 Found 31 hits with Last Name = 'gupta' and Initial = 'ra'
Found 31 hits with Last Name = 'gupta' and Initial = 'ra' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272095
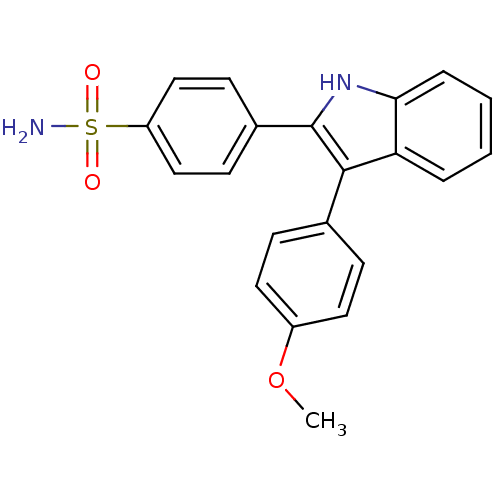
(4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-16-10-6-14(7-11-16)20-18-4-2-3-5-19(18)23-21(20)15-8-12-17(13-9-15)27(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272129
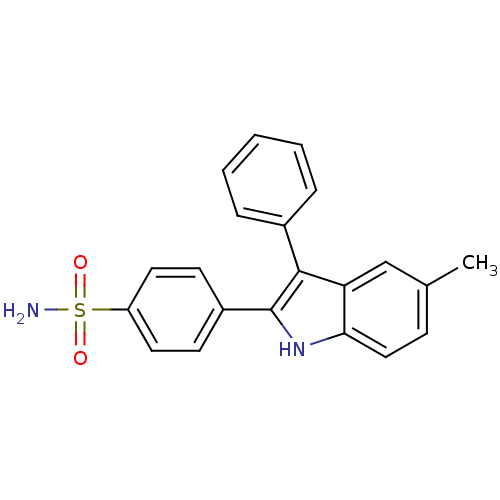
(4-(5-methyl-3-phenyl-1H-indol-2-yl)benzenesulfonam...)Show SMILES Cc1ccc2[nH]c(c(-c3ccccc3)c2c1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O2S/c1-14-7-12-19-18(13-14)20(15-5-3-2-4-6-15)21(23-19)16-8-10-17(11-9-16)26(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272131

(3-(4-fluorophenyl)-2-(4-(methylsulfonyl)phenyl)-1H...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(F)cc1 Show InChI InChI=1S/C21H16FNO2S/c1-26(24,25)17-12-8-15(9-13-17)21-20(14-6-10-16(22)11-7-14)18-4-2-3-5-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272096
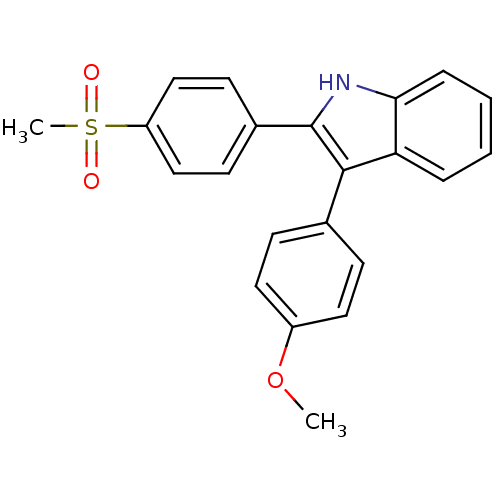
(3-(4-methoxyphenyl)-2-(4-(methylsulfonyl)phenyl)-1...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO3S/c1-26-17-11-7-15(8-12-17)21-19-5-3-4-6-20(19)23-22(21)16-9-13-18(14-10-16)27(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272125
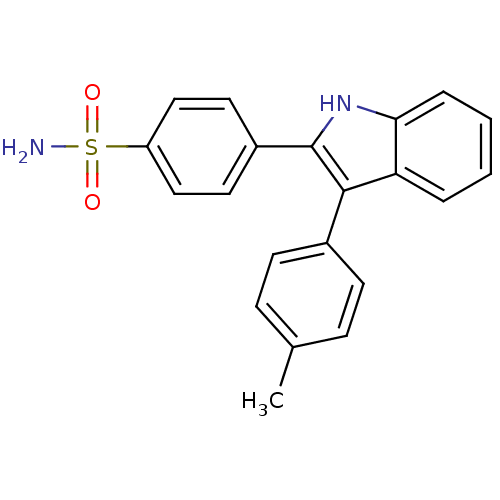
(4-(3-p-tolyl-1H-indol-2-yl)benzenesulfonamide | CH...)Show SMILES Cc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O2S/c1-14-6-8-15(9-7-14)20-18-4-2-3-5-19(18)23-21(20)16-10-12-17(13-11-16)26(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272105
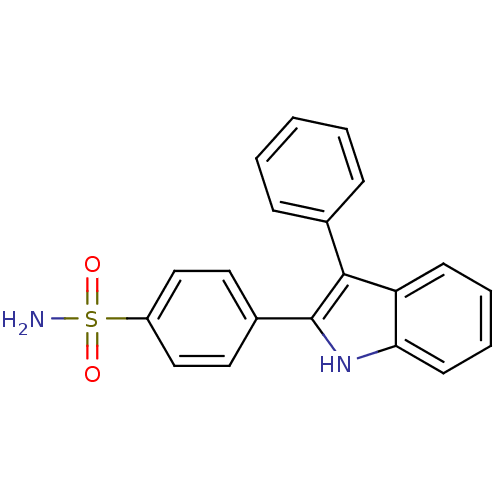
(4-(3-phenyl-1H-indol-2-yl)benzenesulfonamide | CHE...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C20H16N2O2S/c21-25(23,24)16-12-10-15(11-13-16)20-19(14-6-2-1-3-7-14)17-8-4-5-9-18(17)22-20/h1-13,22H,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272126
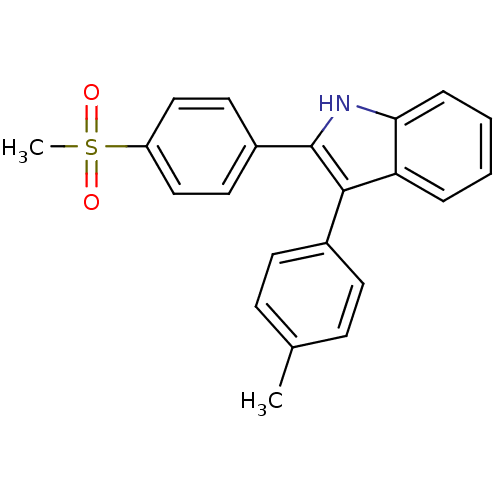
(2-(4-(methylsulfonyl)phenyl)-3-p-tolyl-1H-indole |...)Show SMILES Cc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO2S/c1-15-7-9-16(10-8-15)21-19-5-3-4-6-20(19)23-22(21)17-11-13-18(14-12-17)26(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272110
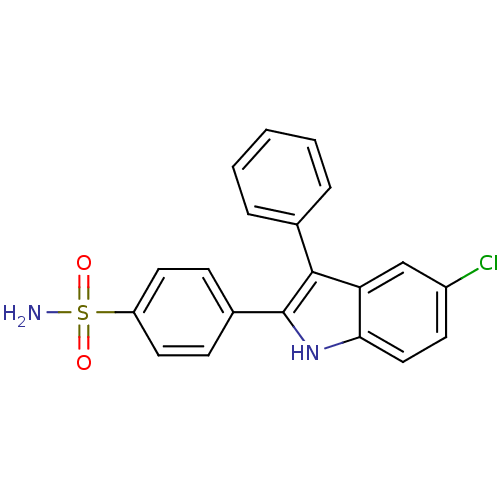
(4-(5-chloro-3-phenyl-1H-indol-2-yl)benzenesulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccccc1 Show InChI InChI=1S/C20H15ClN2O2S/c21-15-8-11-18-17(12-15)19(13-4-2-1-3-5-13)20(23-18)14-6-9-16(10-7-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272097
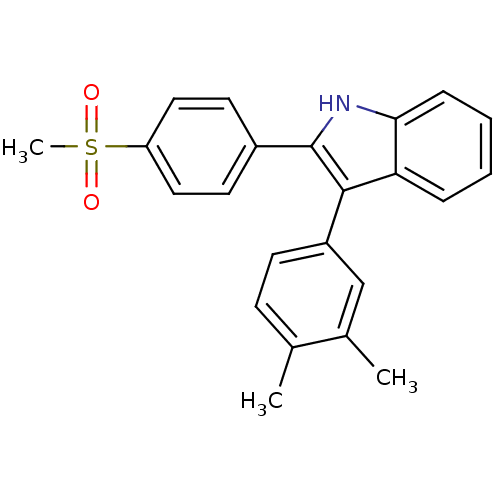
(3-(3,4-dimethylphenyl)-2-(4-(methylsulfonyl)phenyl...)Show SMILES Cc1ccc(cc1C)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C23H21NO2S/c1-15-8-9-18(14-16(15)2)22-20-6-4-5-7-21(20)24-23(22)17-10-12-19(13-11-17)27(3,25)26/h4-14,24H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272128
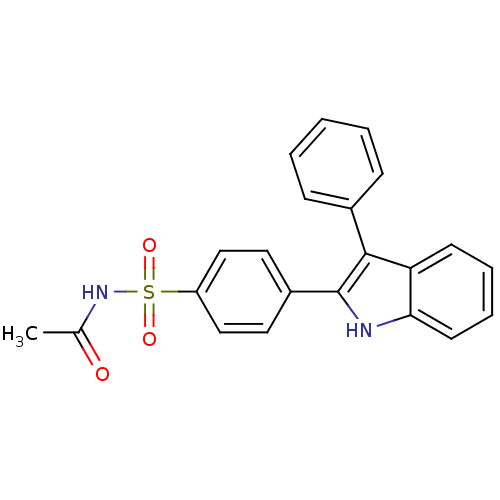
(CHEMBL500943 | N-(4-(3-phenyl-1H-indol-2-yl)phenyl...)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C22H18N2O3S/c1-15(25)24-28(26,27)18-13-11-17(12-14-18)22-21(16-7-3-2-4-8-16)19-9-5-6-10-20(19)23-22/h2-14,23H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272130
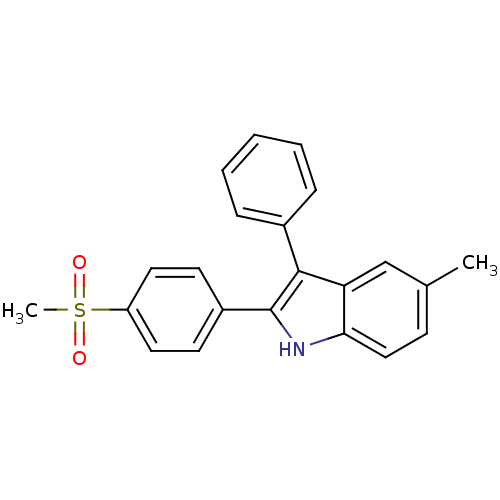
(5-methyl-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...)Show SMILES Cc1ccc2[nH]c(c(-c3ccccc3)c2c1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO2S/c1-15-8-13-20-19(14-15)21(16-6-4-3-5-7-16)22(23-20)17-9-11-18(12-10-17)26(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272111
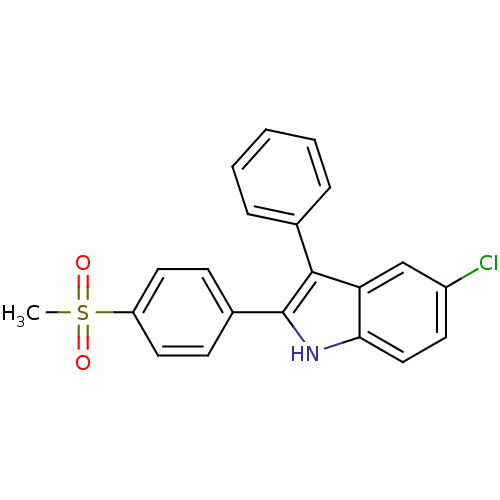
(5-chloro-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccccc1 Show InChI InChI=1S/C21H16ClNO2S/c1-26(24,25)17-10-7-15(8-11-17)21-20(14-5-3-2-4-6-14)18-13-16(22)9-12-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272124
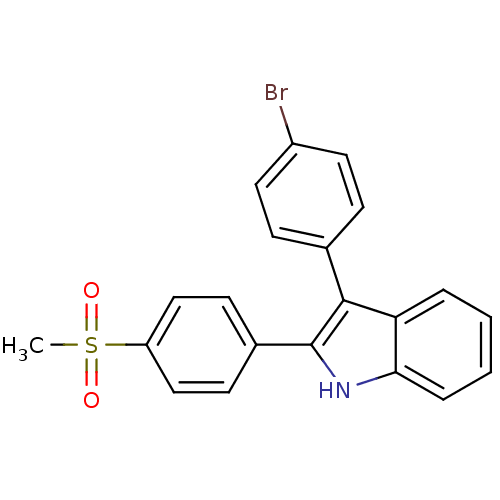
(3-(4-bromophenyl)-2-(4-(methylsulfonyl)phenyl)-1H-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrNO2S/c1-26(24,25)17-12-8-15(9-13-17)21-20(14-6-10-16(22)11-7-14)18-4-2-3-5-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272090

(4-(5-chloro-3-(4-chlorophenyl)-1H-indol-2-yl)benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H14Cl2N2O2S/c21-14-5-1-12(2-6-14)19-17-11-15(22)7-10-18(17)24-20(19)13-3-8-16(9-4-13)27(23,25)26/h1-11,24H,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272106
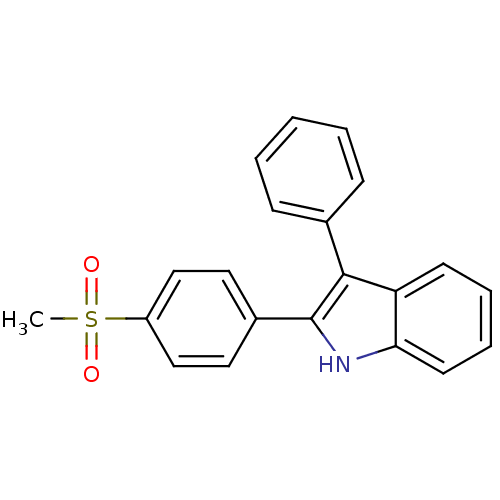
(2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-indole | ...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C21H17NO2S/c1-25(23,24)17-13-11-16(12-14-17)21-20(15-7-3-2-4-8-15)18-9-5-6-10-19(18)22-21/h2-14,22H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272092
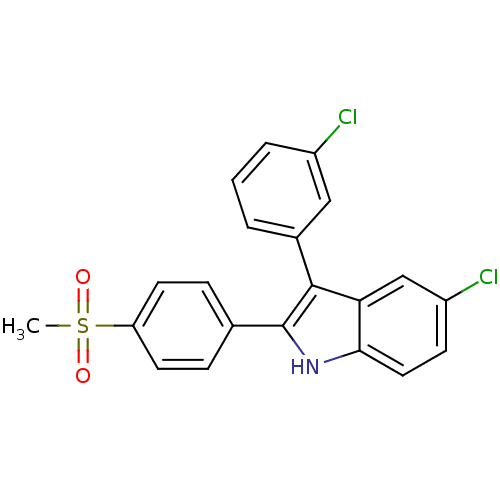
(5-chloro-3-(3-chlorophenyl)-2-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1cccc(Cl)c1 Show InChI InChI=1S/C21H15Cl2NO2S/c1-27(25,26)17-8-5-13(6-9-17)21-20(14-3-2-4-15(22)11-14)18-12-16(23)7-10-19(18)24-21/h2-12,24H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272113
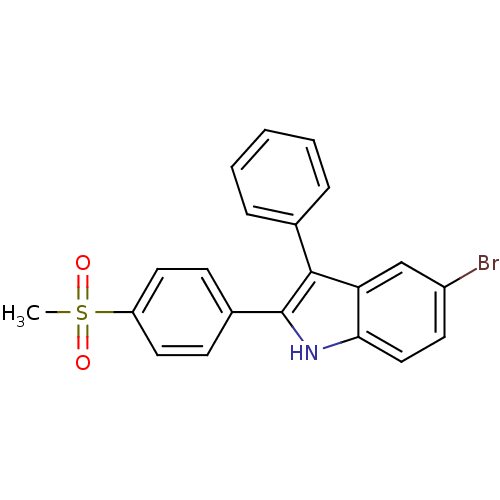
(5-bromo-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-i...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Br)cc2c1-c1ccccc1 Show InChI InChI=1S/C21H16BrNO2S/c1-26(24,25)17-10-7-15(8-11-17)21-20(14-5-3-2-4-6-14)18-13-16(22)9-12-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272127

(4-(3-(3,4-dimethylphenyl)-1H-indol-2-yl)benzenesul...)Show SMILES Cc1ccc(cc1C)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H20N2O2S/c1-14-7-8-17(13-15(14)2)21-19-5-3-4-6-20(19)24-22(21)16-9-11-18(12-10-16)27(23,25)26/h3-13,24H,1-2H3,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272109
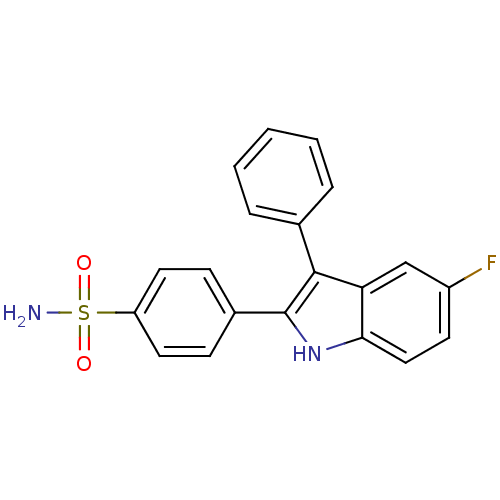
(4-(5-fluoro-3-phenyl-1H-indol-2-yl)benzenesulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(F)cc2c1-c1ccccc1 Show InChI InChI=1S/C20H15FN2O2S/c21-15-8-11-18-17(12-15)19(13-4-2-1-3-5-13)20(23-18)14-6-9-16(10-7-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272114
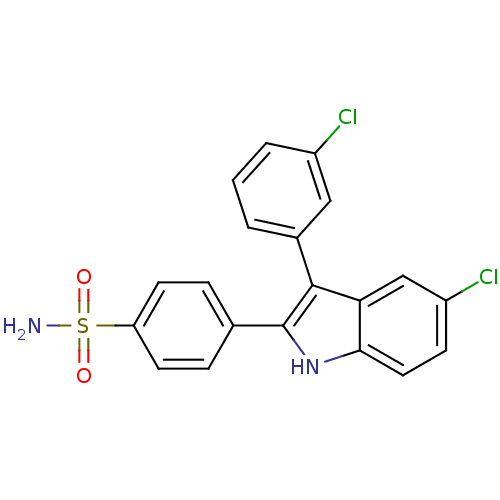
(4-(5-chloro-3-(3-chlorophenyl)-1H-indol-2-yl)benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1cccc(Cl)c1 Show InChI InChI=1S/C20H14Cl2N2O2S/c21-14-3-1-2-13(10-14)19-17-11-15(22)6-9-18(17)24-20(19)12-4-7-16(8-5-12)27(23,25)26/h1-11,24H,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272115
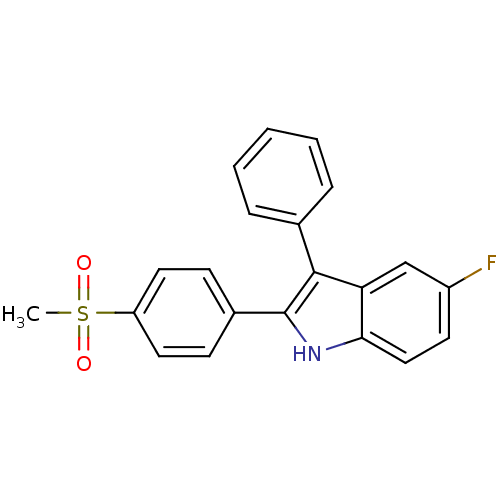
(5-fluoro-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(F)cc2c1-c1ccccc1 Show InChI InChI=1S/C21H16FNO2S/c1-26(24,25)17-10-7-15(8-11-17)21-20(14-5-3-2-4-6-14)18-13-16(22)9-12-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272112
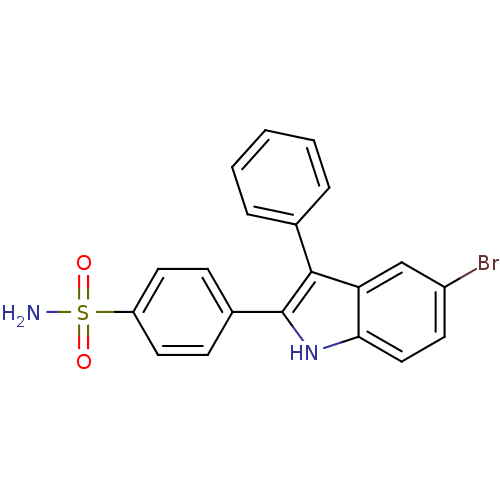
(4-(5-bromo-3-phenyl-1H-indol-2-yl)benzenesulfonami...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Br)cc2c1-c1ccccc1 Show InChI InChI=1S/C20H15BrN2O2S/c21-15-8-11-18-17(12-15)19(13-4-2-1-3-5-13)20(23-18)14-6-9-16(10-7-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272107
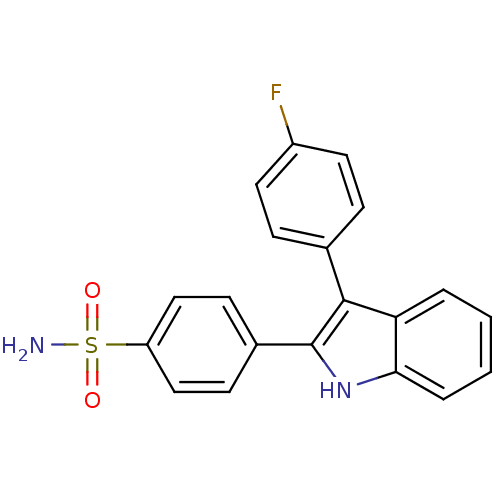
(4-(3-(4-fluorophenyl)-1H-indol-2-yl)benzenesulfona...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(F)cc1 Show InChI InChI=1S/C20H15FN2O2S/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)23-20(19)14-7-11-16(12-8-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272098
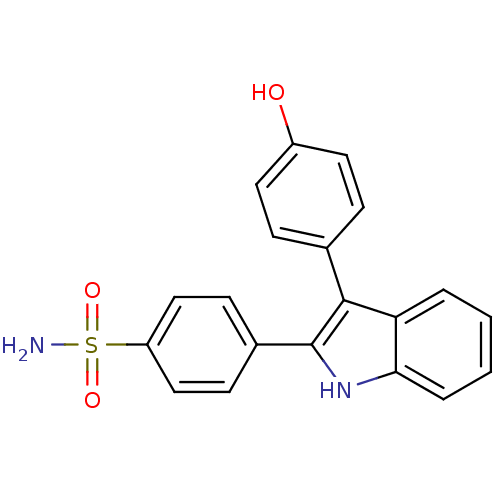
(4-(3-(4-hydroxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(O)cc1 Show InChI InChI=1S/C20H16N2O3S/c21-26(24,25)16-11-7-14(8-12-16)20-19(13-5-9-15(23)10-6-13)17-3-1-2-4-18(17)22-20/h1-12,22-23H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272133
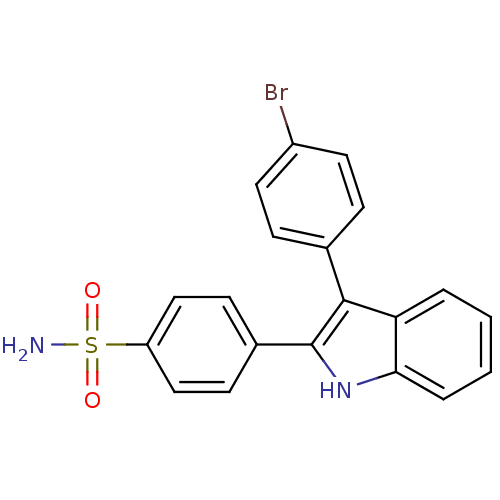
(4-(3-(4-bromophenyl)-1H-indol-2-yl)benzenesulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(Br)cc1 Show InChI InChI=1S/C20H15BrN2O2S/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)23-20(19)14-7-11-16(12-8-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272094
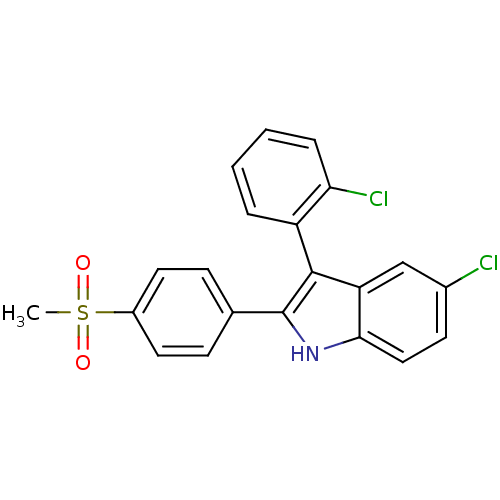
(5-chloro-3-(2-chlorophenyl)-2-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccccc1Cl |(18.72,-39.64,;17.18,-39.64,;17.18,-38.1,;17.19,-41.18,;15.64,-39.64,;14.87,-40.97,;13.33,-40.97,;12.57,-39.64,;13.33,-38.31,;14.86,-38.3,;11.03,-39.64,;10.12,-40.9,;8.64,-40.42,;7.31,-41.19,;5.98,-40.42,;5.98,-38.87,;4.88,-37.77,;7.31,-38.1,;8.64,-38.87,;10.12,-38.39,;10.9,-37.06,;12.44,-37.09,;13.23,-35.77,;12.48,-34.42,;10.93,-34.41,;10.15,-35.73,;8.61,-35.72,)| Show InChI InChI=1S/C21H15Cl2NO2S/c1-27(25,26)15-9-6-13(7-10-15)21-20(16-4-2-3-5-18(16)23)17-12-14(22)8-11-19(17)24-21/h2-12,24H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272108
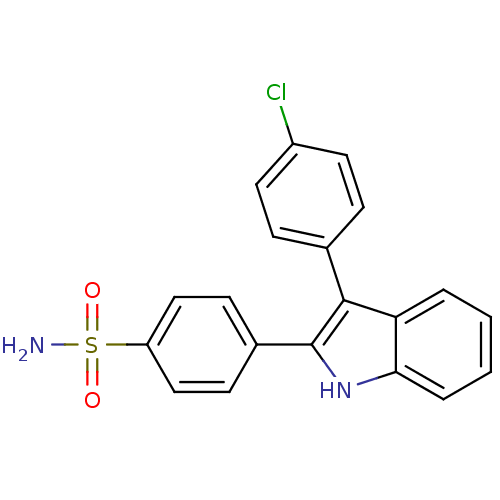
(4-(3-(4-chlorophenyl)-1H-indol-2-yl)benzenesulfona...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H15ClN2O2S/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)23-20(19)14-7-11-16(12-8-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272091
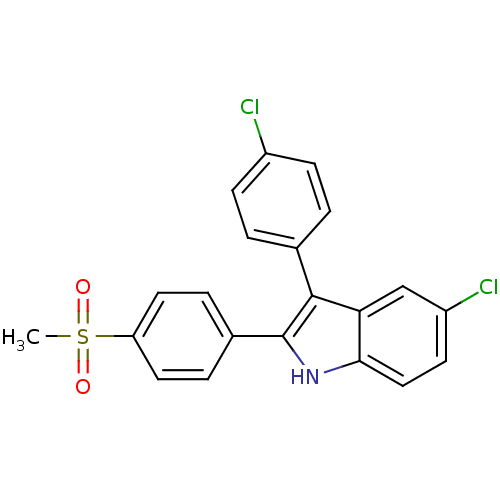
(5-chloro-3-(4-chlorophenyl)-2-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H15Cl2NO2S/c1-27(25,26)17-9-4-14(5-10-17)21-20(13-2-6-15(22)7-3-13)18-12-16(23)8-11-19(18)24-21/h2-12,24H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272093
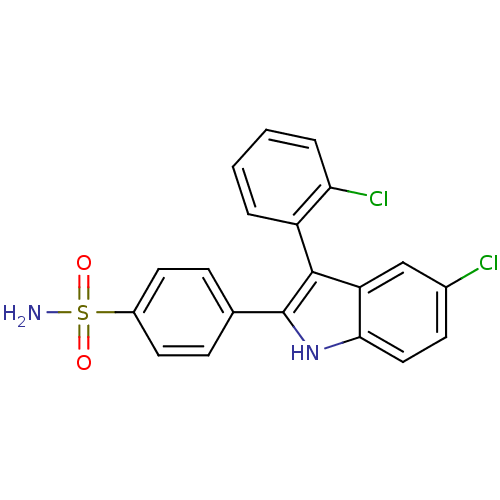
(4-(5-chloro-3-(2-chlorophenyl)-1H-indol-2-yl)benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccccc1Cl |(3.29,-39.95,;1.75,-39.95,;1.75,-38.41,;1.76,-41.49,;.21,-39.95,;-.56,-41.29,;-2.1,-41.29,;-2.86,-39.95,;-2.1,-38.62,;-.57,-38.61,;-4.4,-39.95,;-5.31,-41.21,;-6.79,-40.73,;-8.12,-41.5,;-9.45,-40.73,;-9.45,-39.18,;-10.8,-38.41,;-8.12,-38.41,;-6.79,-39.18,;-5.31,-38.7,;-4.53,-37.38,;-2.99,-37.4,;-2.2,-36.08,;-2.95,-34.73,;-4.5,-34.72,;-5.28,-36.04,;-6.82,-36.03,)| Show InChI InChI=1S/C20H14Cl2N2O2S/c21-13-7-10-18-16(11-13)19(15-3-1-2-4-17(15)22)20(24-18)12-5-8-14(9-6-12)27(23,25)26/h1-11,24H,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272132
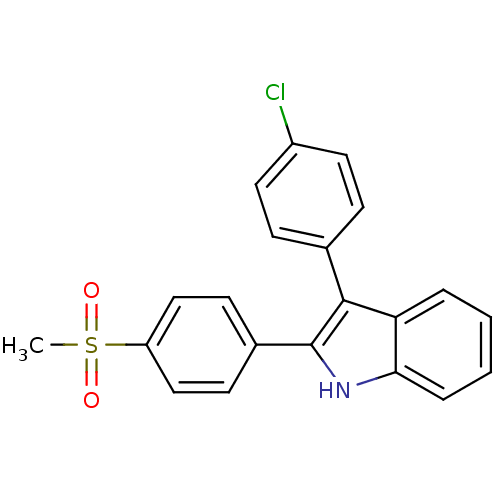
(3-(4-chlorophenyl)-2-(4-(methylsulfonyl)phenyl)-1H...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H16ClNO2S/c1-26(24,25)17-12-8-15(9-13-17)21-20(14-6-10-16(22)11-7-14)18-4-2-3-5-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data