

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23986 (3,4-dihydro-1H-2-benzothiopyran-3-one | isothiochr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23983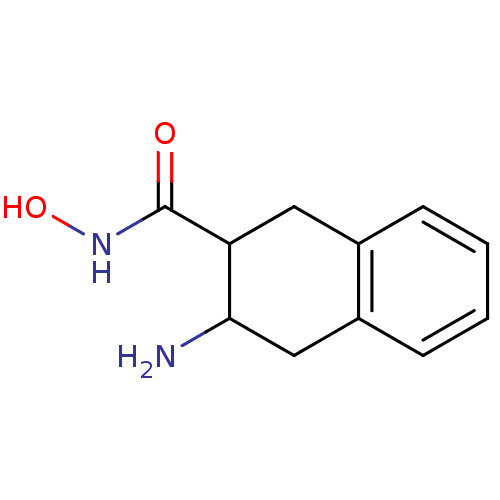 (3-amino-N-hydroxy-1,2,3,4-tetrahydronaphthalene-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23987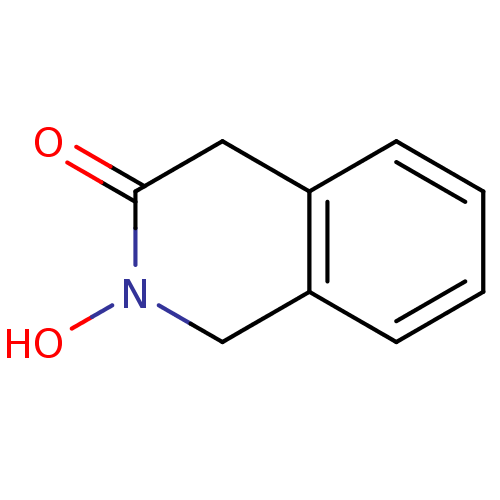 (2-hydroxy-1,2,3,4-tetrahydroisoquinolin-3-one | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23983 (3-amino-N-hydroxy-1,2,3,4-tetrahydronaphthalene-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23978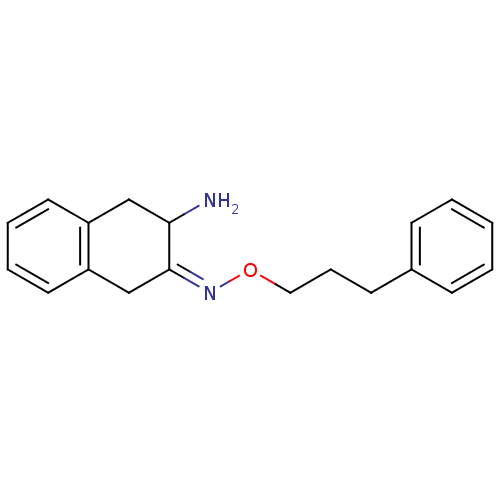 ((3Z)-3-[(3-phenylpropoxy)imino]-1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23979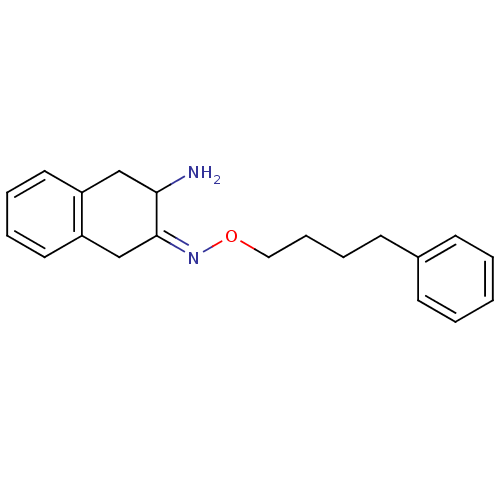 ((3Z)-3-[(4-phenylbutoxy)imino]-1,2,3,4-tetrahydron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Bos taurus (bovine)) | BDBM23983 (3-amino-N-hydroxy-1,2,3,4-tetrahydronaphthalene-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23980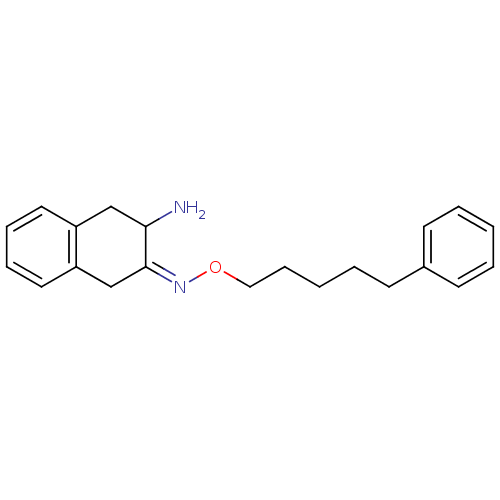 ((3Z)-3-{[(5-phenylpentyl)oxy]imino}-1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23979 ((3Z)-3-[(4-phenylbutoxy)imino]-1,2,3,4-tetrahydron...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23990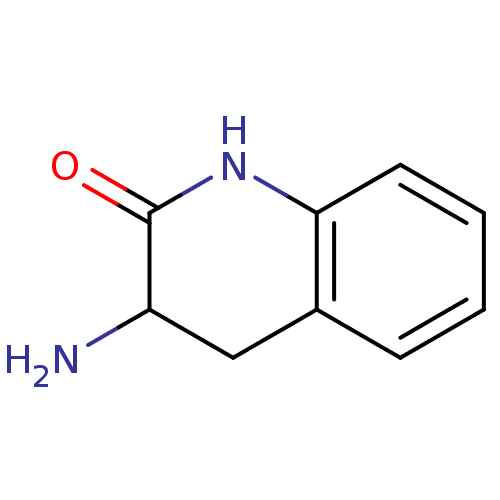 (3-amino-1,2,3,4-tetrahydroquinolin-2-one hydrochlo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23980 ((3Z)-3-{[(5-phenylpentyl)oxy]imino}-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23978 ((3Z)-3-[(3-phenylpropoxy)imino]-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23975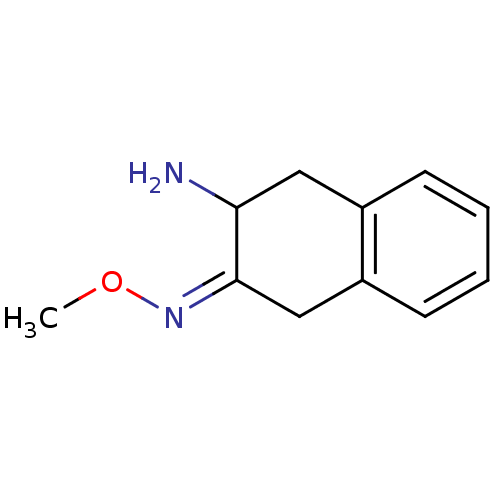 ((3Z)-3-(methoxyimino)-1,2,3,4-tetrahydronaphthalen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23977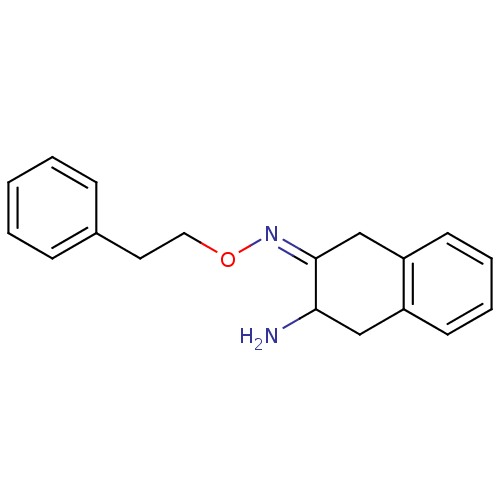 ((3Z)-3-[(2-phenylethoxy)imino]-1,2,3,4-tetrahydron...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Bos taurus (bovine)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 8.0 | 30 |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24285 ((3S)-3-amino-5-[(4-phenoxyphenyl)carbamoyl]pentano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24282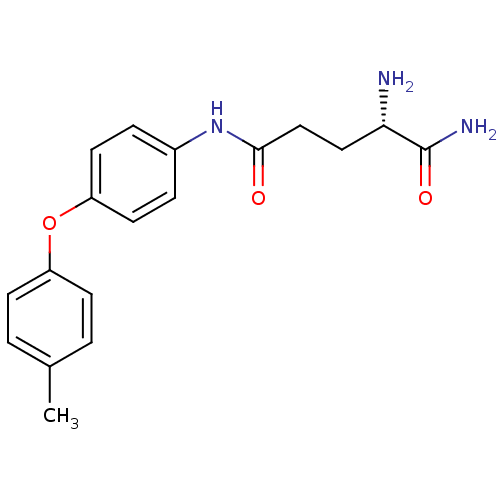 ((2S)-2-amino-N-[4-(4-methylphenoxy)phenyl]pentaned...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24291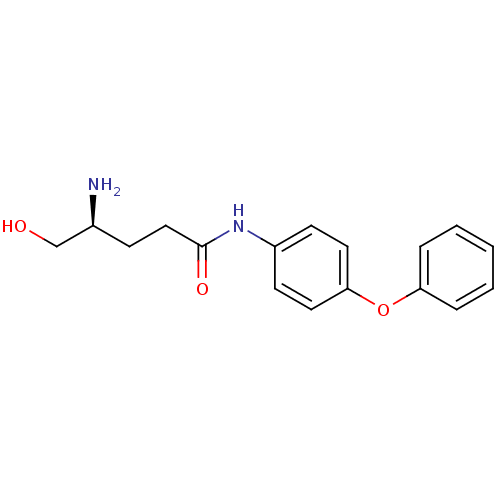 ((4S)-4-amino-5-hydroxy-N-(4-phenoxyphenyl)pentanam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24287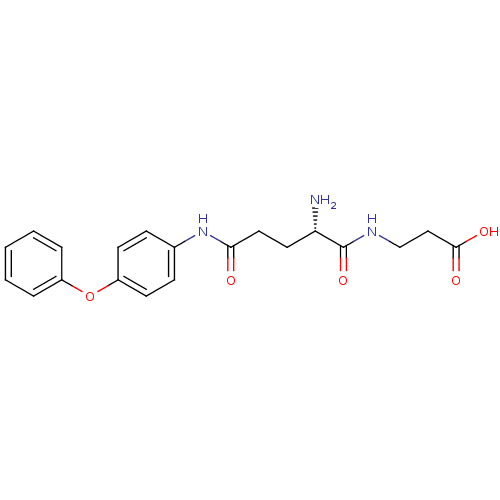 (3-[(2S)-2-amino-N-(4-phenoxyphenyl)pentanediamido]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24277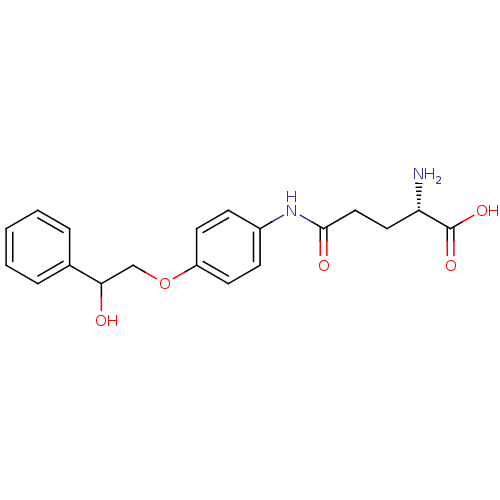 ((2S)-2-amino-4-{[4-(2-hydroxy-2-phenylethoxy)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24276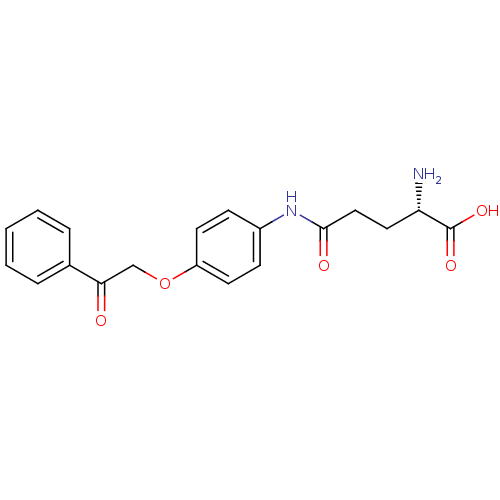 ((2S)-2-amino-4-{[4-(2-oxo-2-phenylethoxy)phenyl]ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24270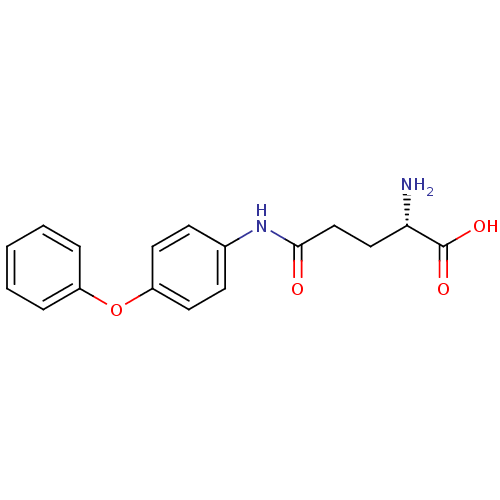 ((2S)-2-amino-4-[(4-phenoxyphenyl)carbamoyl]butanoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24288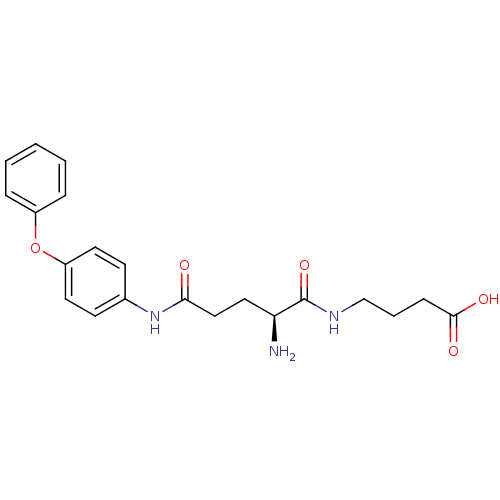 (4-[(2S)-2-amino-N-(4-phenoxyphenyl)pentanediamido]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24289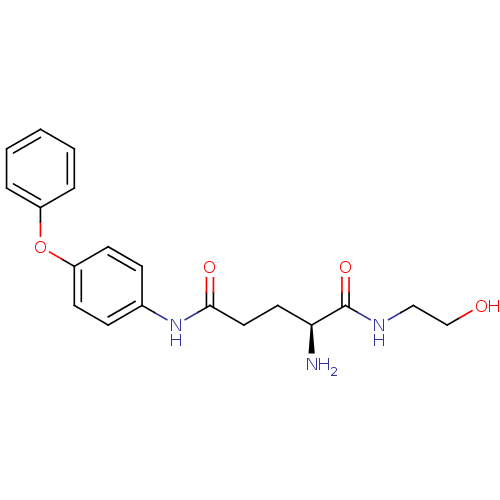 ((2S)-2-amino-N-(2-hydroxyethyl)-N'-(4-phenoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24256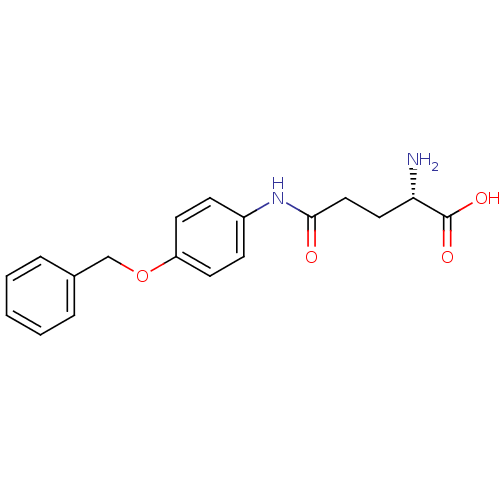 ((2S)-2-amino-4-{[4-(benzyloxy)phenyl]carbamoyl}but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24292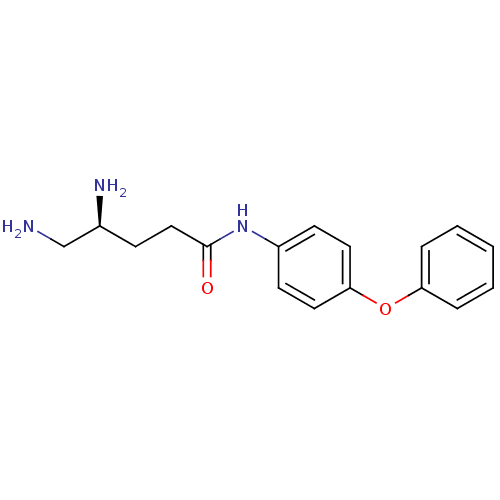 ((4S)-4,5-diamino-N-(4-phenoxyphenyl)pentanamide | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24271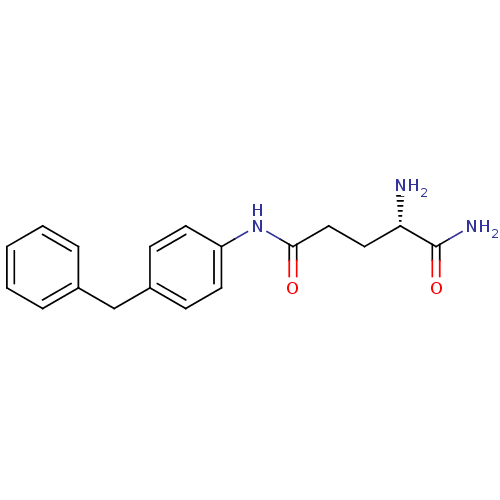 ((2S)-2-amino-N-(4-benzylphenyl)pentanediamide | Mo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24290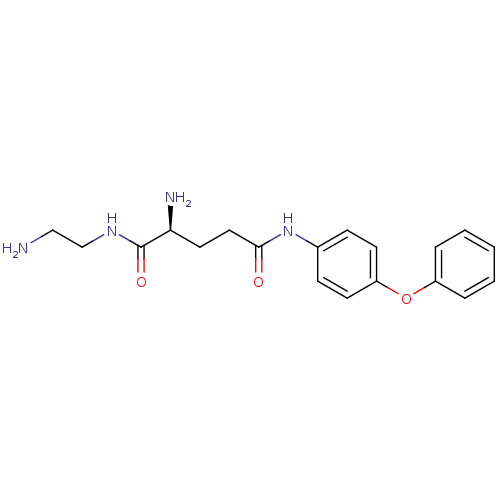 ((2S)-2-amino-N-(2-aminoethyl)-N'-(4-phenoxyphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24283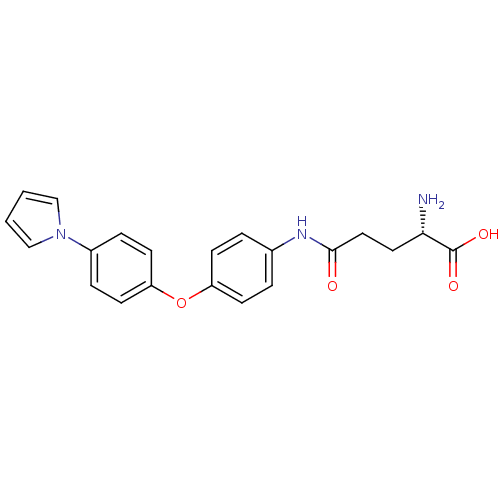 ((2S)-2-amino-4-({4-[4-(1H-pyrrol-1-yl)phenoxy]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24286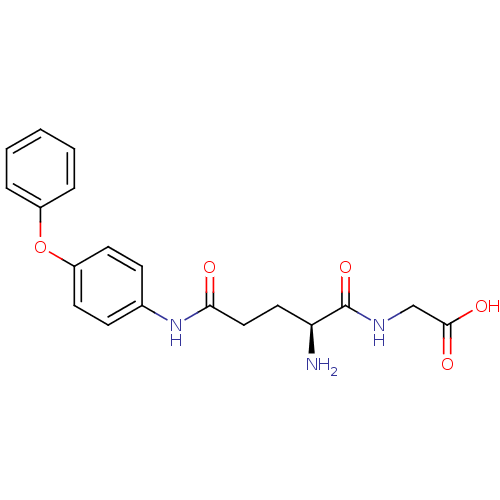 (2-[(2S)-2-amino-N-(4-phenoxyphenyl)pentanediamido]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24261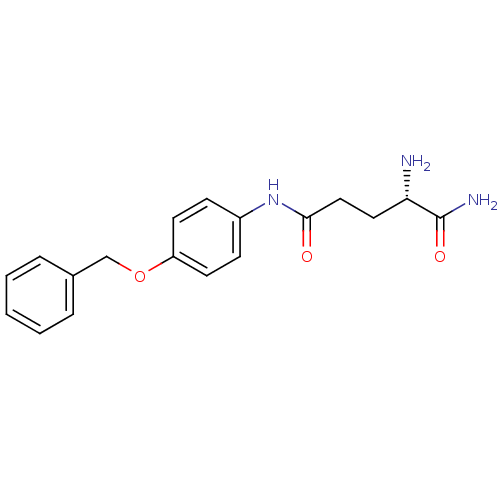 ((2S)-2-amino-N-[4-(benzyloxy)phenyl]pentanediamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24262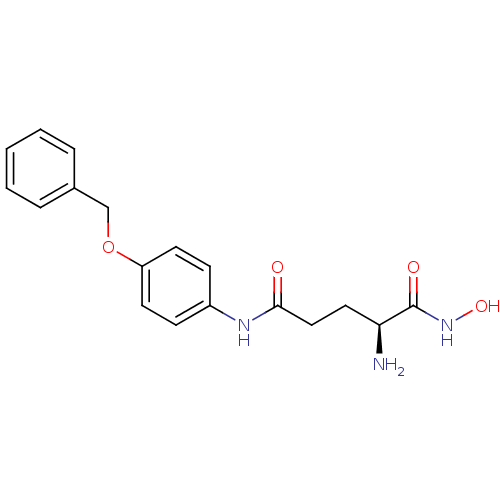 ((2S)-2-amino-N-[4-(benzyloxy)phenyl]-N'-hydroxypen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24284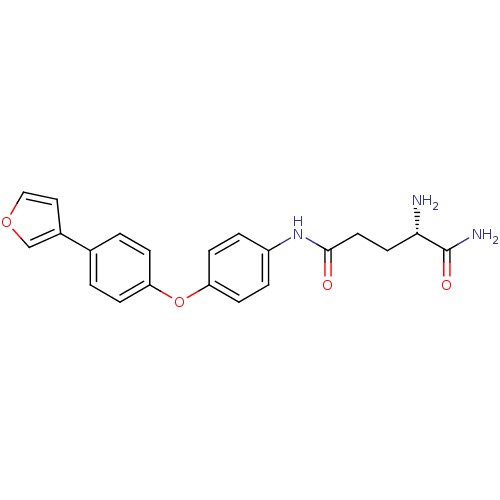 ((2S)-2-amino-N-{4-[4-(furan-3-yl)phenoxy]phenyl}pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24275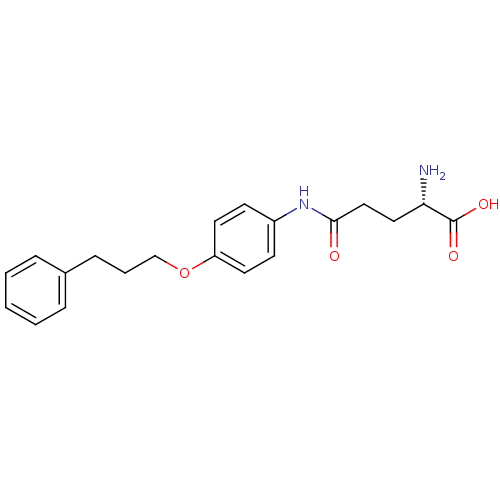 ((2S)-2-amino-4-{[4-(3-phenylpropoxy)phenyl]carbamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24259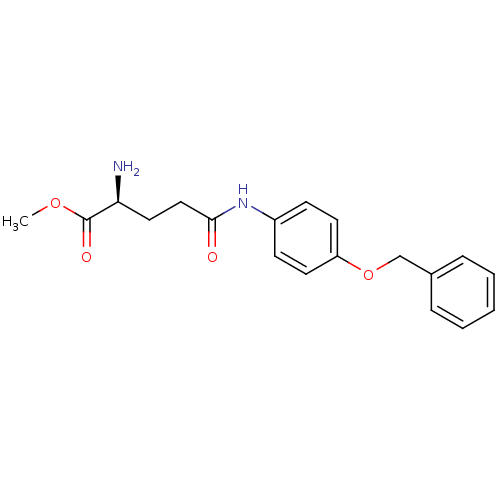 (Modified amino acid analog, 6a | methyl (2S)-2-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24274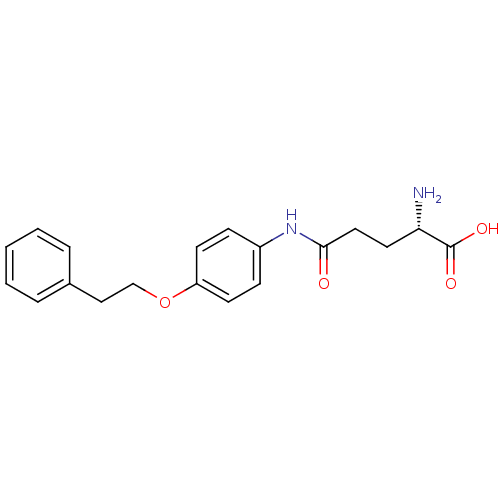 ((2S)-2-amino-4-{[4-(2-phenylethoxy)phenyl]carbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24260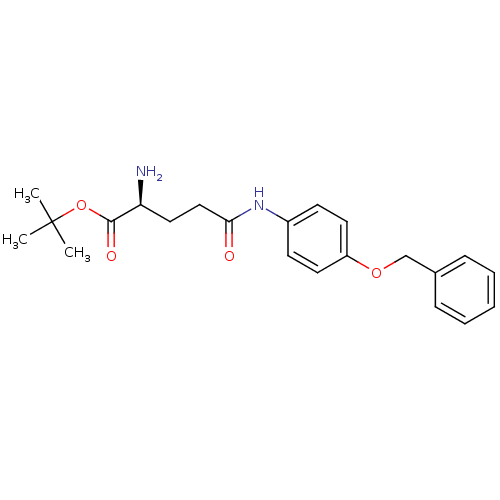 (Modified amino acid analog, 6b | tert-butyl (2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24263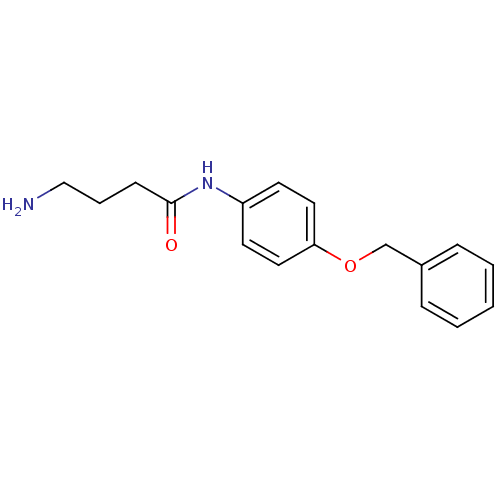 (4-amino-N-[4-(benzyloxy)phenyl]butanamide | Modifi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24298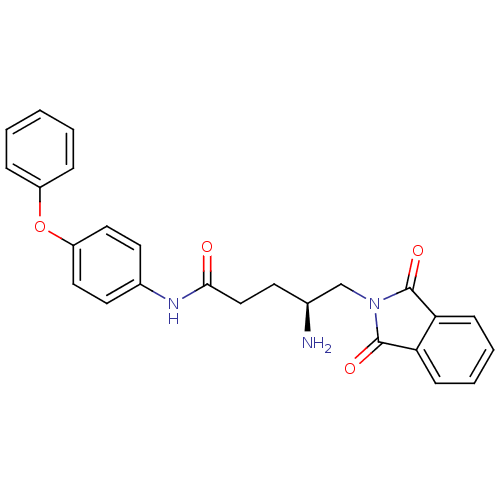 ((4S)-4-amino-5-(1,3-dioxo-2,3-dihydro-1H-isoindol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24294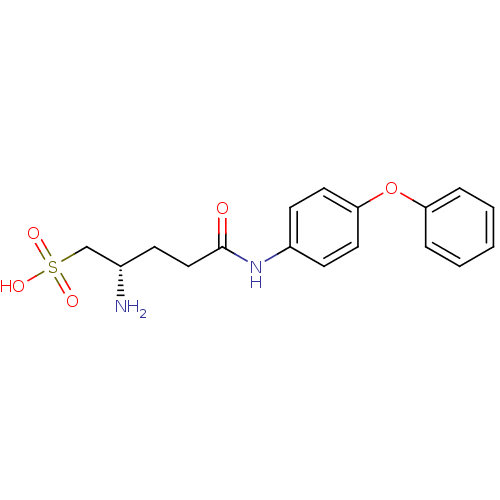 ((2S)-2-amino-4-[(4-phenoxyphenyl)carbamoyl]butane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24244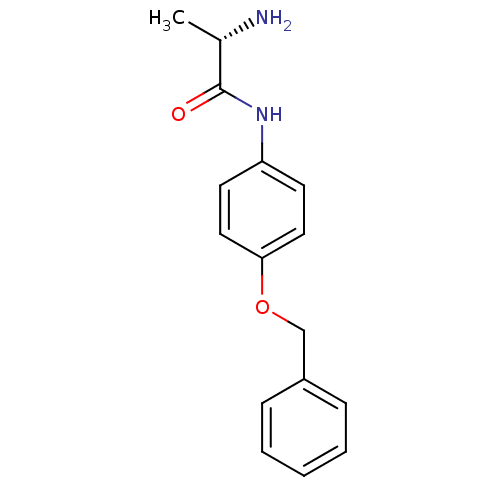 ((2S)-2-amino-N-[4-(benzyloxy)phenyl]propanamide | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24281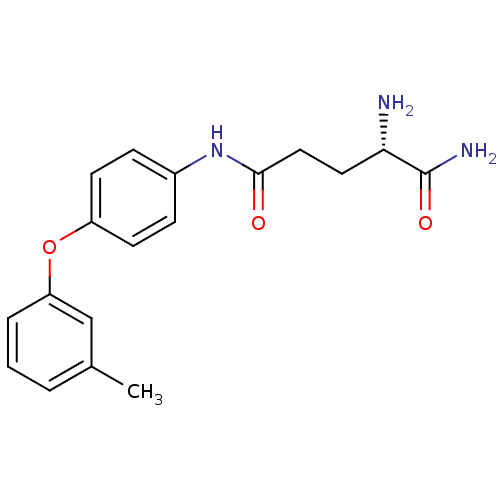 ((2S)-2-amino-N-[4-(3-methylphenoxy)phenyl]pentaned...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24272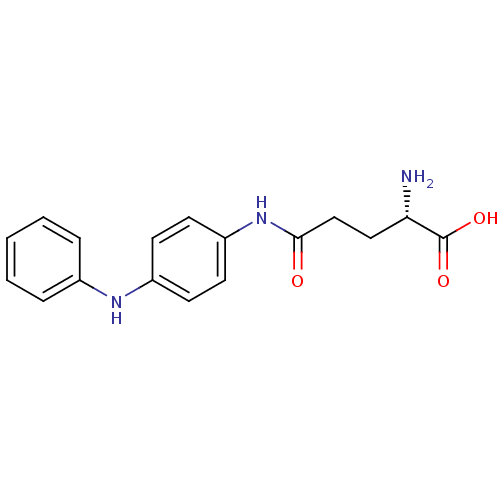 ((2S)-2-amino-4-{[4-(phenylamino)phenyl]carbamoyl}b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24243 (2-amino-N-[4-(benzyloxy)phenyl]acetamide | Amino a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24296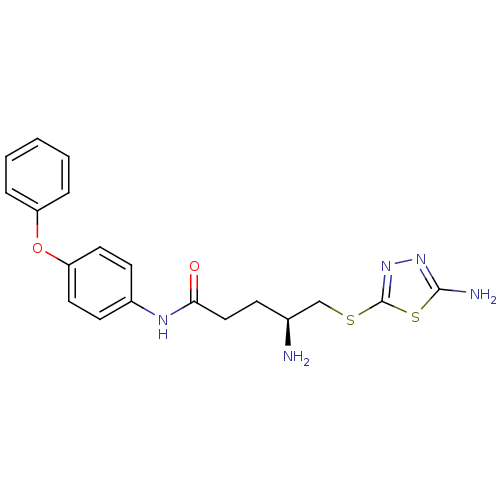 ((4S)-4-amino-5-[(5-amino-1,3,4-thiadiazol-2-yl)sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24297 ((4S)-4-amino-N-(4-phenoxyphenyl)-5-(1H-pyrrol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23973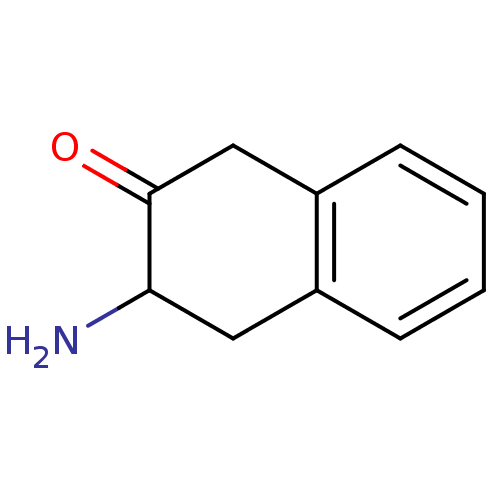 (3-amino-1,2,3,4-tetrahydronaphthalen-2-one hydroch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24251 ((4S)-4-amino-4-{[4-(benzyloxy)phenyl]carbamoyl}but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex Biosciences | Assay Description Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... | Bioorg Med Chem 16: 4963-83 (2008) Article DOI: 10.1016/j.bmc.2008.03.042 BindingDB Entry DOI: 10.7270/Q2BP013C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 132 total ) | Next | Last >> |