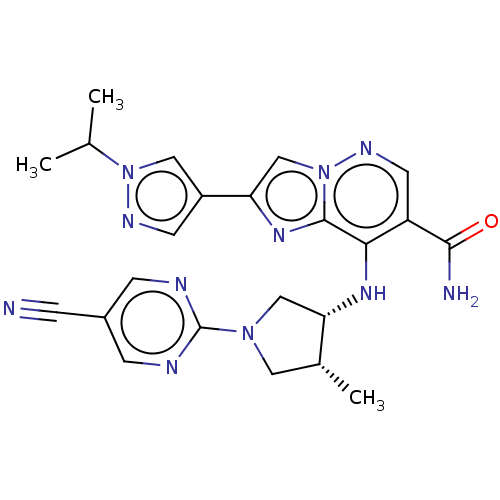 (US9428511, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245709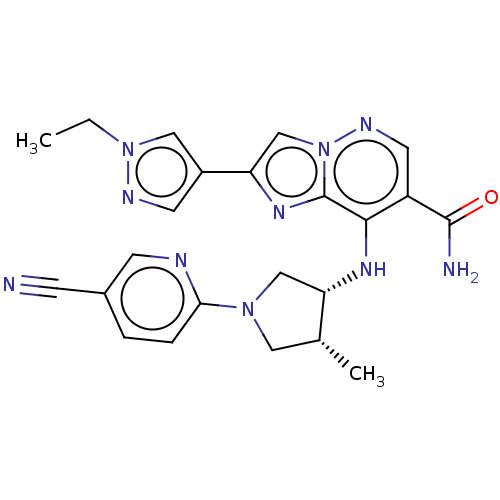 (US9428511, 1 | US9428511, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50509621 (CHEMBL4458215) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of His-tagged TGFBR1 kinase domain T204D mutant (unknown origin) incubated for 1 hr by HTRF analysis | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1609 total ) | Next | Last >> |