 Found 1138 hits with Last Name = 'ha' and Initial = 'ym'
Found 1138 hits with Last Name = 'ha' and Initial = 'ym' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
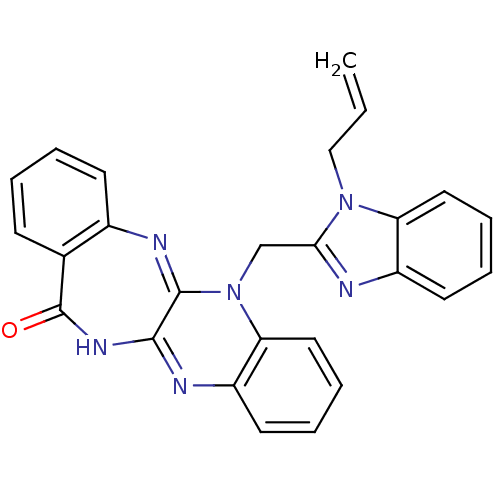
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
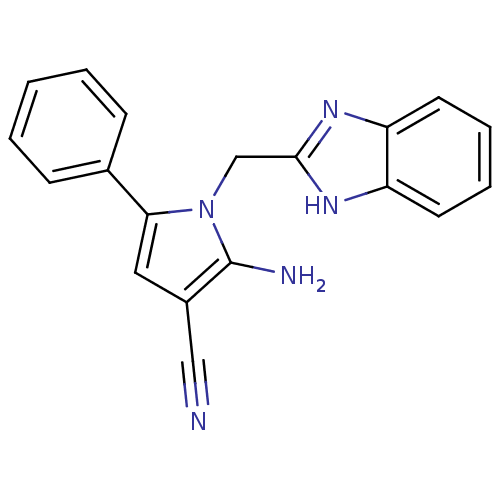
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
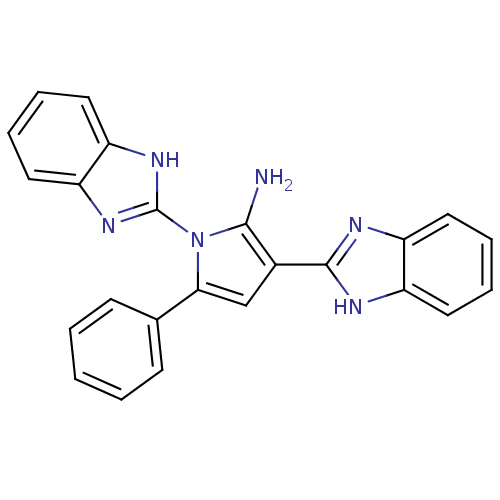
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
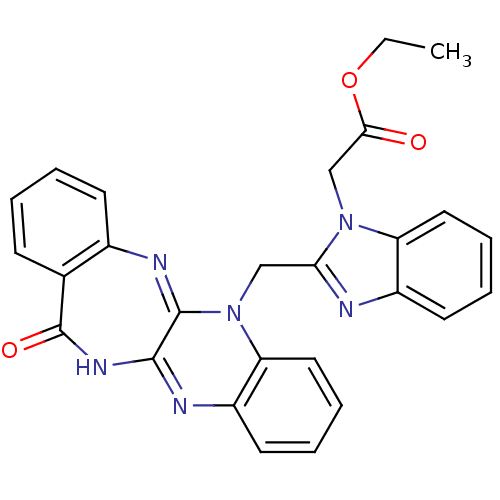
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
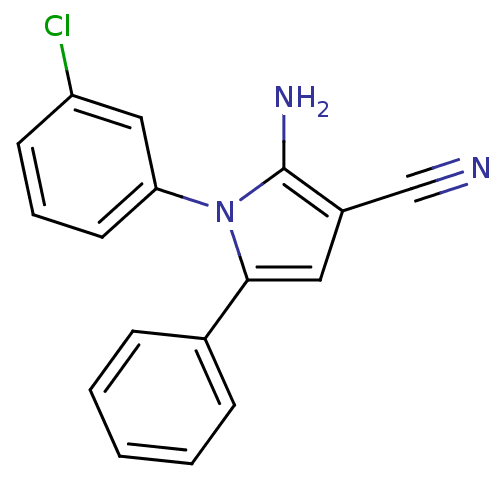
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
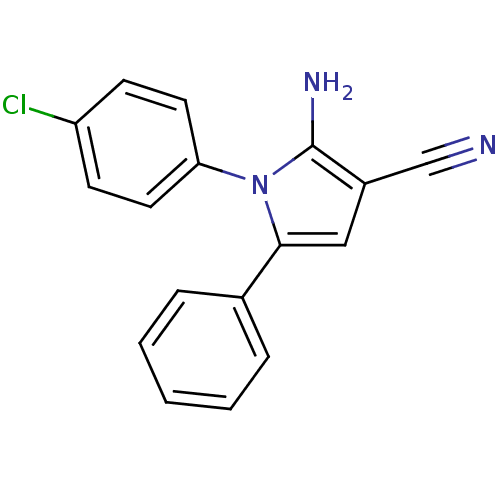
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
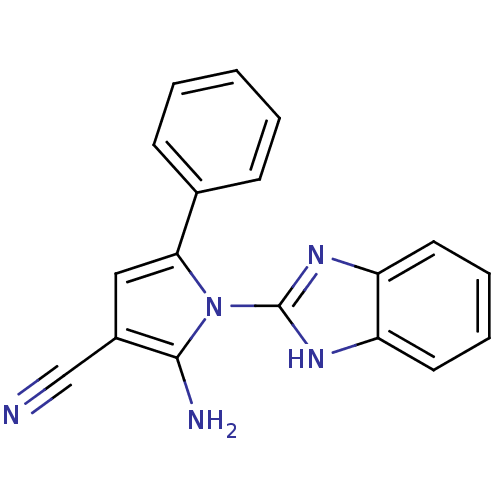
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
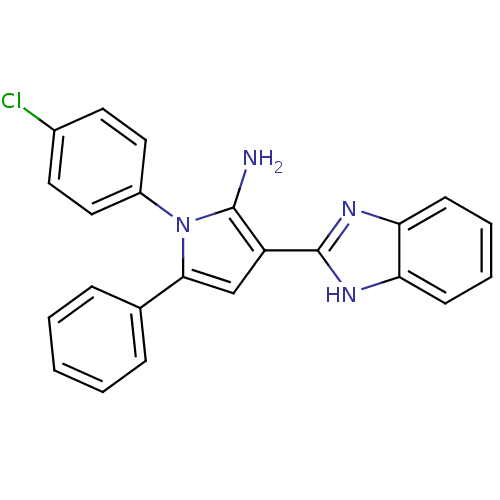
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
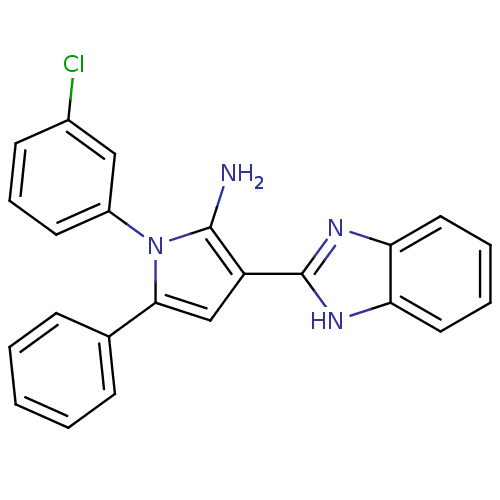
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
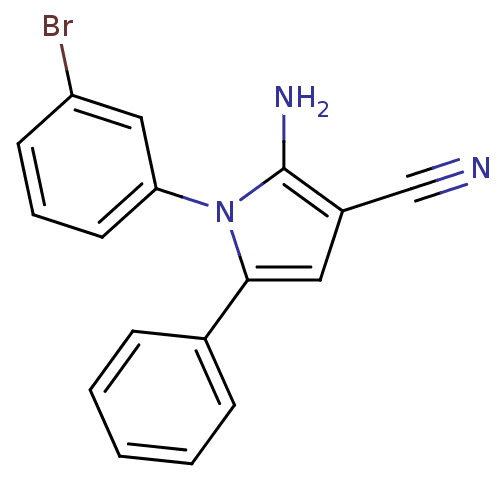
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
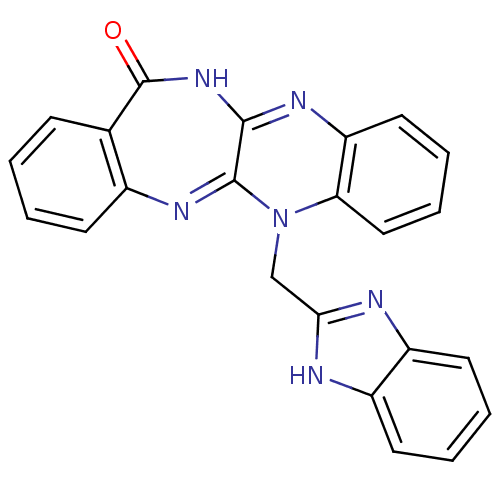
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
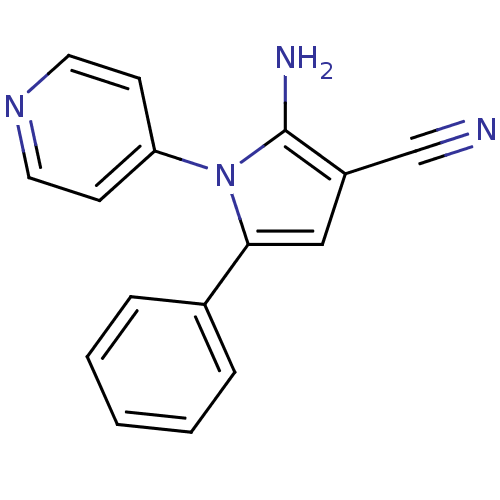
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50343133
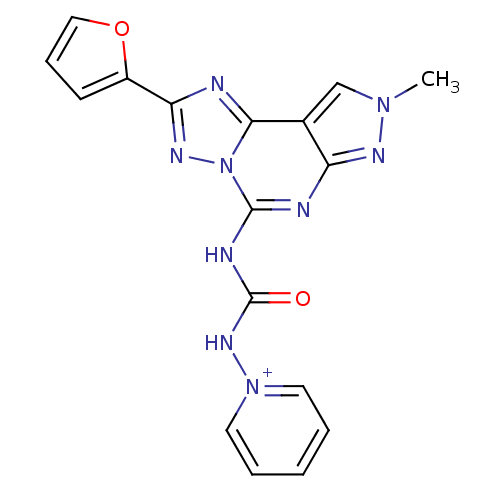
(1-(3-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1...)Show SMILES Cn1cc2c(n1)nc(NC(=O)N[n+]1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C17H13N9O2/c1-24-10-11-13(21-24)19-16(20-17(27)23-25-7-3-2-4-8-25)26-15(11)18-14(22-26)12-6-5-9-28-12/h2-10H,1H3,(H-,19,20,21,23,27)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109481
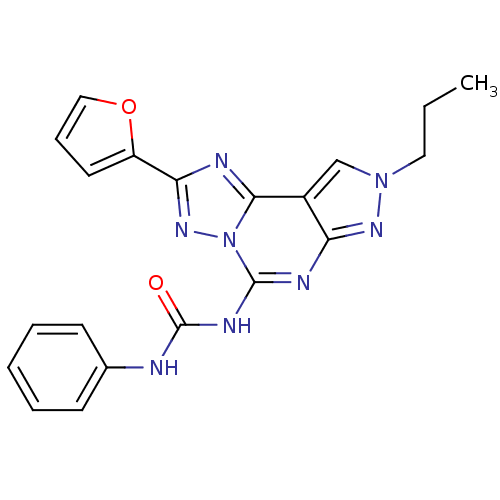
(1-(2-(furan-2-yl)-8-propyl-8H-pyrazolo[4,3-e][1,2,...)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Nc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H18N8O2/c1-2-10-27-12-14-16(25-27)23-19(24-20(29)21-13-7-4-3-5-8-13)28-18(14)22-17(26-28)15-9-6-11-30-15/h3-9,11-12H,2,10H2,1H3,(H2,21,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109461
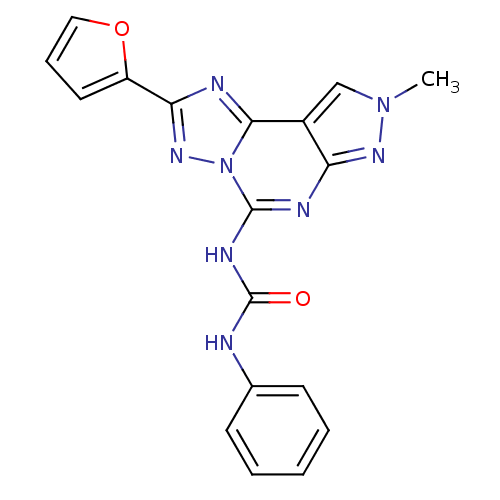
(1-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1,2,...)Show SMILES Cn1cc2c(n1)nc(NC(=O)Nc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C18H14N8O2/c1-25-10-12-14(23-25)21-17(22-18(27)19-11-6-3-2-4-7-11)26-16(12)20-15(24-26)13-8-5-9-28-13/h2-10H,1H3,(H2,19,21,22,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109473
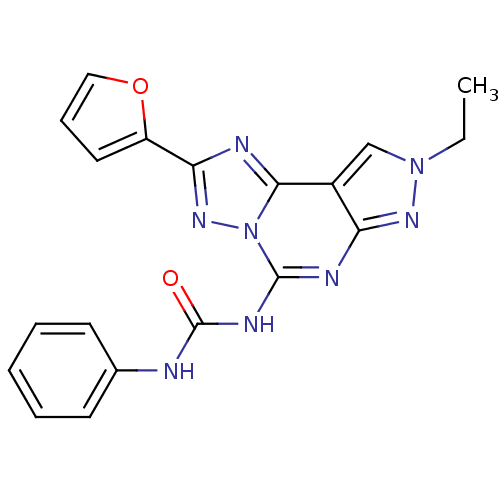
(1-(8-Ethyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES CCn1cc2c(n1)nc(NC(=O)Nc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C19H16N8O2/c1-2-26-11-13-15(24-26)22-18(23-19(28)20-12-7-4-3-5-8-12)27-17(13)21-16(25-27)14-9-6-10-29-14/h3-11H,2H2,1H3,(H2,20,22,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50094691
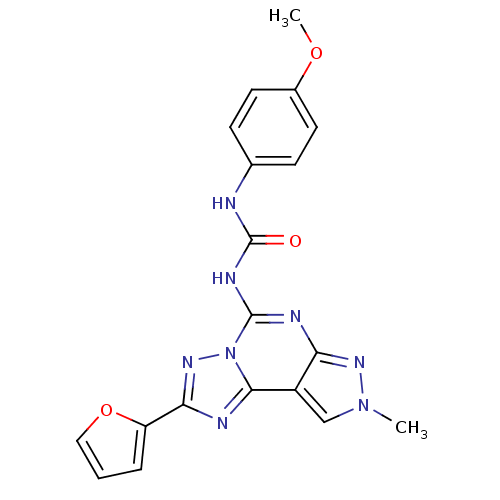
(1-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1,2,...)Show SMILES COc1ccc(NC(=O)Nc2nc3nn(C)cc3c3nc(nn23)-c2ccco2)cc1 Show InChI InChI=1S/C19H16N8O3/c1-26-10-13-15(24-26)22-18(23-19(28)20-11-5-7-12(29-2)8-6-11)27-17(13)21-16(25-27)14-4-3-9-30-14/h3-10H,1-2H3,(H2,20,22,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109456
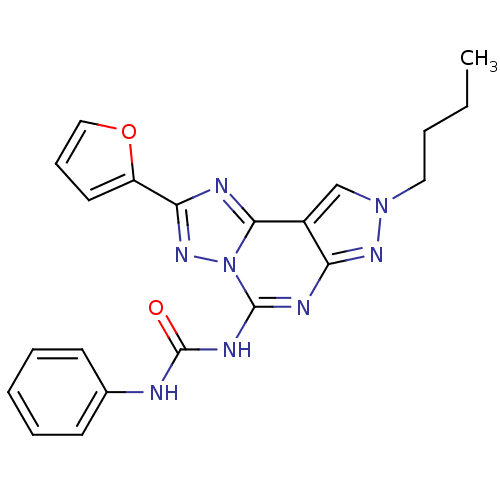
(1-(8-Butyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Nc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H20N8O2/c1-2-3-11-28-13-15-17(26-28)24-20(25-21(30)22-14-8-5-4-6-9-14)29-19(15)23-18(27-29)16-10-7-12-31-16/h4-10,12-13H,2-3,11H2,1H3,(H2,22,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109441
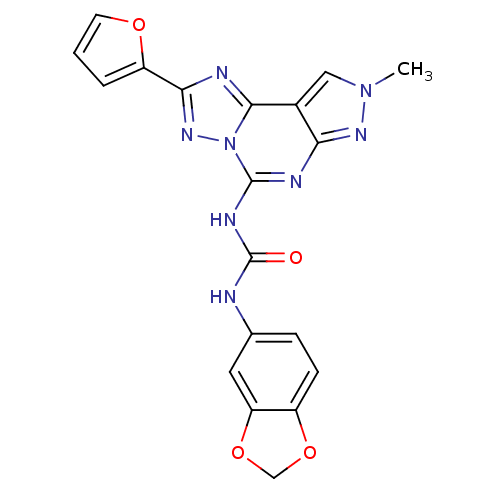
(1-(benzo[d][1,3]dioxol-5-yl)-3-(2-(furan-2-yl)-8-m...)Show SMILES Cn1cc2c(n1)nc(NC(=O)Nc1ccc3OCOc3c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C19H14N8O4/c1-26-8-11-15(24-26)22-18(27-17(11)21-16(25-27)13-3-2-6-29-13)23-19(28)20-10-4-5-12-14(7-10)31-9-30-12/h2-8H,9H2,1H3,(H2,20,22,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109475
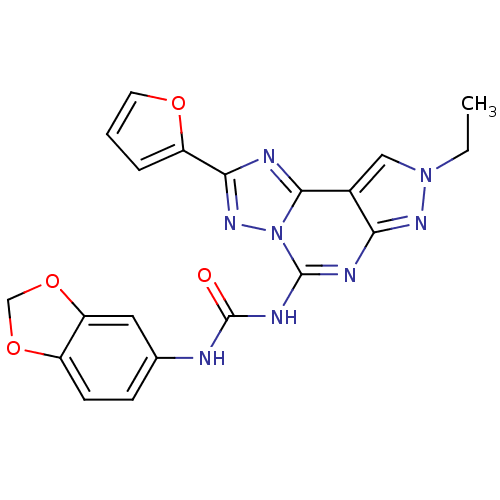
(1-(benzo[d][1,3]dioxol-5-yl)-3-(8-ethyl-2-(furan-2...)Show SMILES CCn1cc2c(n1)nc(NC(=O)Nc1ccc3OCOc3c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H16N8O4/c1-2-27-9-12-16(25-27)23-19(28-18(12)22-17(26-28)14-4-3-7-30-14)24-20(29)21-11-5-6-13-15(8-11)32-10-31-13/h3-9H,2,10H2,1H3,(H2,21,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402379
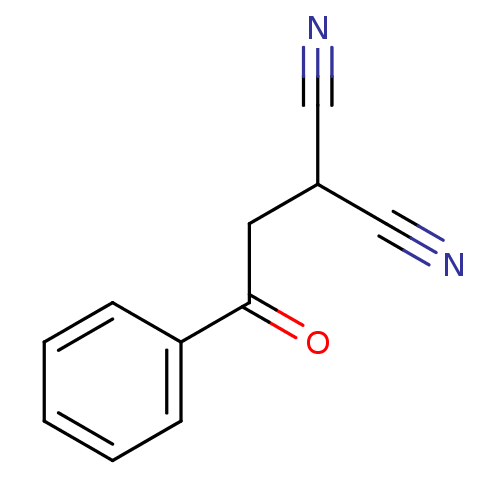
(CHEMBL2206686)Show InChI InChI=1S/C11H8N2O/c12-7-9(8-13)6-11(14)10-4-2-1-3-5-10/h1-5,9H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109446
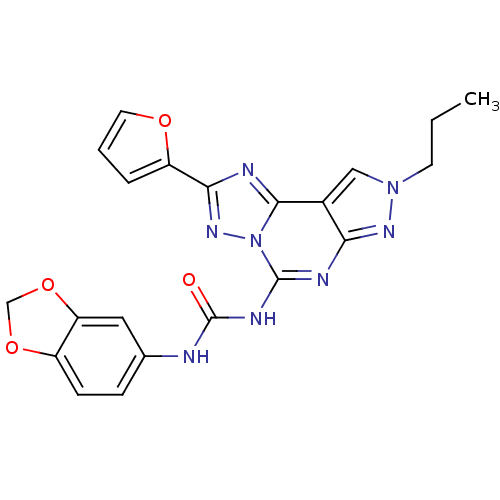
(1-(benzo[d][1,3]dioxol-5-yl)-3-(2-(furan-2-yl)-8-p...)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Nc1ccc3OCOc3c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H18N8O4/c1-2-7-28-10-13-17(26-28)24-20(29-19(13)23-18(27-29)15-4-3-8-31-15)25-21(30)22-12-5-6-14-16(9-12)33-11-32-14/h3-6,8-10H,2,7,11H2,1H3,(H2,22,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50094687

(1-(8-Butyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Nc1ccc(OC)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C22H22N8O3/c1-3-4-11-29-13-16-18(27-29)25-21(26-22(31)23-14-7-9-15(32-2)10-8-14)30-20(16)24-19(28-30)17-6-5-12-33-17/h5-10,12-13H,3-4,11H2,1-2H3,(H2,23,25,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109437
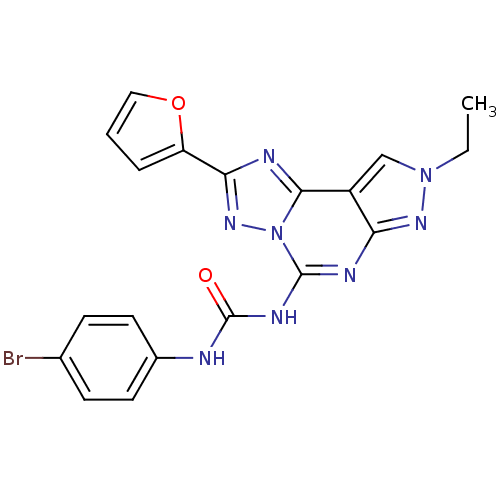
(1-(4-Bromo-phenyl)-3-(8-ethyl-2-furan-2-yl-8H-pyra...)Show SMILES CCn1cc2c(n1)nc(NC(=O)Nc1ccc(Br)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C19H15BrN8O2/c1-2-27-10-13-15(25-27)23-18(24-19(29)21-12-7-5-11(20)6-8-12)28-17(13)22-16(26-28)14-4-3-9-30-14/h3-10H,2H2,1H3,(H2,21,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50094690
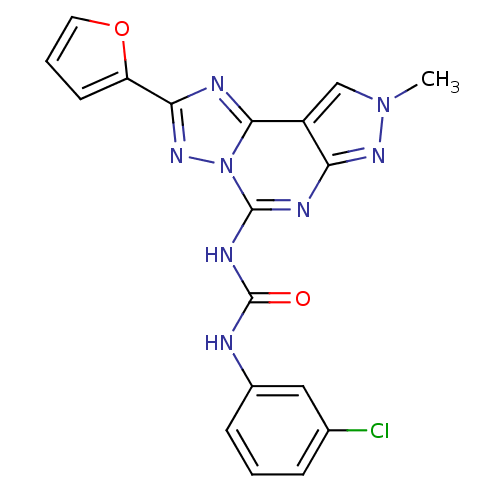
(1-(3-Chloro-phenyl)-3-(2-furan-2-yl-8-methyl-8H-py...)Show SMILES Cn1cc2c(n1)nc(NC(=O)Nc1cccc(Cl)c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C18H13ClN8O2/c1-26-9-12-14(24-26)22-17(23-18(28)20-11-5-2-4-10(19)8-11)27-16(12)21-15(25-27)13-6-3-7-29-13/h2-9H,1H3,(H2,20,22,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109451

(1-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1,2,...)Show SMILES Cn1cc2c(n1)nc(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C18H13N9O4/c1-25-9-12-14(23-25)21-17(26-16(12)20-15(24-26)13-3-2-8-31-13)22-18(28)19-10-4-6-11(7-5-10)27(29)30/h2-9H,1H3,(H2,19,21,22,23,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109435
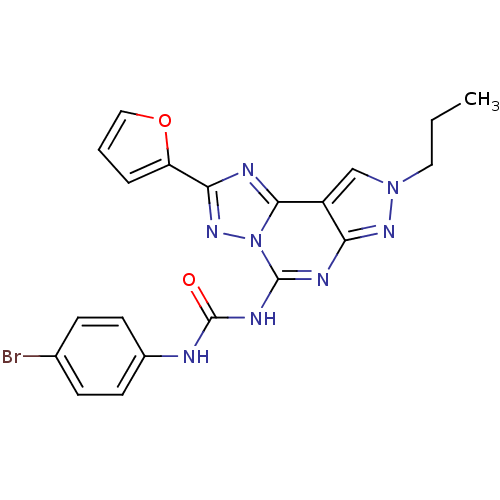
(1-(4-Bromo-phenyl)-3-(2-furan-2-yl-8-propyl-8H-pyr...)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Nc1ccc(Br)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H17BrN8O2/c1-2-9-28-11-14-16(26-28)24-19(25-20(30)22-13-7-5-12(21)6-8-13)29-18(14)23-17(27-29)15-4-3-10-31-15/h3-8,10-11H,2,9H2,1H3,(H2,22,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109477
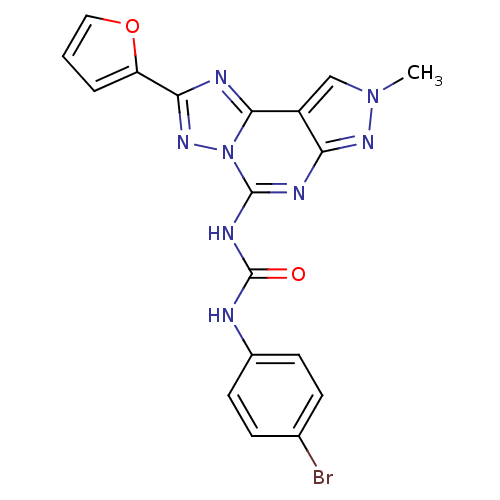
(1-(4-Bromo-phenyl)-3-(2-furan-2-yl-8-methyl-8H-pyr...)Show SMILES Cn1cc2c(n1)nc(NC(=O)Nc1ccc(Br)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C18H13BrN8O2/c1-26-9-12-14(24-26)22-17(23-18(28)20-11-6-4-10(19)5-7-11)27-16(12)21-15(25-27)13-3-2-8-29-13/h2-9H,1H3,(H2,20,22,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109484
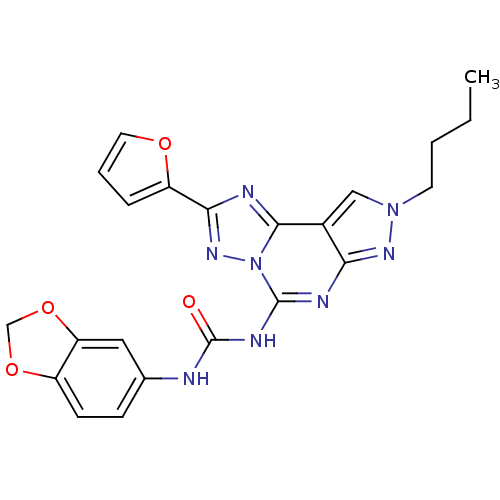
(1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Nc1ccc3OCOc3c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C22H20N8O4/c1-2-3-8-29-11-14-18(27-29)25-21(30-20(14)24-19(28-30)16-5-4-9-32-16)26-22(31)23-13-6-7-15-17(10-13)34-12-33-15/h4-7,9-11H,2-3,8,12H2,1H3,(H2,23,25,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109467
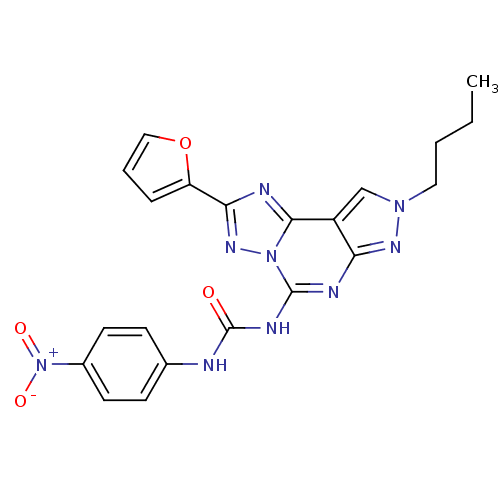
(1-(8-Butyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H19N9O4/c1-2-3-10-28-12-15-17(26-28)24-20(29-19(15)23-18(27-29)16-5-4-11-34-16)25-21(31)22-13-6-8-14(9-7-13)30(32)33/h4-9,11-12H,2-3,10H2,1H3,(H2,22,24,25,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50082418
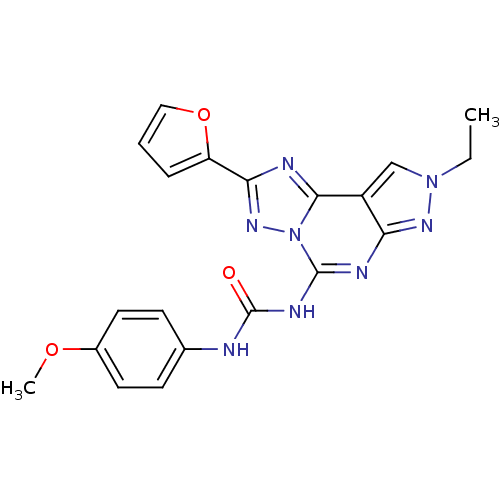
(1-(8-ethyl-2-(furan-2-yl)-8H-pyrazolo[4,3-e][1,2,4...)Show SMILES CCn1cc2c(n1)nc(NC(=O)Nc1ccc(OC)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H18N8O3/c1-3-27-11-14-16(25-27)23-19(24-20(29)21-12-6-8-13(30-2)9-7-12)28-18(14)22-17(26-28)15-5-4-10-31-15/h4-11H,3H2,1-2H3,(H2,21,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50094699
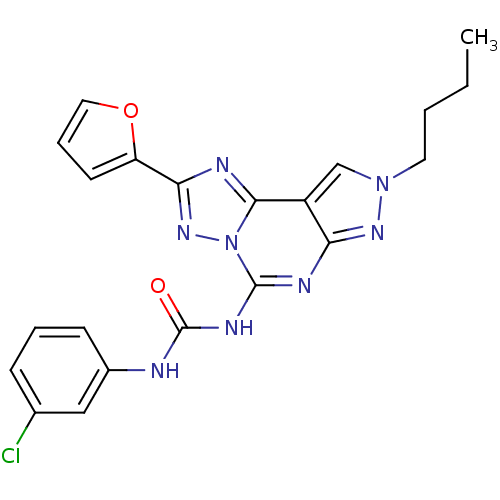
(1-(8-Butyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Nc1cccc(Cl)c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H19ClN8O2/c1-2-3-9-29-12-15-17(27-29)25-20(26-21(31)23-14-7-4-6-13(22)11-14)30-19(15)24-18(28-30)16-8-5-10-32-16/h4-8,10-12H,2-3,9H2,1H3,(H2,23,25,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50053929
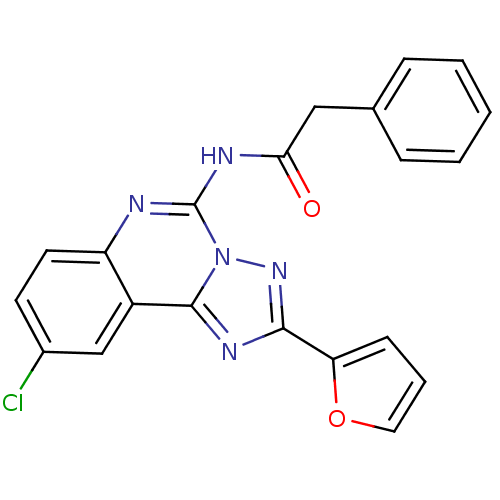
(CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...)Show SMILES Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 Show InChI InChI=1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109471
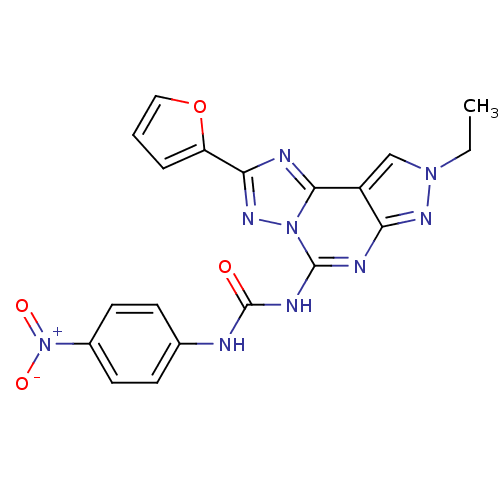
(1-(8-Ethyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES CCn1cc2c(n1)nc(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C19H15N9O4/c1-2-26-10-13-15(24-26)22-18(27-17(13)21-16(25-27)14-4-3-9-32-14)23-19(29)20-11-5-7-12(8-6-11)28(30)31/h3-10H,2H2,1H3,(H2,20,22,23,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109453
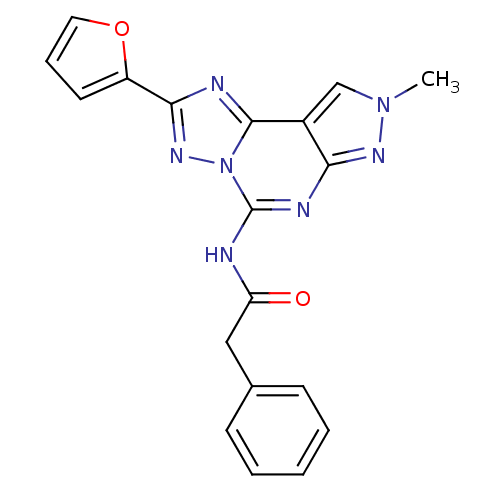
(CHEMBL341376 | N-(2-(furan-2-yl)-8-methyl-8H-pyraz...)Show SMILES Cn1cc2c(n1)nc(NC(=O)Cc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C19H15N7O2/c1-25-11-13-16(23-25)22-19(20-15(27)10-12-6-3-2-4-7-12)26-18(13)21-17(24-26)14-8-5-9-28-14/h2-9,11H,10H2,1H3,(H,20,22,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109448
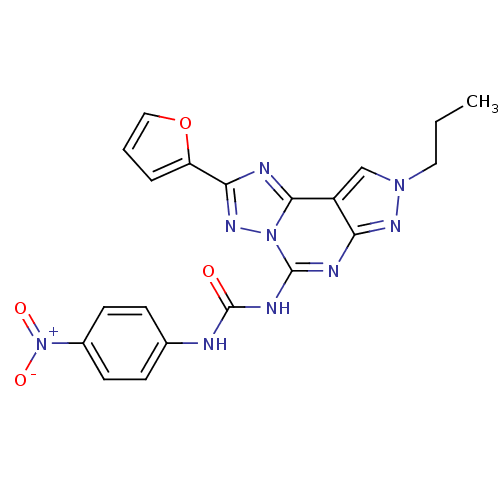
(1-(2-(furan-2-yl)-8-propyl-8H-pyrazolo[4,3-e][1,2,...)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Nc1ccc(cc1)[N+]([O-])=O)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H17N9O4/c1-2-9-27-11-14-16(25-27)23-19(28-18(14)22-17(26-28)15-4-3-10-33-15)24-20(30)21-12-5-7-13(8-6-12)29(31)32/h3-8,10-11H,2,9H2,1H3,(H2,21,23,24,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM85619
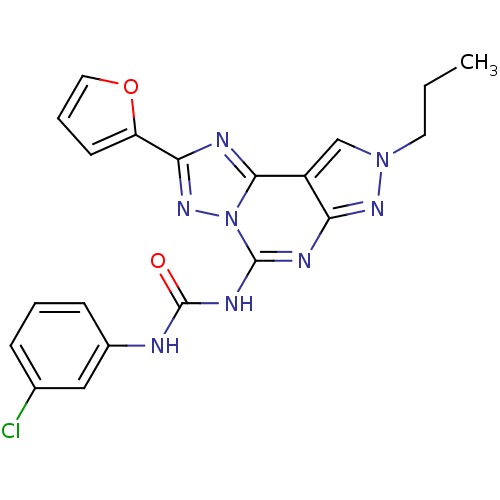
(1-(3-chlorophenyl)-3-[2-(furan-2-yl)-8-propyl-8H-p...)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Nc1cccc(Cl)c1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H17ClN8O2/c1-2-8-28-11-14-16(26-28)24-19(25-20(30)22-13-6-3-5-12(21)10-13)29-18(14)23-17(27-29)15-7-4-9-31-15/h3-7,9-11H,2,8H2,1H3,(H2,22,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109476
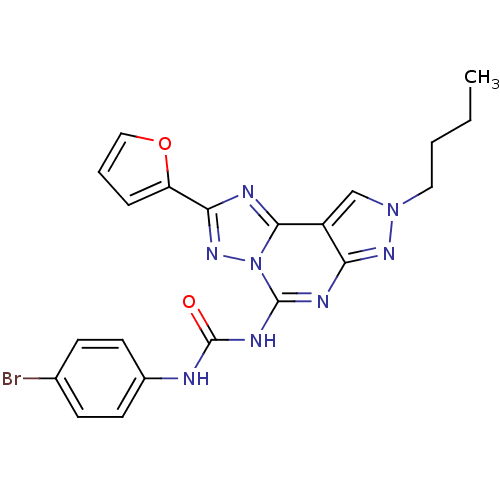
(1-(4-Bromo-phenyl)-3-(8-butyl-2-furan-2-yl-8H-pyra...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Nc1ccc(Br)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H19BrN8O2/c1-2-3-10-29-12-15-17(27-29)25-20(26-21(31)23-14-8-6-13(22)7-9-14)30-19(15)24-18(28-30)16-5-4-11-32-16/h4-9,11-12H,2-3,10H2,1H3,(H2,23,25,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109449
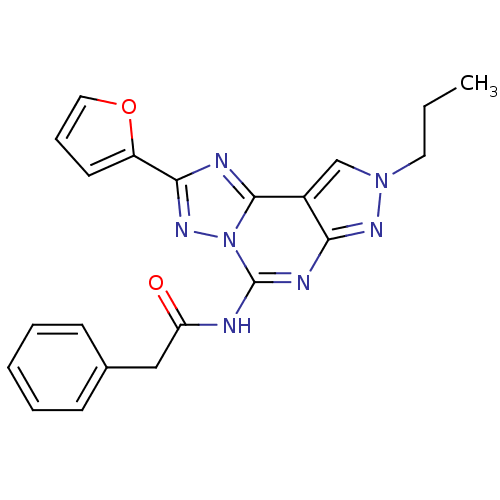
(CHEMBL349464 | N-(2-(furan-2-yl)-8-propyl-8H-pyraz...)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Cc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H19N7O2/c1-2-10-27-13-15-18(25-27)24-21(22-17(29)12-14-7-4-3-5-8-14)28-20(15)23-19(26-28)16-9-6-11-30-16/h3-9,11,13H,2,10,12H2,1H3,(H,22,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109482
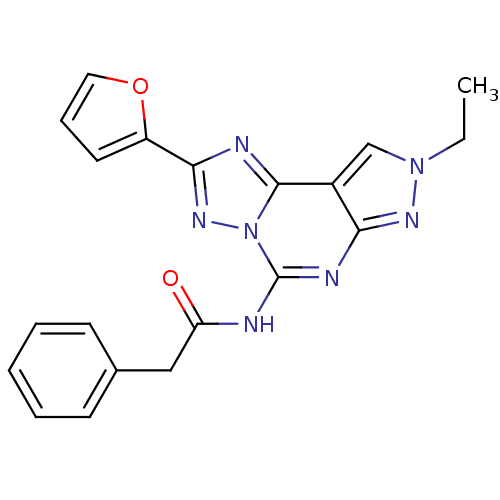
(CHEMBL165073 | N-(8-Ethyl-2-furan-2-yl-8H-pyrazolo...)Show SMILES CCn1cc2c(n1)nc(NC(=O)Cc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C20H17N7O2/c1-2-26-12-14-17(24-26)23-20(21-16(28)11-13-7-4-3-5-8-13)27-19(14)22-18(25-27)15-9-6-10-29-15/h3-10,12H,2,11H2,1H3,(H,21,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50109485
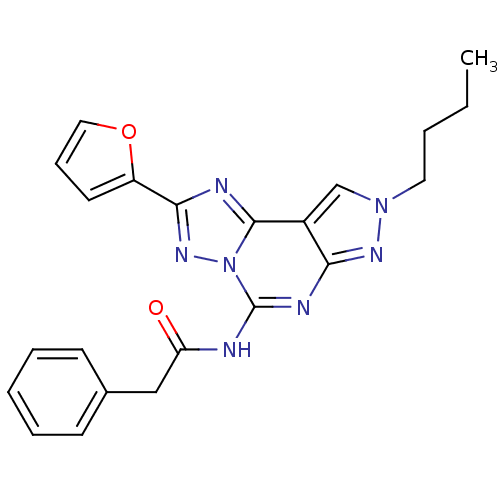
(CHEMBL163968 | N-(8-Butyl-2-furan-2-yl-8H-pyrazolo...)Show SMILES CCCCn1cc2c(n1)nc(NC(=O)Cc1ccccc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C22H21N7O2/c1-2-3-11-28-14-16-19(26-28)25-22(23-18(30)13-15-8-5-4-6-9-15)29-21(16)24-20(27-29)17-10-7-12-31-17/h4-10,12,14H,2-3,11,13H2,1H3,(H,23,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402368
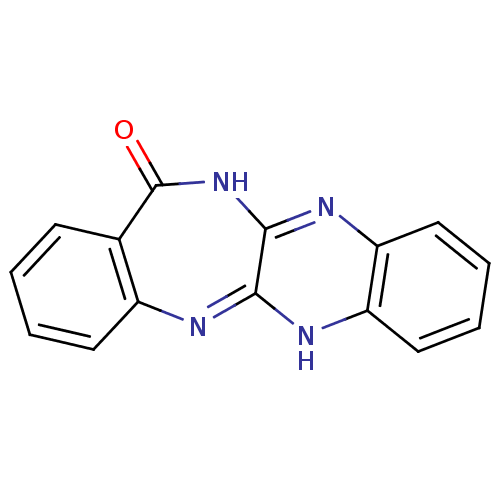
(CHEMBL2206682)Show InChI InChI=1S/C15H10N4O/c20-15-9-5-1-2-6-10(9)16-13-14(19-15)18-12-8-4-3-7-11(12)17-13/h1-8H,(H,16,17)(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50086159
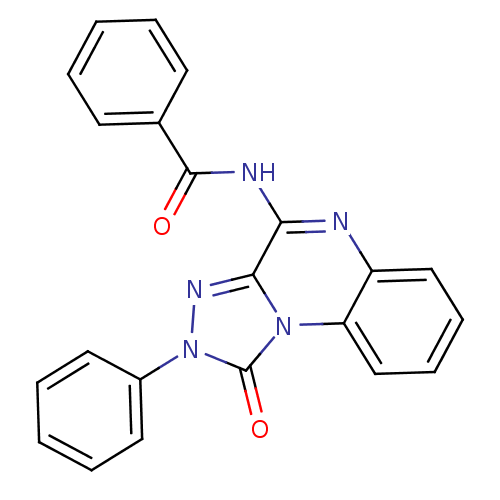
(CHEMBL16488 | N-(1-Oxo-2-phenyl-1,2-dihydro-[1,2,4...)Show SMILES O=C(Nc1nc2ccccc2n2c1nn(-c1ccccc1)c2=O)c1ccccc1 Show InChI InChI=1S/C22H15N5O2/c28-21(15-9-3-1-4-10-15)24-19-20-25-27(16-11-5-2-6-12-16)22(29)26(20)18-14-8-7-13-17(18)23-19/h1-14H,(H,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
Bioorg Med Chem Lett 21: 2898-905 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.073
BindingDB Entry DOI: 10.7270/Q2VH5P5Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data