

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4690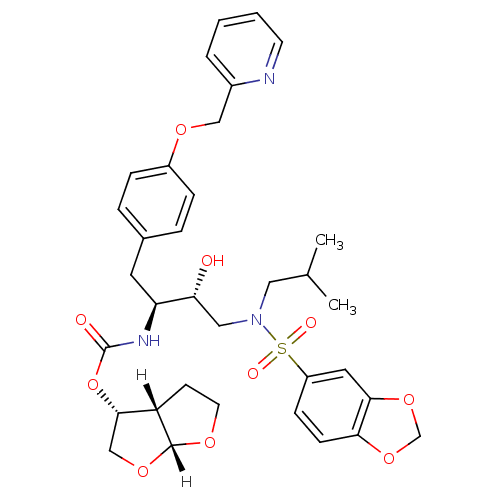 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW0385 from HIV1 protease | Bioorg Med Chem Lett 16: 1788-94 (2006) Article DOI: 10.1016/j.bmcl.2006.01.035 BindingDB Entry DOI: 10.7270/Q2WS8V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW0385 from HIV1 protease | Bioorg Med Chem Lett 16: 1788-94 (2006) Article DOI: 10.1016/j.bmcl.2006.01.035 BindingDB Entry DOI: 10.7270/Q2WS8V1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Antagonist activity at 5HT2A receptor | J Med Chem 55: 5749-59 (2012) Article DOI: 10.1021/jm300338m BindingDB Entry DOI: 10.7270/Q2FQ9XQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor | J Med Chem 55: 5749-59 (2012) Article DOI: 10.1021/jm300338m BindingDB Entry DOI: 10.7270/Q2FQ9XQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35650 (pyrimidylpyrrole, 10c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35641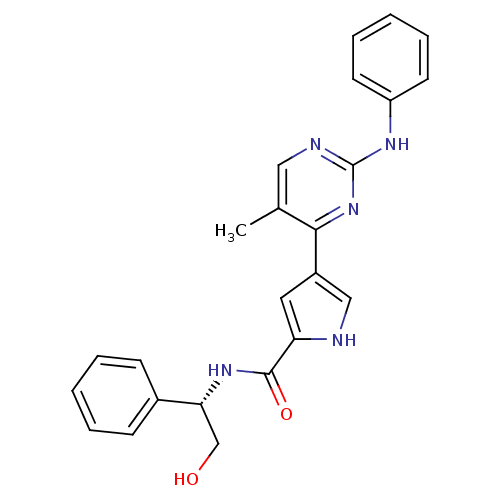 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15645 (N-((S)-1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35647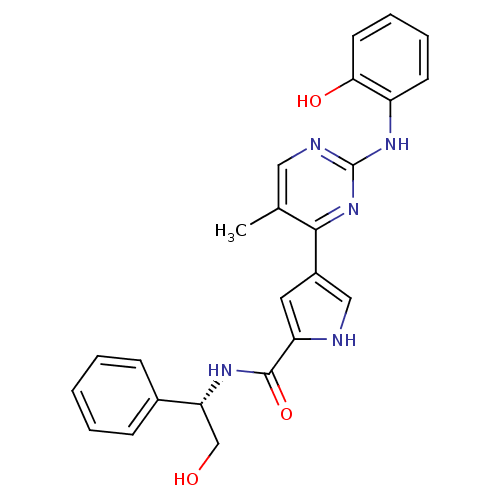 (erk000636 | pyrimidylpyrrole, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35663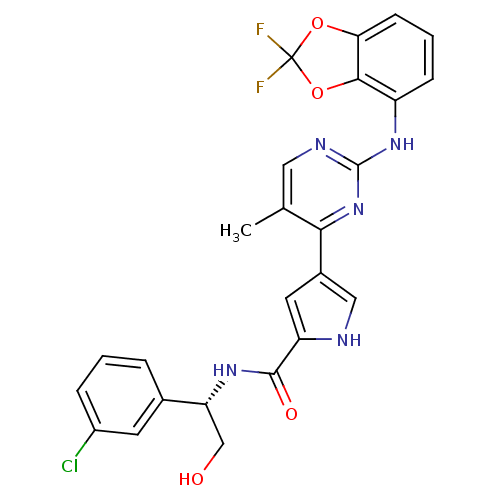 (pyrimidylpyrrole, 11k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35662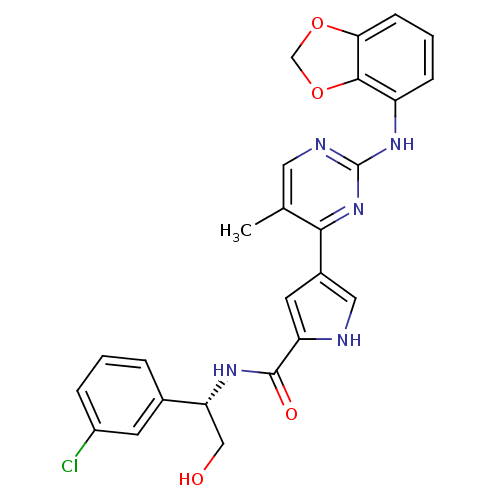 (pyrimidylpyrrole, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35660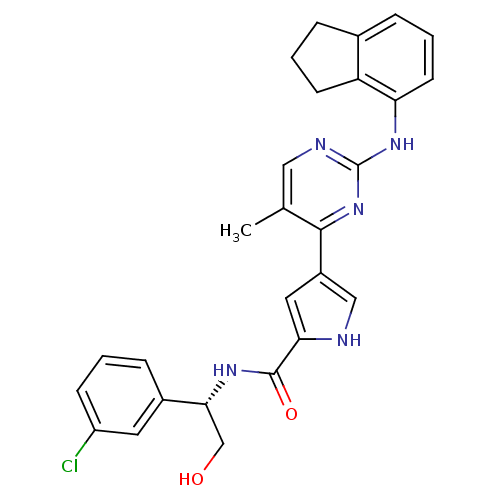 (pyrimidylpyrrole, 11h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35659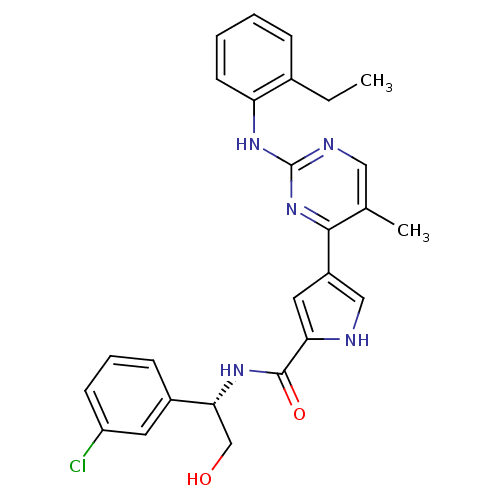 (pyrimidylpyrrole, 11g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35657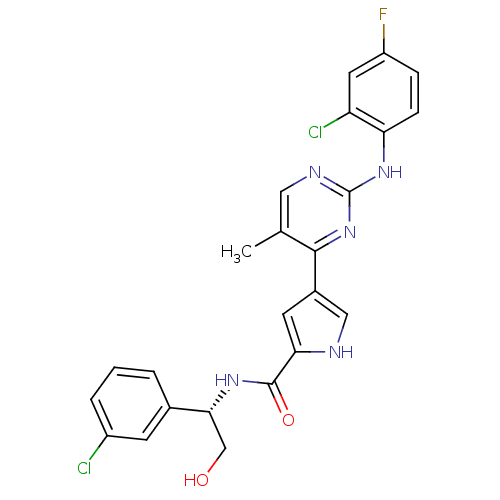 (pyrimidylpyrrole, 11e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35654 (erk000526 | pyrimidylpyrrole, 11b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35651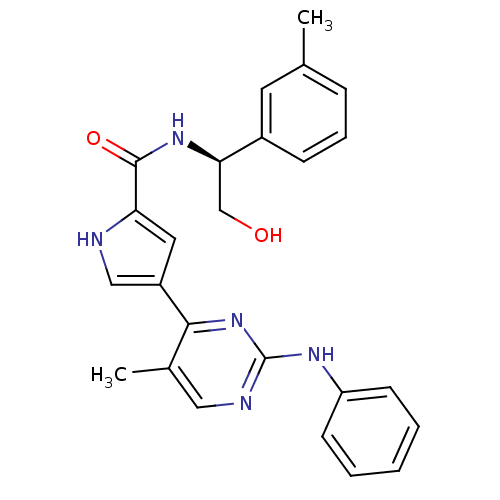 (pyrimidylpyrrole, 10d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35642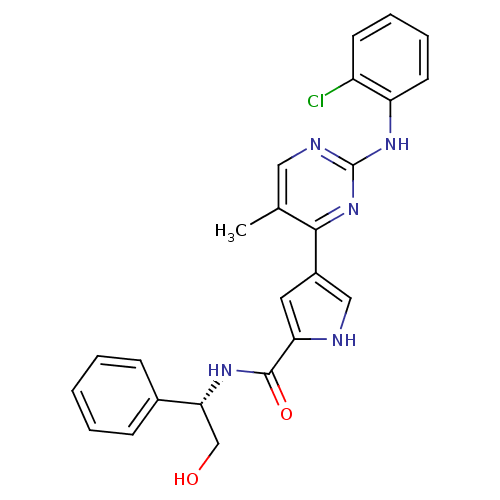 (pyrimidylpyrrole, 9a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35649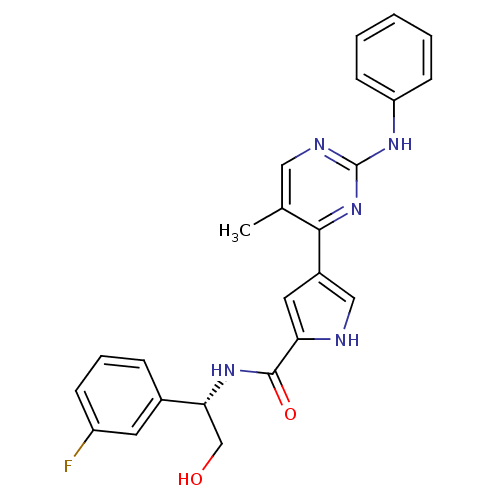 (erk000537 | pyrimidylpyrrole, 10b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35645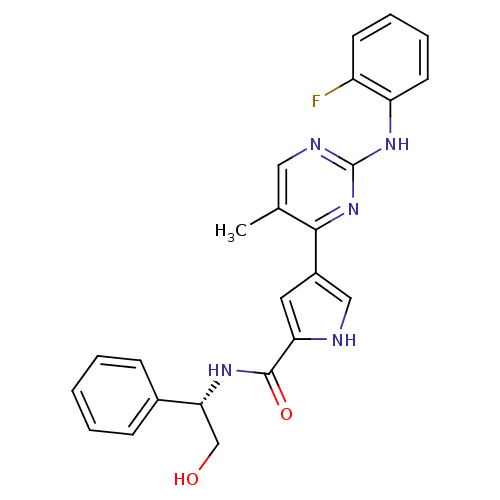 (pyrimidylpyrrole, 9d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35643 (pyrimidylpyrrole, 9b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Antagonist activity at 5HT2A receptor | J Med Chem 55: 5749-59 (2012) Article DOI: 10.1021/jm300338m BindingDB Entry DOI: 10.7270/Q2FQ9XQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35665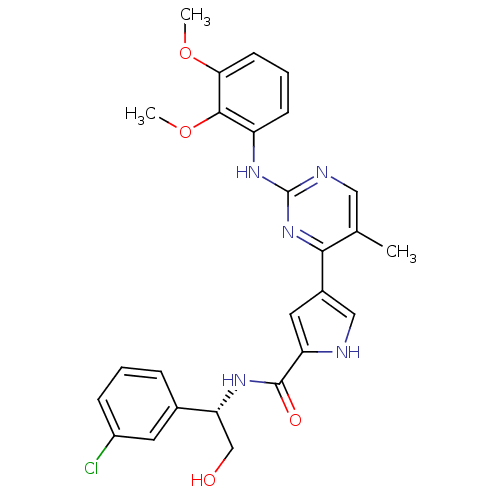 (erk000617 | pyrimidylpyrrole, 11m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35644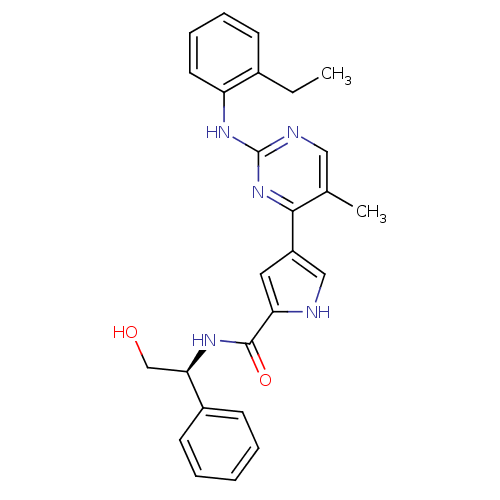 (erk000524 | pyrimidylpyrrole, 9c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35646 (erk000506 | pyrimidylpyrrole, 9e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35647 (erk000636 | pyrimidylpyrrole, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35656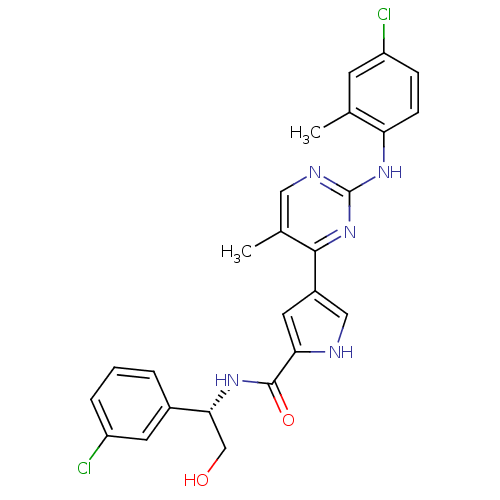 (erk000650 | pyrimidylpyrrole, 11d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35658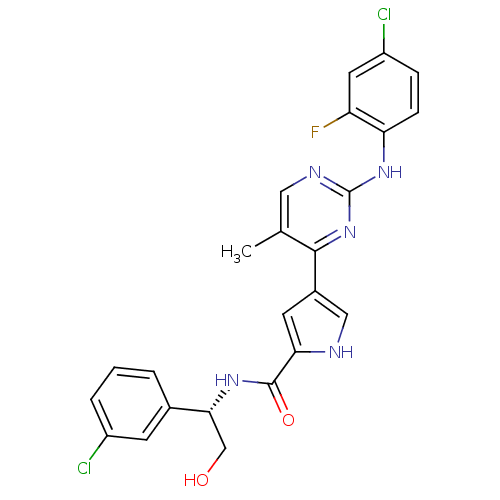 (erk000651 | pyrimidylpyrrole, 11f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35649 (erk000537 | pyrimidylpyrrole, 10b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15644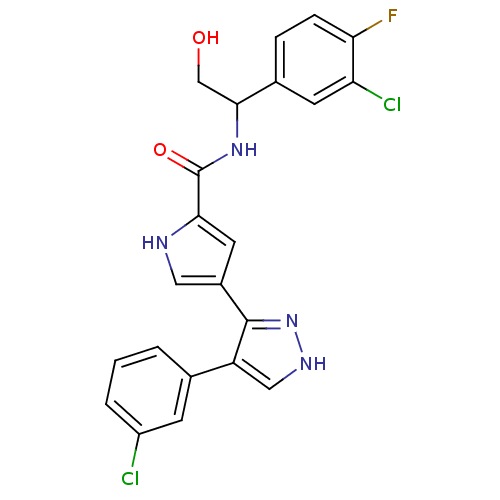 (N-(1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15643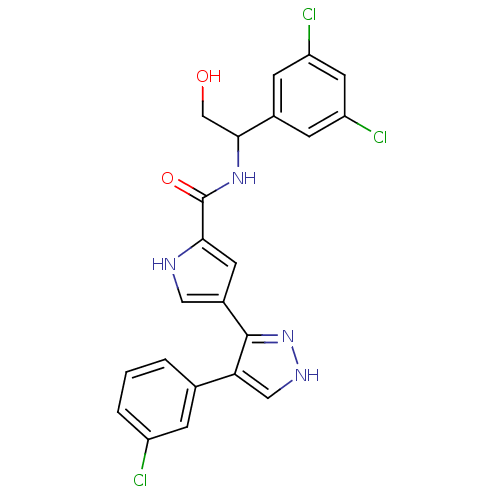 (4-(4-(3-Chlorophenyl)-1H-pyrazol-3-yl)-N-(1-(3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35641 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121622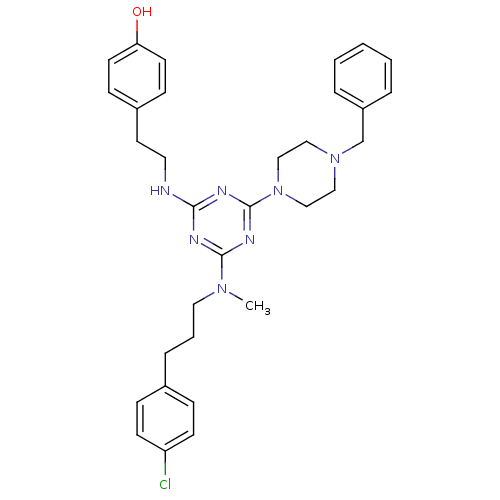 (4-[2-(4-(4-Benzyl-piperazin-1-yl)-6-{[3-(4-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM35664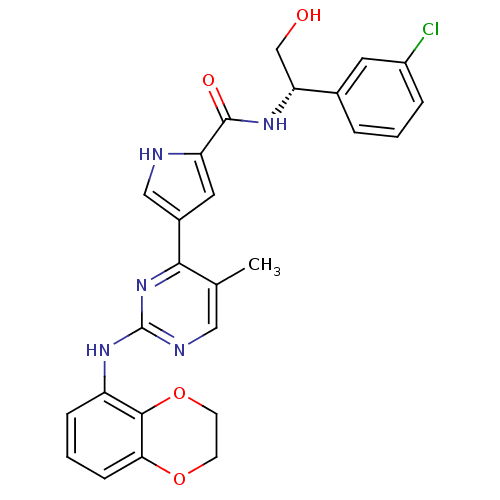 (erk000628 | pyrimidylpyrrole, 11l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35661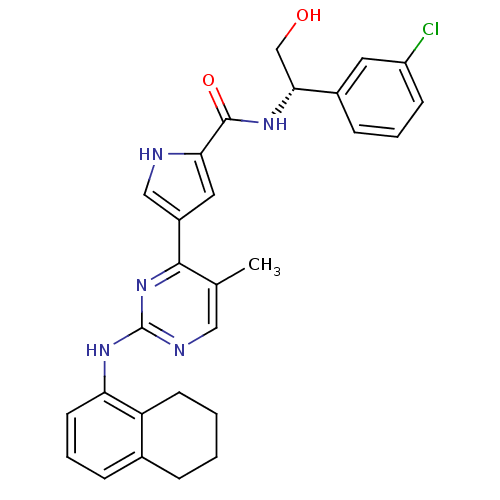 (erk000595 | pyrimidylpyrrole, 11i) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15638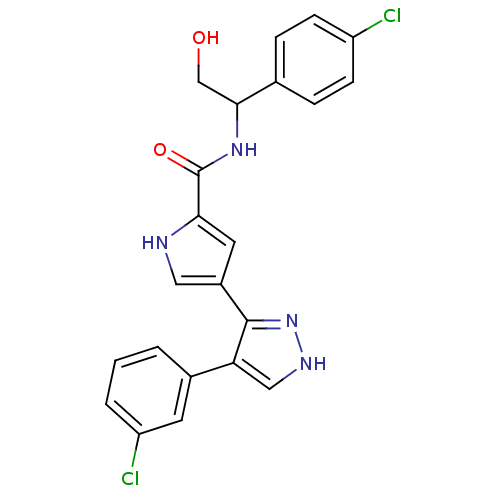 (4-(4-(3-Chlorophenyl)-1H-pyrazol-3-yl)-N-(1-(4-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121598 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121608 (4-[2-(4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121590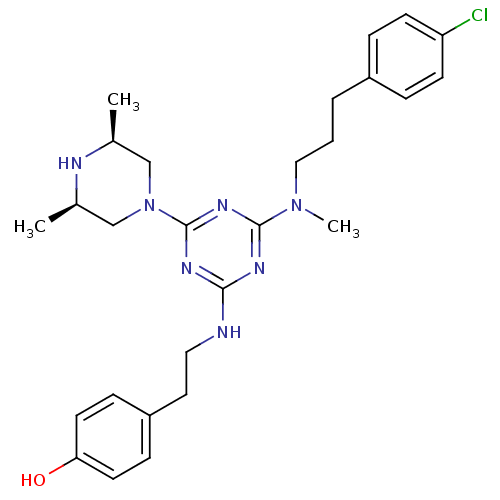 (4-{2-[4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121589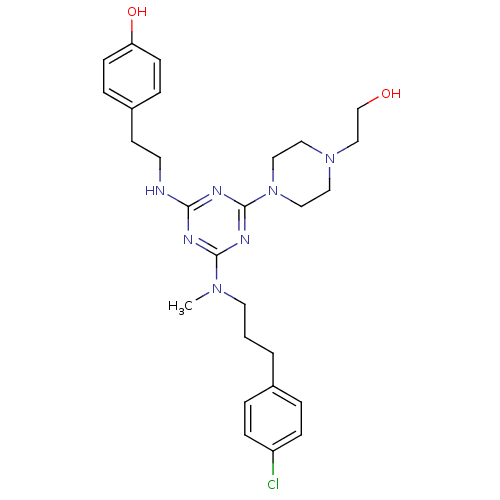 (4-(2-{4-{[3-(4-Chloro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM35662 (pyrimidylpyrrole, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121602 (4-[2-(4-{[3-(4-Fluoro-phenyl)-propyl]-methyl-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35664 (erk000628 | pyrimidylpyrrole, 11l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM35641 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121626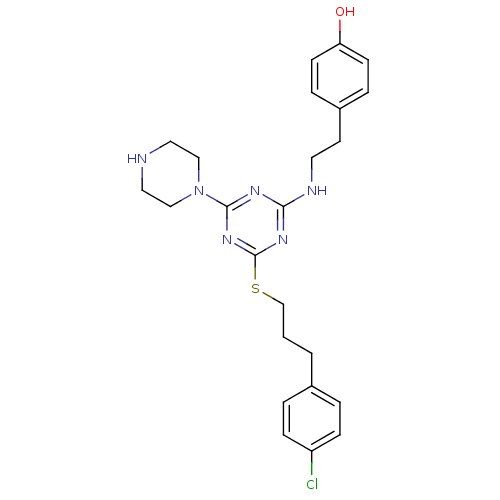 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM35641 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM35650 (pyrimidylpyrrole, 10c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315314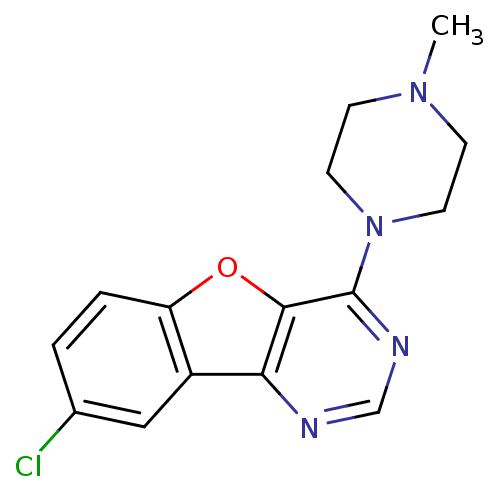 (8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121584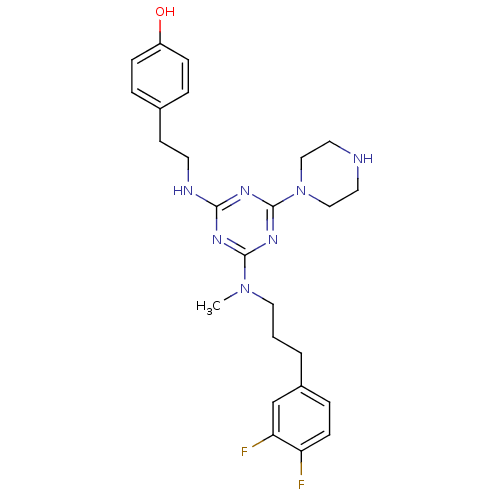 (4-[2-(4-{[3-(3,4-Difluoro-phenyl)-propyl]-methyl-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15642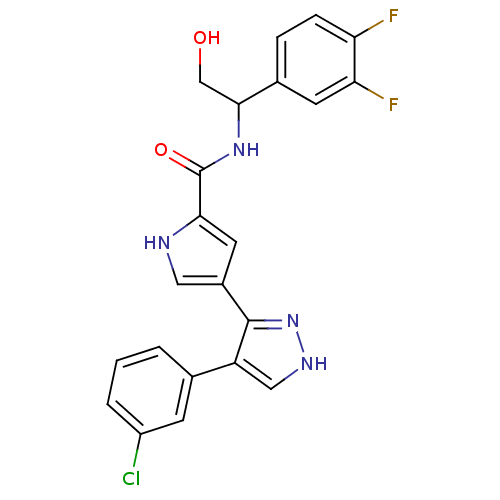 (4-(4-(3-Chlorophenyl)-1H-pyrazol-3-yl)-N-(1-(3,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 866 total ) | Next | Last >> |