 Found 7400 hits with Last Name = 'hand' and Initial = 'a'
Found 7400 hits with Last Name = 'hand' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024382

(CHEMBL189690)Show SMILES CCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27)/t23-,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024388

(CHEMBL190621)Show SMILES CCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(Oc2ccc(N)cc2)cc1 Show InChI InChI=1S/C24H26N3O5P/c1-2-31-33(30,22-13-11-21(12-14-22)32-20-9-7-19(25)8-10-20)27-16-18-6-4-3-5-17(18)15-23(27)24(28)26-29/h3-14,23,29H,2,15-16,25H2,1H3,(H,26,28)/t23-,33?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nicotinic receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4749-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.142
BindingDB Entry DOI: 10.7270/Q2QC04GV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024390

(CHEMBL372702)Show SMILES CCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H25N2O4P/c1-2-30-31(29,22-14-12-19(13-15-22)18-8-4-3-5-9-18)26-17-21-11-7-6-10-20(21)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27)/t23-,31?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024402
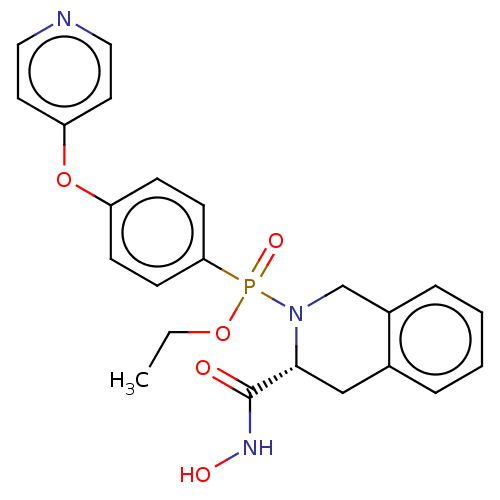
(CHEMBL190232)Show SMILES CCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C23H24N3O5P/c1-2-30-32(29,21-9-7-19(8-10-21)31-20-11-13-24-14-12-20)26-16-18-6-4-3-5-17(18)15-22(26)23(27)25-28/h3-14,22,28H,2,15-16H2,1H3,(H,25,27)/t22-,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36608

(Rapamycin C-7, analog 1)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42-,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50602641

(VRT 043198 | Vrt 043198)Show SMILES CC(C)(C)[C@H](NC(=O)c1ccc(N)c(Cl)c1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114002
BindingDB Entry DOI: 10.7270/Q2JH3R8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
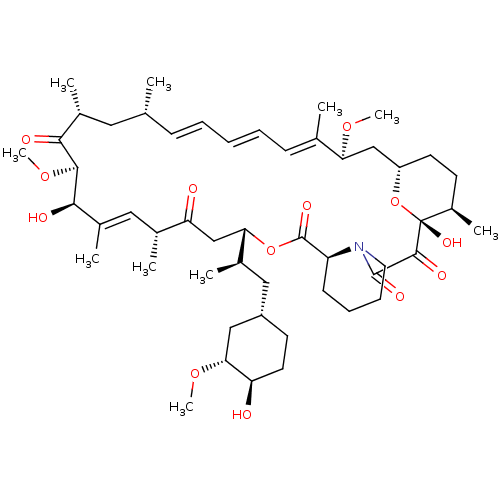
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36612
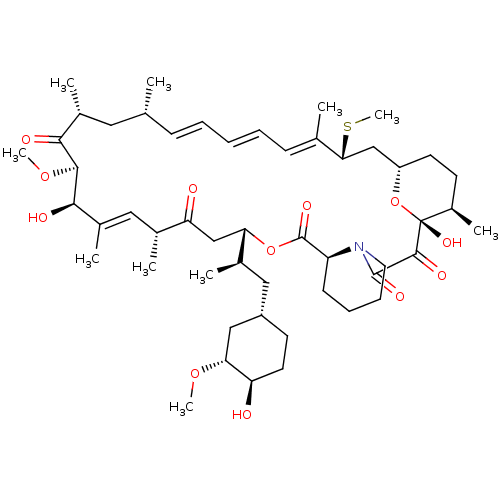
(Rapamycin C-7, analog 6a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36613
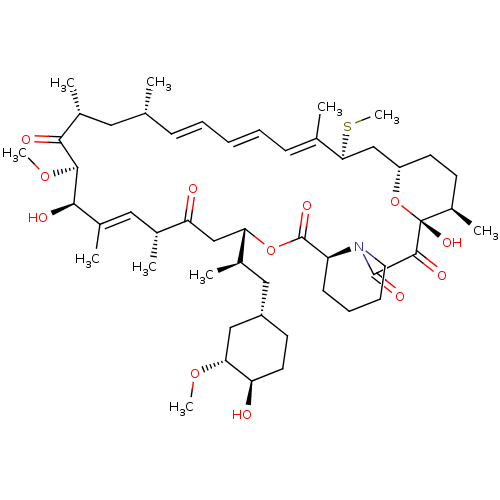
(Rapamycin C-7, analog 6b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44+,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36627

(Rapamycin C-7, analog 16a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccc(Cl)cc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82ClNO13/c1-34-15-11-10-12-16-35(2)48(70-33-41-19-22-43(58)23-20-41)31-44-24-18-40(7)57(67,72-44)54(64)55(65)59-26-14-13-17-45(59)56(66)71-49(37(4)29-42-21-25-46(60)50(30-42)68-8)32-47(61)36(3)28-39(6)52(63)53(69-9)51(62)38(5)27-34/h10-12,15-16,19-20,22-23,28,34,36-38,40,42,44-46,48-50,52-53,60,63,67H,13-14,17-18,21,24-27,29-33H2,1-9H3/b12-10+,15-11+,35-16+,39-28+/t34-,36-,37-,38-,40-,42+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309873

(4-(6-Chlorooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...)Show SMILES Clc1cnc2nc(oc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(-1.41,-19.11,;-1.97,-20.56,;-.99,-21.77,;-1.55,-23.21,;-3.08,-23.45,;-3.94,-24.73,;-5.43,-24.32,;-5.49,-22.78,;-4.05,-22.24,;-3.49,-20.8,;-6.76,-25.09,;-6.39,-26.56,;-7.4,-25.84,;-8.78,-25.91,;-8.85,-24.2,;-8.23,-23.06,;-8.17,-24.47,;-9.47,-25.17,;-9.77,-26.61,)| Show InChI InChI=1S/C13H15ClN4O/c14-9-7-11-12(15-8-9)16-13(19-11)18-6-5-17-3-1-10(18)2-4-17/h7-8,10H,1-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36623
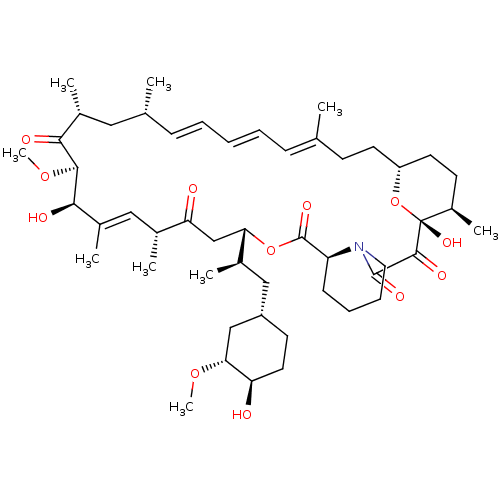
(Rapamycin C-7, analog 12)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\CC[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C50H77NO12/c1-30-15-11-10-12-16-31(2)25-34(5)44(54)46(61-9)45(55)35(6)26-32(3)41(53)29-42(33(4)27-37-20-23-40(52)43(28-37)60-8)62-49(58)39-17-13-14-24-51(39)48(57)47(56)50(59)36(7)19-22-38(63-50)21-18-30/h10-12,15-16,26,31-34,36-40,42-43,45-46,52,55,59H,13-14,17-25,27-29H2,1-9H3/b11-10+,16-12+,30-15+,35-26+/t31-,32-,33-,34-,36-,37+,38-,39+,40-,42+,43-,45-,46+,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024401
(CHEMBL190712)Show SMILES CCOP(=O)(N1CC2Nc3ccccc3C2C[C@@H]1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N3O5P/c1-3-29-30(27,15-10-8-14(28-2)9-11-15)24-13-19-17(12-20(24)21(25)23-26)16-6-4-5-7-18(16)22-19/h4-11,17,19-20,22,26H,3,12-13H2,1-2H3,(H,23,25)/t17?,19?,20-,30?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Mus musculus (house mouse)) | BDBM50309864

(4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...)Show SMILES Clc1ccc2oc(nc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(33.87,4.67,;32.34,4.44,;31.36,5.65,;29.84,5.41,;29.28,3.97,;27.84,3.42,;27.9,1.89,;29.4,1.48,;30.25,2.76,;31.78,2.99,;26.57,1.11,;26.94,-.35,;25.94,.37,;24.56,.3,;24.48,2.01,;25.1,3.15,;25.16,1.74,;23.87,1.03,;23.57,-.41,)| Show InChI InChI=1S/C14H16ClN3O/c15-10-1-2-13-12(9-10)16-14(19-13)18-8-7-17-5-3-11(18)4-6-17/h1-2,9,11H,3-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36622

(Rapamycin C-7, analog 11b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc[nH]2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H80N2O12/c1-32-16-11-10-12-17-33(2)41(42-18-15-24-55-42)30-40-22-20-38(7)54(64,68-40)51(61)52(62)56-25-14-13-19-43(56)53(63)67-46(35(4)28-39-21-23-44(57)47(29-39)65-8)31-45(58)34(3)27-37(6)49(60)50(66-9)48(59)36(5)26-32/h10-12,15-18,24,27,32,34-36,38-41,43-44,46-47,49-50,55,57,60,64H,13-14,19-23,25-26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43+,44-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309897

(2-(1,4-Diazabicyclo[3.2.2]nonan-4-yl)-5-(pyrrolidi...)Show SMILES C1CCN(C1)c1ccc2oc(nc2n1)N1CCN2CCC1CC2 |TLB:10:14:19.18:21.22,(20.91,-33.41,;20.89,-31.87,;19.42,-31.41,;18.52,-32.67,;19.45,-33.91,;16.98,-32.69,;16,-31.49,;14.48,-31.72,;13.92,-33.16,;12.48,-33.7,;12.54,-35.24,;14.04,-35.65,;14.89,-34.37,;16.41,-34.13,;11.22,-36.01,;11.58,-37.48,;10.58,-36.75,;9.2,-36.82,;9.13,-35.12,;9.75,-33.98,;9.81,-35.39,;8.51,-36.09,;8.21,-37.53,)| Show InChI InChI=1S/C17H23N5O/c1-2-8-21(7-1)15-4-3-14-16(18-15)19-17(23-14)22-12-11-20-9-5-13(22)6-10-20/h3-4,13H,1-2,5-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309864

(4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...)Show SMILES Clc1ccc2oc(nc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(33.87,4.67,;32.34,4.44,;31.36,5.65,;29.84,5.41,;29.28,3.97,;27.84,3.42,;27.9,1.89,;29.4,1.48,;30.25,2.76,;31.78,2.99,;26.57,1.11,;26.94,-.35,;25.94,.37,;24.56,.3,;24.48,2.01,;25.1,3.15,;25.16,1.74,;23.87,1.03,;23.57,-.41,)| Show InChI InChI=1S/C14H16ClN3O/c15-10-1-2-13-12(9-10)16-14(19-13)18-8-7-17-5-3-11(18)4-6-17/h1-2,9,11H,3-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024380
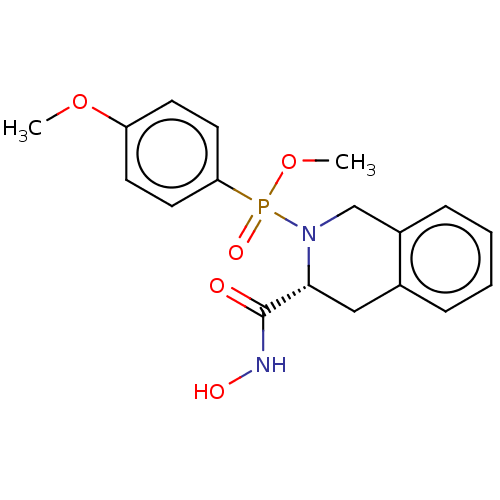
(CHEMBL193124)Show SMILES COc1ccc(cc1)P(=O)(OC)N1Cc2ccccc2C[C@@H]1C(=O)NO Show InChI InChI=1S/C18H21N2O5P/c1-24-15-7-9-16(10-8-15)26(23,25-2)20-12-14-6-4-3-5-13(14)11-17(20)18(21)19-22/h3-10,17,22H,11-12H2,1-2H3,(H,19,21)/t17-,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309872
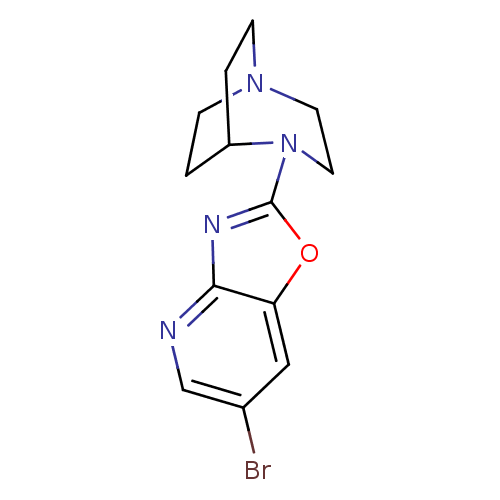
(4-(6-Bromooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...)Show SMILES Brc1cnc2nc(oc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(31.84,-7.59,;31.28,-9.04,;32.26,-10.25,;31.7,-11.69,;30.17,-11.93,;29.32,-13.21,;27.82,-12.8,;27.76,-11.26,;29.2,-10.72,;29.76,-9.28,;26.49,-13.57,;26.86,-15.04,;25.85,-14.32,;24.48,-14.39,;24.4,-12.68,;25.02,-11.54,;25.08,-12.95,;23.79,-13.65,;23.49,-15.09,)| Show InChI InChI=1S/C13H15BrN4O/c14-9-7-11-12(15-8-9)16-13(19-11)18-6-5-17-3-1-10(18)2-4-17/h7-8,10H,1-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309865

(4-(5-Bromobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2]...)Show SMILES Brc1ccc2oc(nc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(.18,-3.78,;-1.35,-4.01,;-2.33,-2.8,;-3.85,-3.04,;-4.41,-4.48,;-5.85,-5.03,;-5.79,-6.56,;-4.3,-6.97,;-3.44,-5.69,;-1.92,-5.46,;-7.12,-7.33,;-6.75,-8.8,;-7.76,-8.08,;-9.13,-8.15,;-9.21,-6.44,;-8.59,-5.3,;-8.53,-6.71,;-9.82,-7.41,;-10.12,-8.86,)| Show InChI InChI=1S/C14H16BrN3O/c15-10-1-2-13-12(9-10)16-14(19-13)18-8-7-17-5-3-11(18)4-6-17/h1-2,9,11H,3-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309880

(4-(6-Phenyloxazolo[5,4-b]pyridin-2-yl)-1,4-diazabi...)Show SMILES C1CN2CCC1N(CC2)c1nc2cc(cnc2o1)-c1ccccc1 |TLB:9:6:0.1:4.3,(-10.27,-43.52,;-10.9,-44.66,;-10.81,-46.36,;-11.81,-47.07,;-11.51,-45.63,;-10.21,-44.93,;-8.8,-45.55,;-8.44,-47.01,;-9.44,-46.29,;-7.48,-44.78,;-5.99,-45.19,;-5.14,-43.91,;-3.61,-43.68,;-3.05,-42.24,;-4.03,-41.03,;-5.55,-41.27,;-6.1,-42.7,;-7.54,-43.25,;-1.71,-41.48,;-.39,-42.27,;.96,-41.51,;.97,-39.96,;-.37,-39.18,;-1.71,-39.94,)| Show InChI InChI=1S/C19H20N4O/c1-2-4-14(5-3-1)15-12-17-18(20-13-15)24-19(21-17)23-11-10-22-8-6-16(23)7-9-22/h1-5,12-13,16H,6-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309879
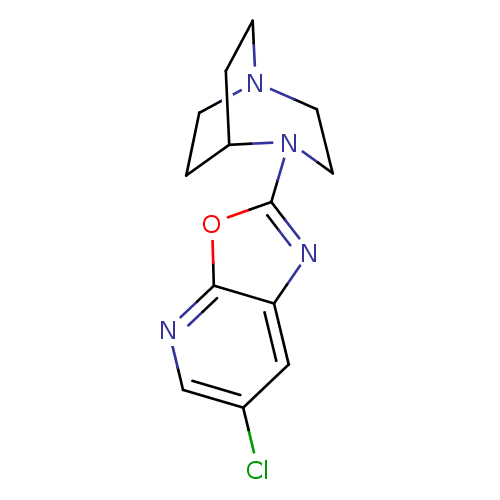
(2-(6-Chlorooxazolo[5,4-b]pyridin-2-yl)-2,5-diazabi...)Show SMILES Clc1cnc2oc(nc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(32.86,-26.98,;31.33,-27.22,;30.35,-26.01,;28.83,-26.25,;28.27,-27.69,;26.83,-28.23,;26.89,-29.77,;28.39,-30.18,;29.24,-28.9,;30.77,-28.66,;25.56,-30.54,;25.93,-32.01,;24.92,-31.29,;23.55,-31.36,;23.47,-29.65,;24.09,-28.51,;24.15,-29.92,;22.85,-30.62,;22.55,-32.06,)| Show InChI InChI=1S/C13H15ClN4O/c14-9-7-11-12(15-8-9)19-13(16-11)18-6-5-17-3-1-10(18)2-4-17/h7-8,10H,1-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36619
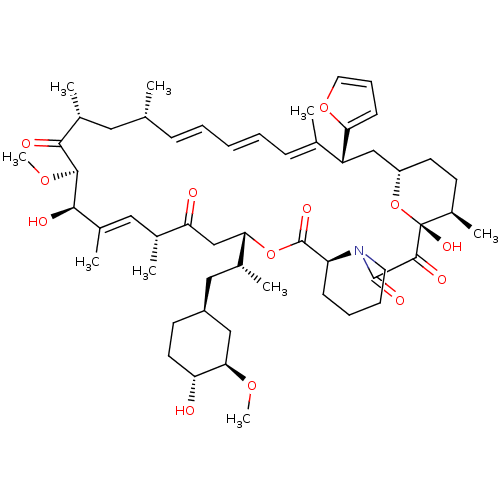
(Rapamycin C-7, analog 10a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41-,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309888
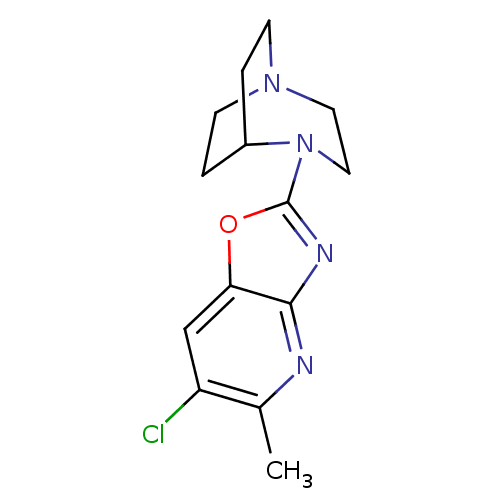
(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309892

(2-(5-Phenyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabi...)Show SMILES C1CN2CCC1N(CC2)c1nc2nc(ccc2o1)-c1ccccc1 |TLB:9:6:0.1:4.3,(-8.33,-22.75,;-8.95,-23.89,;-8.87,-25.59,;-9.86,-26.3,;-9.56,-24.86,;-8.27,-24.16,;-6.86,-24.78,;-6.49,-26.25,;-7.5,-25.53,;-5.53,-24.01,;-4.04,-24.42,;-3.19,-23.14,;-1.66,-22.9,;-1.1,-21.46,;-2.07,-20.26,;-3.6,-20.5,;-4.15,-21.93,;-5.59,-22.48,;.25,-20.71,;1.57,-21.5,;2.92,-20.74,;2.94,-19.2,;1.6,-18.41,;.26,-19.17,)| Show InChI InChI=1S/C19H20N4O/c1-2-4-14(5-3-1)16-6-7-17-18(20-16)21-19(24-17)23-13-12-22-10-8-15(23)9-11-22/h1-7,15H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024400
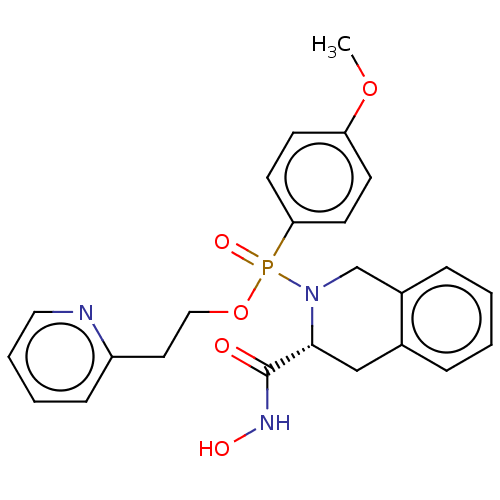
(CHEMBL192719)Show SMILES COc1ccc(cc1)P(=O)(OCCc1ccccn1)N1Cc2ccccc2C[C@@H]1C(=O)NO Show InChI InChI=1S/C24H26N3O5P/c1-31-21-9-11-22(12-10-21)33(30,32-15-13-20-8-4-5-14-25-20)27-17-19-7-3-2-6-18(19)16-23(27)24(28)26-29/h2-12,14,23,29H,13,15-17H2,1H3,(H,26,28)/t23-,33?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Mus musculus (house mouse)) | BDBM50309866

(4-(5-Methylbenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...)Show SMILES Cc1ccc2oc(nc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(11.7,-3.08,;10.17,-3.31,;9.19,-2.1,;7.67,-2.34,;7.11,-3.78,;5.67,-4.33,;5.73,-5.86,;7.23,-6.27,;8.08,-4.99,;9.61,-4.76,;4.4,-6.63,;4.77,-8.1,;3.76,-7.38,;2.39,-7.45,;2.31,-5.74,;2.93,-4.6,;2.99,-6.01,;1.7,-6.71,;1.4,-8.15,)| Show InChI InChI=1S/C15H19N3O/c1-11-2-3-14-13(10-11)16-15(19-14)18-9-8-17-6-4-12(18)5-7-17/h2-3,10,12H,4-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309893
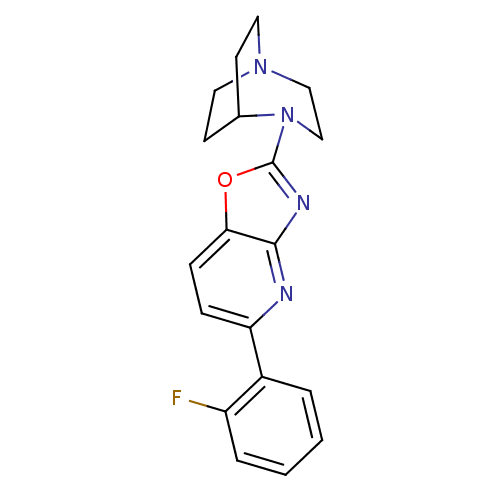
(2-(5-(2-Fluorophenyl)oxazolo[4,5-b]pyridin-2-yl)-2...)Show SMILES Fc1ccccc1-c1ccc2oc(nc2n1)N1CCN2CCC1CC2 |TLB:12:16:21.20:23.24,(13.59,-22.93,;13.62,-21.39,;14.96,-20.63,;14.98,-19.08,;13.64,-18.29,;12.3,-19.05,;12.29,-20.59,;10.94,-21.35,;9.97,-20.14,;8.45,-20.38,;7.89,-21.81,;6.45,-22.36,;6.51,-23.89,;8,-24.3,;8.86,-23.02,;10.38,-22.79,;5.18,-24.67,;5.55,-26.13,;4.55,-25.41,;3.17,-25.48,;3.1,-23.78,;3.71,-22.63,;3.77,-24.05,;2.48,-24.75,;2.18,-26.19,)| Show InChI InChI=1S/C19H19FN4O/c20-15-4-2-1-3-14(15)16-5-6-17-18(21-16)22-19(25-17)24-12-11-23-9-7-13(24)8-10-23/h1-6,13H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36610
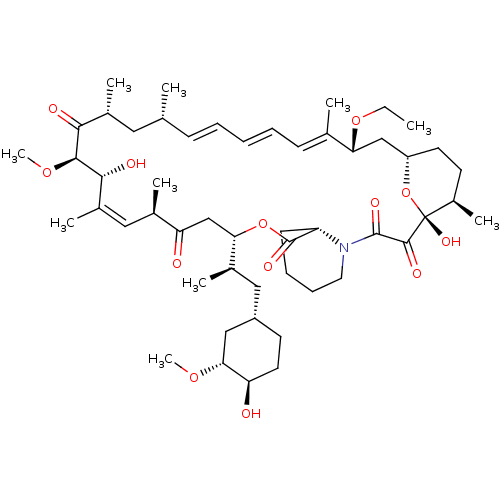
(Rapamycin C-7, analog 5a)Show SMILES CCO[C@@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43-,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36615
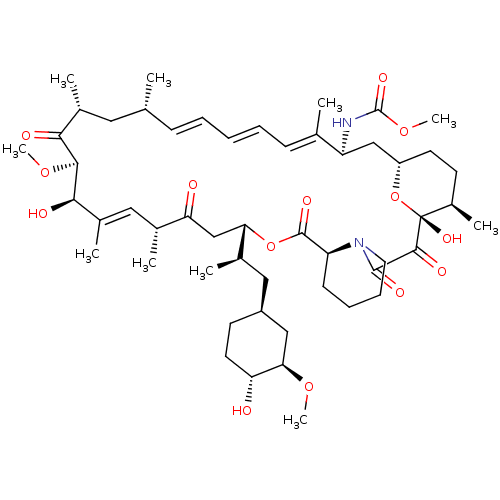
(Rapamycin C-7, analog 7b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024393
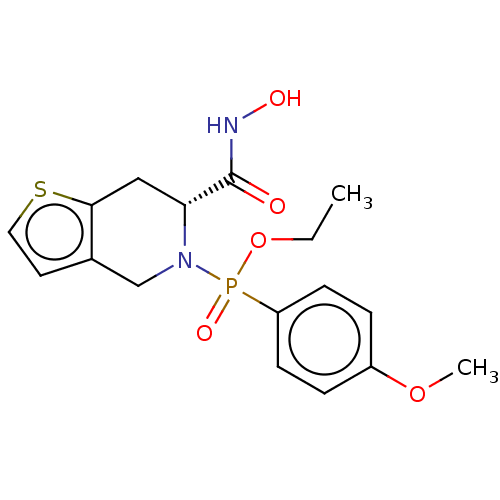
(CHEMBL426449)Show SMILES CCOP(=O)(N1Cc2ccsc2C[C@@H]1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C17H21N2O5PS/c1-3-24-25(22,14-6-4-13(23-2)5-7-14)19-11-12-8-9-26-16(12)10-15(19)17(20)18-21/h4-9,15,21H,3,10-11H2,1-2H3,(H,18,20)/t15-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Mus musculus (house mouse)) | BDBM50309863
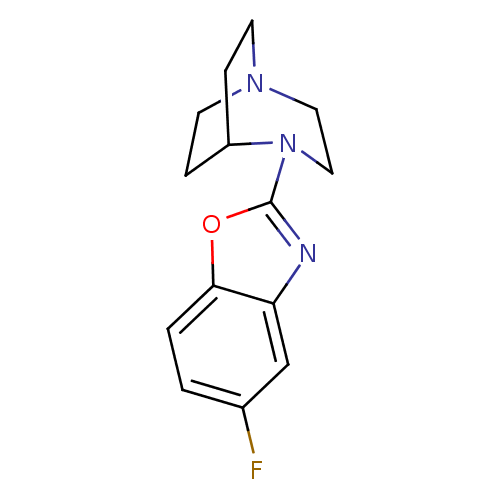
(4-(5-Fluorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...)Show SMILES Fc1ccc2oc(nc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(22.74,5.07,;21.21,4.83,;20.23,6.04,;18.71,5.8,;18.15,4.37,;16.71,3.82,;16.77,2.29,;18.27,1.88,;19.12,3.16,;20.65,3.39,;15.44,1.51,;15.81,.04,;14.8,.77,;13.43,.7,;13.35,2.4,;13.97,3.55,;14.03,2.13,;12.74,1.43,;12.44,-.01,)| Show InChI InChI=1S/C14H16FN3O/c15-10-1-2-13-12(9-10)16-14(19-13)18-8-7-17-5-3-11(18)4-6-17/h1-2,9,11H,3-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36611

(Rapamycin C-7, analog 5b)Show SMILES CCO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43+,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309886
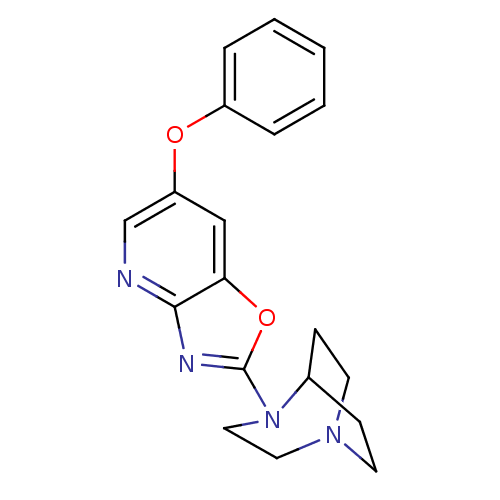
(2-(6-Phenoxyoxazolo[4,5-b]pyridin-2-yl)-2,5-diazab...)Show SMILES C1CN2CCC1N(CC2)c1nc2ncc(Oc3ccccc3)cc2o1 |TLB:9:6:0.1:4.3,(12.67,-1.62,;12.06,-2.77,;12.13,-4.47,;11.15,-5.17,;11.44,-3.73,;12.74,-3.03,;14.14,-3.65,;14.51,-5.12,;13.51,-4.4,;15.47,-2.88,;16.96,-3.29,;17.81,-2.01,;19.34,-1.78,;19.9,-.34,;18.92,.87,;19.48,2.32,;20.83,3.08,;22.17,2.29,;23.52,3.05,;23.53,4.6,;22.18,5.39,;20.84,4.62,;17.4,.63,;16.85,-.81,;15.41,-1.35,)| Show InChI InChI=1S/C19H20N4O2/c1-2-4-15(5-3-1)24-16-12-17-18(20-13-16)21-19(25-17)23-11-10-22-8-6-14(23)7-9-22/h1-5,12-14H,6-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36614
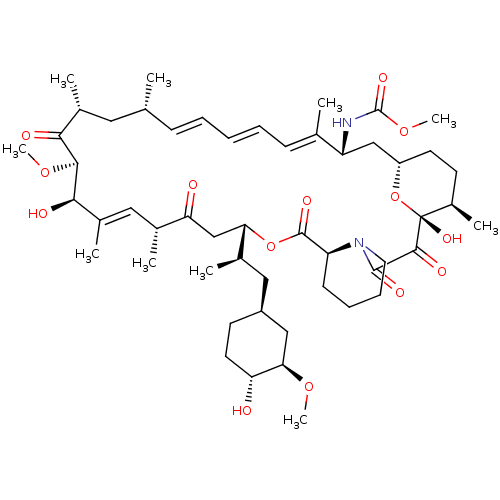
(Rapamycin C-7, analog 7a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39-,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024383
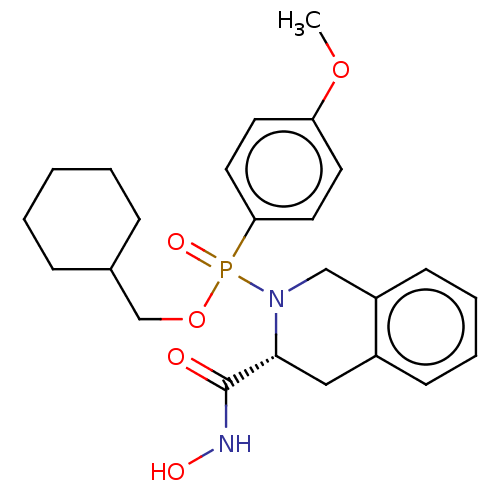
(CHEMBL192121)Show SMILES COc1ccc(cc1)P(=O)(OCC1CCCCC1)N1Cc2ccccc2C[C@@H]1C(=O)NO Show InChI InChI=1S/C24H31N2O5P/c1-30-21-11-13-22(14-12-21)32(29,31-17-18-7-3-2-4-8-18)26-16-20-10-6-5-9-19(20)15-23(26)24(27)25-28/h5-6,9-14,18,23,28H,2-4,7-8,15-17H2,1H3,(H,25,27)/t23-,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36625
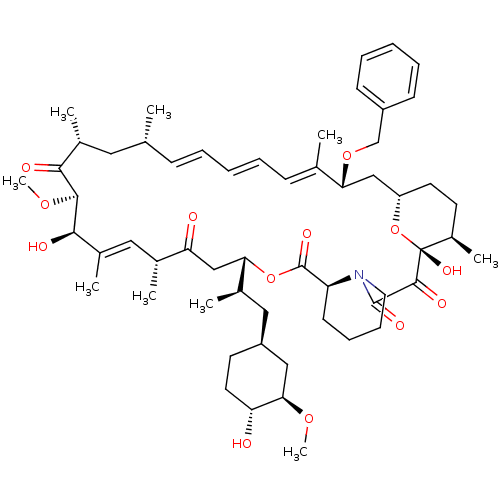
(Rapamycin C-7, analog 14a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccccc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H83NO13/c1-35-18-12-10-13-19-36(2)48(69-34-42-20-14-11-15-21-42)32-44-25-23-41(7)57(66,71-44)54(63)55(64)58-27-17-16-22-45(58)56(65)70-49(38(4)30-43-24-26-46(59)50(31-43)67-8)33-47(60)37(3)29-40(6)52(62)53(68-9)51(61)39(5)28-35/h10-15,18-21,29,35,37-39,41,43-46,48-50,52-53,59,62,66H,16-17,22-28,30-34H2,1-9H3/b13-10+,18-12+,36-19+,40-29+/t35-,37-,38-,39-,41-,43+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50171860
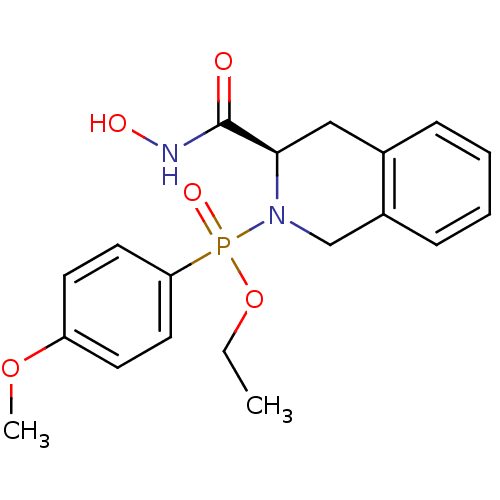
(((R)-3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin...)Show SMILES CCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C19H23N2O5P/c1-3-26-27(24,17-10-8-16(25-2)9-11-17)21-13-15-7-5-4-6-14(15)12-18(21)19(22)20-23/h4-11,18,23H,3,12-13H2,1-2H3,(H,20,22)/t18-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50171860

(((R)-3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin...)Show SMILES CCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C19H23N2O5P/c1-3-26-27(24,17-10-8-16(25-2)9-11-17)21-13-15-7-5-4-6-14(15)12-18(21)19(22)20-23/h4-11,18,23H,3,12-13H2,1-2H3,(H,20,22)/t18-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition constant for human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024385
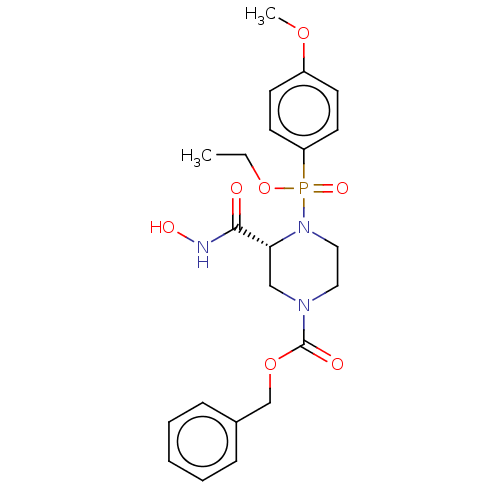
(CHEMBL365588)Show SMILES CCOP(=O)(N1CCN(C[C@@H]1C(=O)NO)C(=O)OCc1ccccc1)c1ccc(OC)cc1 Show InChI InChI=1S/C22H28N3O7P/c1-3-32-33(29,19-11-9-18(30-2)10-12-19)25-14-13-24(15-20(25)21(26)23-28)22(27)31-16-17-7-5-4-6-8-17/h4-12,20,28H,3,13-16H2,1-2H3,(H,23,26)/t20-,33?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309891
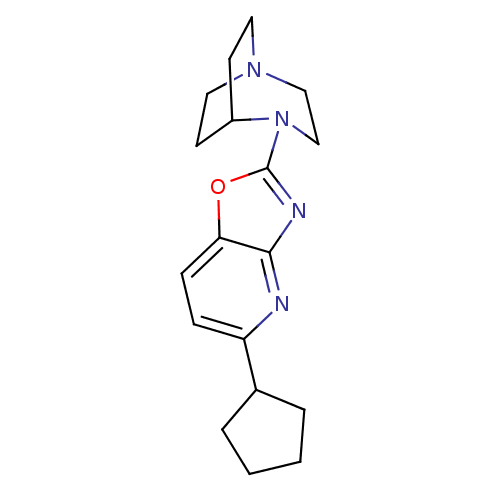
(2-(5-Cyclopentyloxazolo[4,5-b]pyridin-2-yl)-2,5-di...)Show SMILES C1CCC(C1)c1ccc2oc(nc2n1)N1CCN2CCC1CC2 |TLB:10:14:19.18:21.22,(36.18,-10.06,;35.94,-8.53,;34.41,-8.28,;33.71,-9.66,;34.8,-10.75,;32.18,-9.9,;31.21,-8.69,;29.69,-8.93,;29.13,-10.36,;27.69,-10.91,;27.75,-12.44,;29.24,-12.85,;30.1,-11.57,;31.62,-11.34,;26.42,-13.21,;26.79,-14.68,;25.79,-13.96,;24.41,-14.03,;24.34,-12.33,;24.95,-11.18,;25.02,-12.59,;23.72,-13.3,;23.42,-14.73,)| Show InChI InChI=1S/C18H24N4O/c1-2-4-13(3-1)15-5-6-16-17(19-15)20-18(23-16)22-12-11-21-9-7-14(22)8-10-21/h5-6,13-14H,1-4,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36620

(Rapamycin C-7, analog 10b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309882
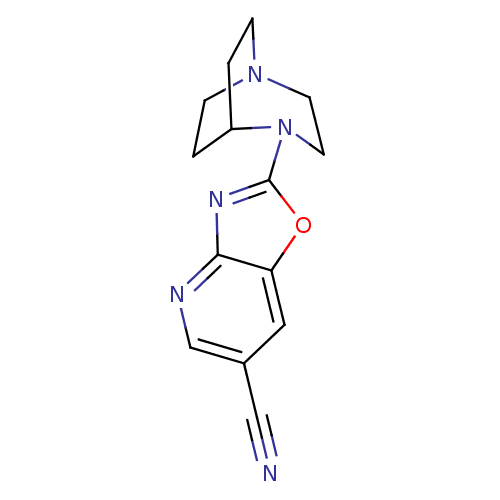
(4-(6-Cyanooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...)Show SMILES N#Cc1cnc2nc(oc2c1)N1CCN2CCC1CC2 |TLB:7:11:16.15:18.19,(18.05,-37.42,;17.5,-38.87,;16.94,-40.32,;17.92,-41.53,;17.36,-42.97,;15.83,-43.2,;14.98,-44.48,;13.48,-44.07,;13.42,-42.54,;14.86,-41.99,;15.42,-40.56,;12.15,-44.85,;12.52,-46.31,;11.51,-45.59,;10.14,-45.66,;10.06,-43.96,;10.68,-42.81,;10.74,-44.22,;9.45,-44.93,;9.15,-46.37,)| Show InChI InChI=1S/C14H15N5O/c15-8-10-7-12-13(16-9-10)17-14(20-12)19-6-5-18-3-1-11(19)2-4-18/h7,9,11H,1-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024389
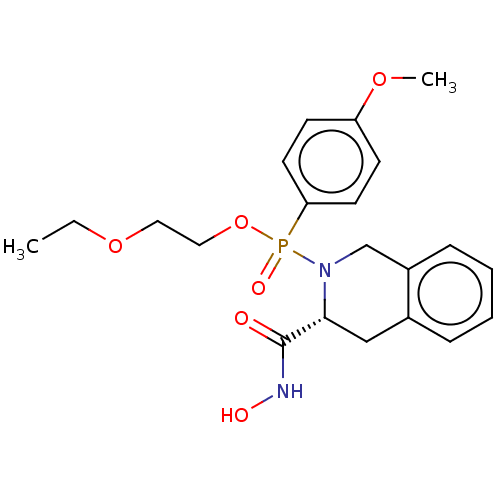
(CHEMBL370032)Show SMILES CCOCCOP(=O)(N1Cc2ccccc2C[C@@H]1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H27N2O6P/c1-3-28-12-13-29-30(26,19-10-8-18(27-2)9-11-19)23-15-17-7-5-4-6-16(17)14-20(23)21(24)22-25/h4-11,20,25H,3,12-15H2,1-2H3,(H,22,24)/t20-,30?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024381
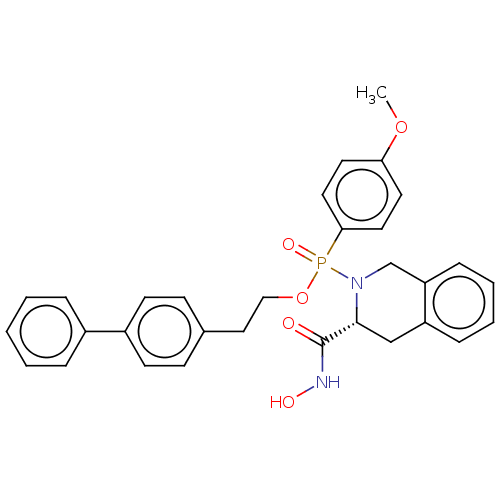
(CHEMBL190713)Show SMILES COc1ccc(cc1)P(=O)(OCCc1ccc(cc1)-c1ccccc1)N1Cc2ccccc2C[C@@H]1C(=O)NO Show InChI InChI=1S/C31H31N2O5P/c1-37-28-15-17-29(18-16-28)39(36,33-22-27-10-6-5-9-26(27)21-30(33)31(34)32-35)38-20-19-23-11-13-25(14-12-23)24-7-3-2-4-8-24/h2-18,30,35H,19-22H2,1H3,(H,32,34)/t30-,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50024397
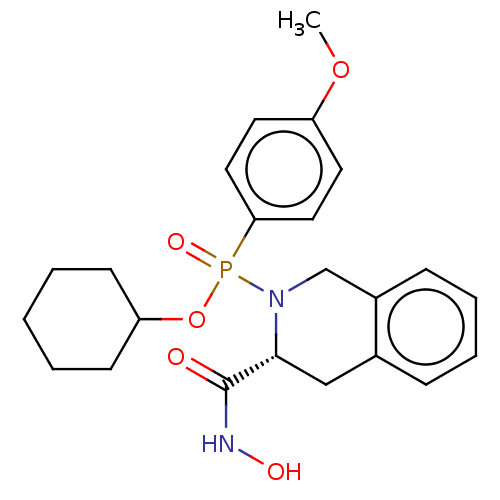
(CHEMBL372470)Show SMILES COc1ccc(cc1)P(=O)(OC1CCCCC1)N1Cc2ccccc2C[C@@H]1C(=O)NO Show InChI InChI=1S/C23H29N2O5P/c1-29-19-11-13-21(14-12-19)31(28,30-20-9-3-2-4-10-20)25-16-18-8-6-5-7-17(18)15-22(25)23(26)24-27/h5-8,11-14,20,22,27H,2-4,9-10,15-16H2,1H3,(H,24,26)/t22-,31?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of MOCAc-Pro-Leu-Gly-Leu-A2p (Dnp)-Ala-Arg-NH2 binding to human matrix metalloprotease-9 (MMP-9) |
J Med Chem 48: 5437-47 (2005)
Article DOI: 10.1021/jm049050v
BindingDB Entry DOI: 10.7270/Q2Z89BZ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50309875
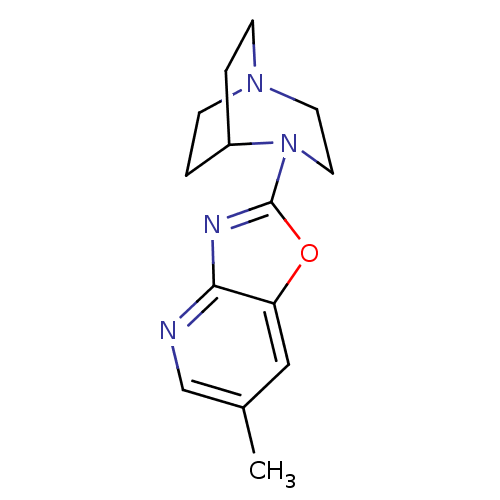
(4-(6-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...)Show SMILES Cc1cnc2nc(oc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(18.8,-18.14,;18.24,-19.59,;19.22,-20.8,;18.66,-22.24,;17.13,-22.47,;16.28,-23.76,;14.78,-23.34,;14.72,-21.81,;16.16,-21.26,;16.72,-19.83,;13.45,-24.12,;13.82,-25.59,;12.81,-24.86,;11.44,-24.93,;11.36,-23.23,;11.98,-22.08,;12.04,-23.5,;10.75,-24.2,;10.45,-25.64,)| Show InChI InChI=1S/C14H18N4O/c1-10-8-12-13(15-9-10)16-14(19-12)18-7-6-17-4-2-11(18)3-5-17/h8-9,11H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36618

(Rapamycin C-7, analog 9)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2c(OC)cc(OC)cc2OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C59H87NO15/c1-34-18-14-13-15-19-35(2)44(52-50(71-10)31-43(69-8)32-51(52)72-11)30-42-23-21-40(7)59(68,75-42)56(65)57(66)60-25-17-16-20-45(60)58(67)74-48(37(4)28-41-22-24-46(61)49(29-41)70-9)33-47(62)36(3)27-39(6)54(64)55(73-12)53(63)38(5)26-34/h13-15,18-19,27,31-32,34,36-38,40-42,44-46,48-49,54-55,61,64,68H,16-17,20-26,28-30,33H2,1-12H3/b15-13+,18-14+,35-19+,39-27+/t34-,36-,37-,38-,40-,41+,42+,44+,45+,46-,48+,49-,54-,55+,59-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36626

(Rapamycin C-7, analog 15a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2cccc(c2)N(=O)=O)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82N2O15/c1-34-16-11-10-12-17-35(2)48(72-33-42-18-15-19-43(29-42)59(68)69)31-44-23-21-40(7)57(67,74-44)54(64)55(65)58-25-14-13-20-45(58)56(66)73-49(37(4)28-41-22-24-46(60)50(30-41)70-8)32-47(61)36(3)27-39(6)52(63)53(71-9)51(62)38(5)26-34/h10-12,15-19,27,29,34,36-38,40-41,44-46,48-50,52-53,60,63,67H,13-14,20-26,28,30-33H2,1-9H3/b12-10+,16-11+,35-17+,39-27+/t34-,36-,37-,38-,40-,41+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data