 Found 1211 hits with Last Name = 'handzlik' and Initial = 'j'
Found 1211 hits with Last Name = 'handzlik' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50535225
(CHEMBL4516622)Show SMILES C[C@@H]1CCCN1[C@H]1C[C@@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r,wU:8.11,1.0,wD:6.6,(34.83,-7.94,;35.15,-9.44,;34.11,-10.59,;34.88,-11.92,;36.39,-11.6,;36.55,-10.07,;37.88,-9.3,;39.37,-9.7,;39.77,-8.21,;38.28,-7.81,;41.1,-7.45,;41.1,-5.9,;42.43,-5.13,;43.77,-5.9,;43.77,-7.45,;42.43,-8.22,;45.09,-5.12,;46.43,-5.89,;47.76,-5.12,;47.75,-3.58,;46.41,-2.81,;45.08,-3.59,;49.08,-2.8,;50.41,-2.03,)| Show InChI InChI=1S/C22H24N2/c1-16-3-2-12-24(16)22-13-21(14-22)20-10-8-19(9-11-20)18-6-4-17(15-23)5-7-18/h4-11,16,21-22H,2-3,12-14H2,1H3/t16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human H3R |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting |
Bioorg Med Chem 19: 1349-60 (2011)
Article DOI: 10.1016/j.bmc.2010.11.051
BindingDB Entry DOI: 10.7270/Q2TT4TS3 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting |
Bioorg Med Chem 19: 1349-60 (2011)
Article DOI: 10.1016/j.bmc.2010.11.051
BindingDB Entry DOI: 10.7270/Q2TT4TS3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50393167

(CHEMBL2153721)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C1C2CC3OC(=O)C1C3C2 |TLB:22:24:26:19.20,21:20:24.17:26,THB:6:17:26:19.20| Show InChI InChI=1S/C19H24N4O4/c1-3-5-22-16-14(17(24)23(6-4-2)19(22)26)20-15(21-16)12-9-7-10-11(8-9)27-18(25)13(10)12/h9-13H,3-8H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from adenosine A1 receptor in rat brain cortical membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595
((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human H3R expressed in rat C6 cells |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044615

(CHEMBL3329435)Show InChI InChI=1S/C18H20N4O2S/c1-14-20-18-16(21-12-10-19-11-13-21)8-5-9-17(18)22(14)25(23,24)15-6-3-2-4-7-15/h2-9,19H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM34152
(CHEMBL362628 | E-6801)Show SMILES CN(C)CCc1c[nH]c2ccc(NS(=O)(=O)c3c(Cl)nc4sccn34)cc12 Show InChI InChI=1S/C17H18ClN5O2S2/c1-22(2)6-5-11-10-19-14-4-3-12(9-13(11)14)21-27(24,25)16-15(18)20-17-23(16)7-8-26-17/h3-4,7-10,19,21H,5-6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415977
(CHEMBL1085113)Show InChI InChI=1S/C18H20N2O3S/c19-18(21)20-12-14-6-4-5-13-11-16(9-10-17(13)14)24(22,23)15-7-2-1-3-8-15/h1-3,7-11,14H,4-6,12H2,(H3,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
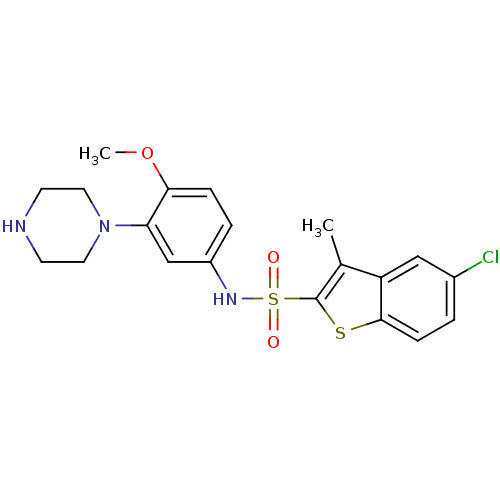
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells after 1 hr by liquid scintillation counting method |
Eur J Med Chem 135: 117-124 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.033
BindingDB Entry DOI: 10.7270/Q2QN696C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins |
Bioorg Med Chem 19: 2850-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.046
BindingDB Entry DOI: 10.7270/Q2X63N8N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50419052

(SB-399885)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Cl)cc(Cl)c1OC Show InChI InChI=1S/C18H21Cl2N3O4S/c1-26-17-4-3-13(11-16(17)23-7-5-21-6-8-23)28(24,25)22-15-10-12(19)9-14(20)18(15)27-2/h3-4,9-11,21-22H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells |
Eur J Med Chem 135: 117-124 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.033
BindingDB Entry DOI: 10.7270/Q2QN696C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004518
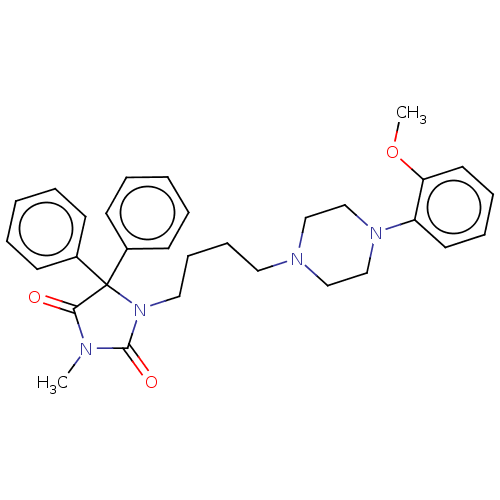
(CHEMBL2079256)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)N(C)C(=O)C2(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H36N4O3/c1-32-29(36)31(25-13-5-3-6-14-25,26-15-7-4-8-16-26)35(30(32)37)20-12-11-19-33-21-23-34(24-22-33)27-17-9-10-18-28(27)38-2/h3-10,13-18H,11-12,19-24H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in HEK293 cell membrane after 1 hr by Microbeta scintillation countin... |
Eur J Med Chem 78: 324-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.065
BindingDB Entry DOI: 10.7270/Q2J104Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
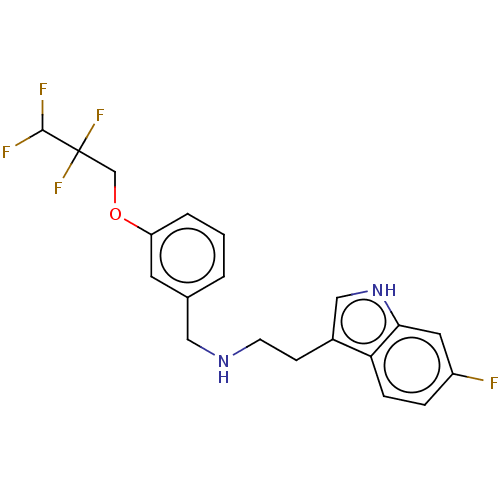
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] raclopride from human recombinant D2L receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50606660
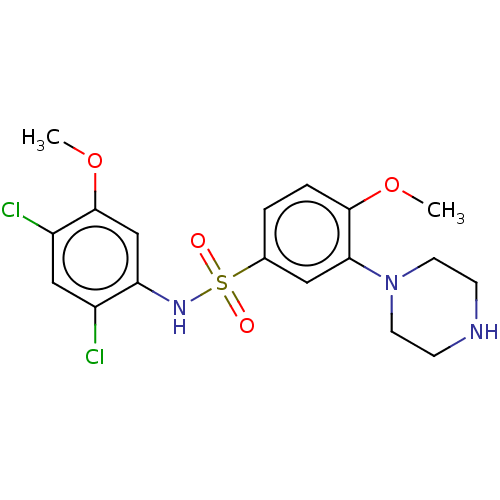
(CHEMBL5220473)Show SMILES COc1cc(NS(=O)(=O)c2ccc(OC)c(c2)N2CCNCC2)c(Cl)cc1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50606652
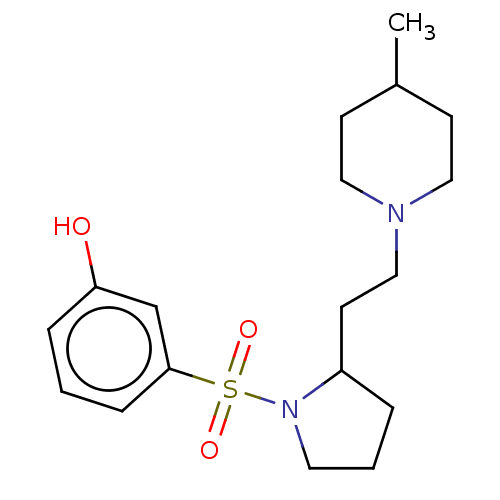
(CHEMBL5218527) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50606653
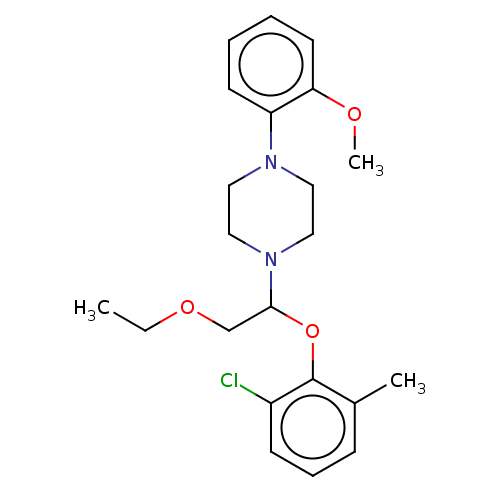
(CHEMBL5219333) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044616
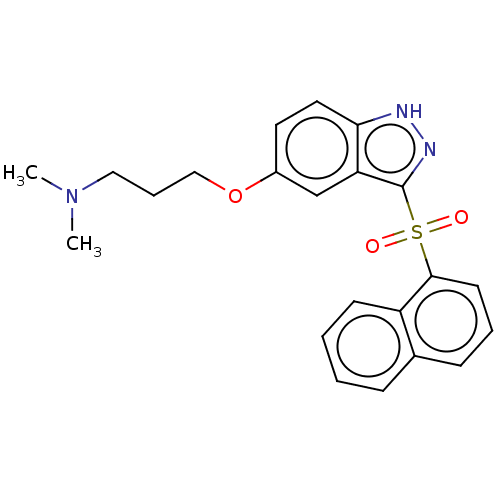
(Cerlapirdine | PF-05212365 | SAM-531 | WAY-262531)Show SMILES CN(C)CCCOc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H23N3O3S/c1-25(2)13-6-14-28-17-11-12-20-19(15-17)22(24-23-20)29(26,27)21-10-5-8-16-7-3-4-9-18(16)21/h3-5,7-12,15H,6,13-14H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM30712
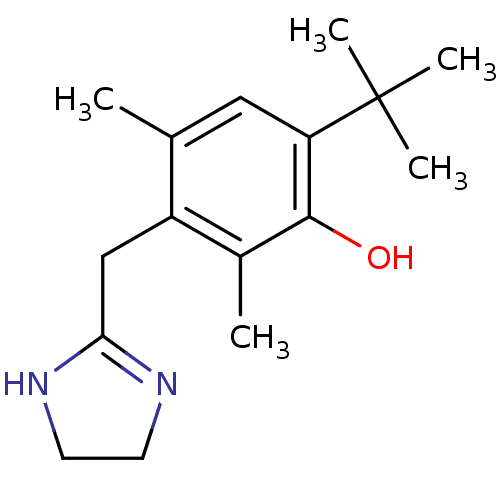
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-clonidine from alpha2-adrenergic receptor in rat brain cortex after 30 mins by Microbeta scintillation counting method |
Eur J Med Chem 147: 102-114 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.093
BindingDB Entry DOI: 10.7270/Q29P346S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human full-length histamine H4 receptor expressed in HEK293 cells after 60 mins |
Bioorg Med Chem 19: 2850-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.046
BindingDB Entry DOI: 10.7270/Q2X63N8N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50400902

(1-(2-(2,4-dimethylphenylsulfanyl)phenyl)piperazine...)Show InChI InChI=1S/C18H22N2S/c1-14-7-8-17(15(2)13-14)21-18-6-4-3-5-16(18)20-11-9-19-10-12-20/h3-8,13,19H,9-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50606655

(CHEMBL5219924)Show SMILES O=C1C2C3CCC(C3)C2C(=O)N1CC1CCCCC1CN1CCN(CC1)c1nsc2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50533199
(CHEMBL4527011)Show SMILES [H][C@]12CCN(c3ccc(cc3)-c3ccc(cc3)-n3ncccc3=O)[C@@]1([H])CN(C)C2 |r| Show InChI InChI=1S/C23H24N4O/c1-25-15-19-12-14-26(22(19)16-25)20-8-4-17(5-9-20)18-6-10-21(11-7-18)27-23(28)3-2-13-24-27/h2-11,13,19,22H,12,14-16H2,1H3/t19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3R expressed in rat C6 cells incubated for 20 mins by [35S]GTPgammaS binding assay |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50606655

(CHEMBL5219924)Show SMILES O=C1C2C3CCC(C3)C2C(=O)N1CC1CCCCC1CN1CCN(CC1)c1nsc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50606656
(CHEMBL4117763)Show SMILES COc1ccc2n(cc(CN3CCNCC3)c2c1)S(=O)(=O)c1ccccc1Br | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-MeHA from rat brain H3 receptor |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21358

(2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...)Show SMILES NCCc1cn(c2ccccc12)S(=O)(=O)c1c(Cl)nc2sccn12 Show InChI InChI=1S/C15H13ClN4O2S2/c16-13-14(19-7-8-23-15(19)18-13)24(21,22)20-9-10(5-6-17)11-3-1-2-4-12(11)20/h1-4,7-9H,5-6,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins |
Bioorg Med Chem 19: 2850-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.046
BindingDB Entry DOI: 10.7270/Q2X63N8N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human striatal full length H3 receptor after 60 mins by gamma counting method |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50458523

(JNJ-18038683)Show InChI InChI=1S/C20H20ClN3/c21-17-8-6-16(7-9-17)20-18-10-12-22-13-11-19(18)24(23-20)14-15-4-2-1-3-5-15/h1-9,22H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM81790
(Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...)Show InChI InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50274767
(CHEMBL4125735)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N[C@H]3CCNC3)nc3ccccc3c12 |r| Show InChI InChI=1S/C21H19ClN4O2S/c22-14-4-3-5-16(12-14)29(27,28)26-11-9-18-20(26)17-6-1-2-7-19(17)25-21(18)24-15-8-10-23-13-15/h1-7,9,11-12,15,23H,8,10,13H2,(H,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HEK293 cells incubated for 1 hr by radioligand binding assay |
Eur J Med Chem 178: 740-751 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.022
BindingDB Entry DOI: 10.7270/Q22F7RSS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50004756
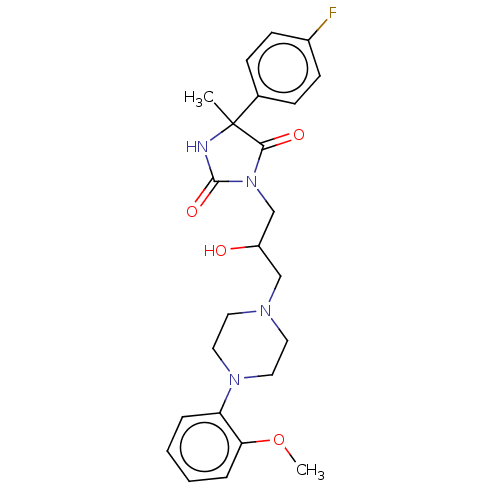
(CHEMBL3233679)Show SMILES Cl.COc1ccccc1N1CCN(CC(O)CN2C(=O)NC(C)(C2=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C24H29FN4O4.ClH/c1-24(17-7-9-18(25)10-8-17)22(31)29(23(32)26-24)16-19(30)15-27-11-13-28(14-12-27)20-5-3-4-6-21(20)33-2;/h3-10,19,30H,11-16H2,1-2H3,(H,26,32);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7 receptor expressed in HEK293 cell membrane after 1 hr by Microbeta scintillation counting anal... |
Eur J Med Chem 78: 324-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.065
BindingDB Entry DOI: 10.7270/Q2J104Q0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM81790

(Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...)Show InChI InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50400902

(1-(2-(2,4-dimethylphenylsulfanyl)phenyl)piperazine...)Show InChI InChI=1S/C18H22N2S/c1-14-7-8-17(15(2)13-14)21-18-6-4-3-5-16(18)20-11-9-19-10-12-20/h3-8,13,19H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50004587

(8-Adamantan-1-yl-1,3-dipropyl-3,7-dihydro-purine-2...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CC3CC(CC(C3)C1)C2 |TLB:24:19:26:25.23.22,24:23:26:18.19.20,20:21:25:18.19.24,THB:20:19:25:26.21.22| Show InChI InChI=1S/C21H30N4O2/c1-3-5-24-17-16(18(26)25(6-4-2)20(24)27)22-19(23-17)21-10-13-7-14(11-21)9-15(8-13)12-21/h13-15H,3-12H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from adenosine A1 receptor in rat brain cortical membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50524111

(CHEMBL4452569)Show InChI InChI=1S/C13H11FIN3/c1-2-18-7-16-6-11(18)8-5-17-10-4-3-9(15)13(14)12(8)10/h3-7,17H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044601
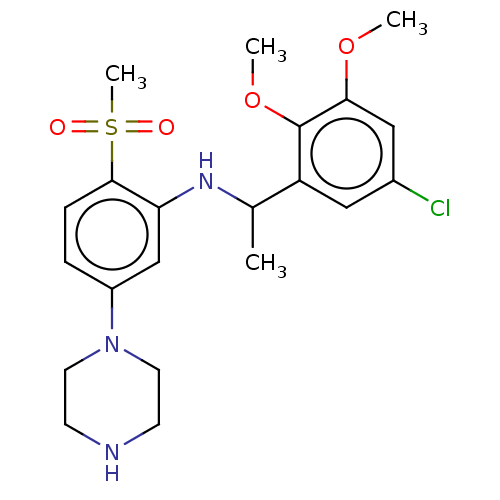
(CHEMBL3329445)Show SMILES COc1cc(Cl)cc(C(C)Nc2cc(ccc2S(C)(=O)=O)N2CCNCC2)c1OC Show InChI InChI=1S/C21H28ClN3O4S/c1-14(17-11-15(22)12-19(28-2)21(17)29-3)24-18-13-16(25-9-7-23-8-10-25)5-6-20(18)30(4,26)27/h5-6,11-14,23-24H,7-10H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human full-length histamine H4 receptor expressed in HEK293 cells after 60 mins |
Bioorg Med Chem 19: 2850-8 (2011)
Article DOI: 10.1016/j.bmc.2011.03.046
BindingDB Entry DOI: 10.7270/Q2X63N8N |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50004518

(CHEMBL2079256)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)N(C)C(=O)C2(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H36N4O3/c1-32-29(36)31(25-13-5-3-6-14-25,26-15-7-4-8-16-26)35(30(32)37)20-12-11-19-33-21-23-34(24-22-33)27-17-9-10-18-28(27)38-2/h3-10,13-18H,11-12,19-24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by microbeta scintillation counting |
Bioorg Med Chem 20: 2290-303 (2012)
Article DOI: 10.1016/j.bmc.2012.02.009
BindingDB Entry DOI: 10.7270/Q2XW4NP9 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50004518

(CHEMBL2079256)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)N(C)C(=O)C2(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H36N4O3/c1-32-29(36)31(25-13-5-3-6-14-25,26-15-7-4-8-16-26)35(30(32)37)20-12-11-19-33-21-23-34(24-22-33)27-17-9-10-18-28(27)38-2/h3-10,13-18H,11-12,19-24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by microbeta scintillation counting |
Bioorg Med Chem 20: 2290-303 (2012)
Article DOI: 10.1016/j.bmc.2012.02.009
BindingDB Entry DOI: 10.7270/Q2XW4NP9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50300828
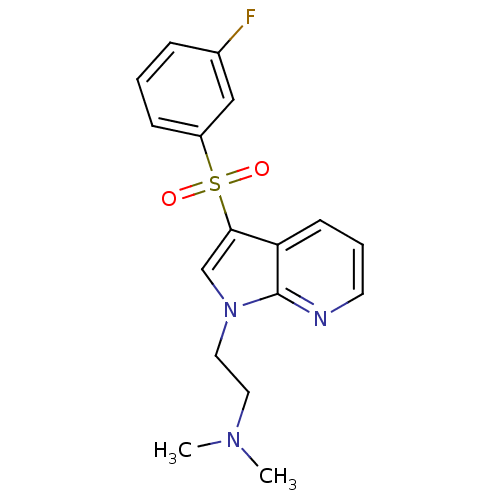
(2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...)Show InChI InChI=1S/C17H18FN3O2S/c1-20(2)9-10-21-12-16(15-7-4-8-19-17(15)21)24(22,23)14-6-3-5-13(18)11-14/h3-8,11-12H,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128275
BindingDB Entry DOI: 10.7270/Q2VQ36SJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50524116
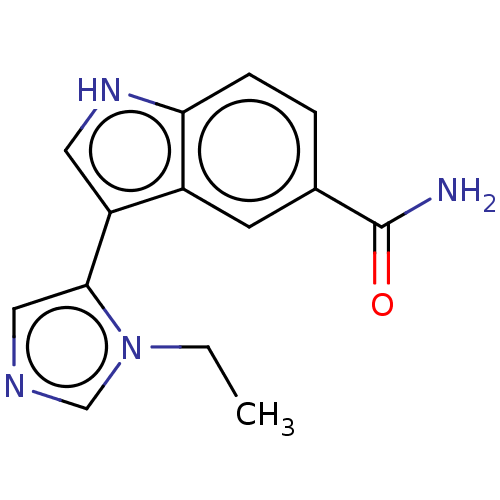
(CHEMBL4469847)Show InChI InChI=1S/C14H14N4O/c1-2-18-8-16-7-13(18)11-6-17-12-4-3-9(14(15)19)5-10(11)12/h3-8,17H,2H2,1H3,(H2,15,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50176050
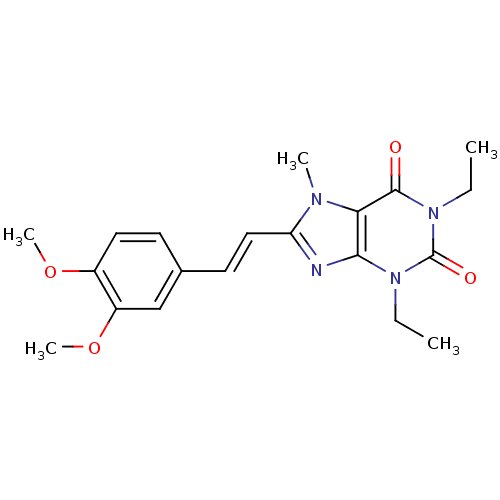
(8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...)Show SMILES CCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]MSX-2 from adenosine A2A receptor in rat brain striatal membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50272040
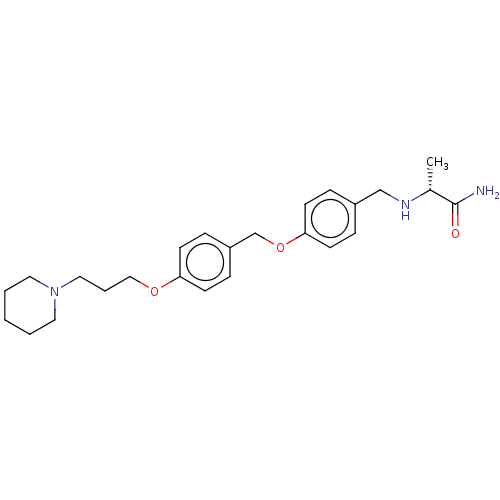
(CHEMBL4129950)Show SMILES C[C@@H](NCc1ccc(OCc2ccc(OCCCN3CCCCC3)cc2)cc1)C(N)=O |r| Show InChI InChI=1S/C25H35N3O3/c1-20(25(26)29)27-18-21-6-10-24(11-7-21)31-19-22-8-12-23(13-9-22)30-17-5-16-28-14-3-2-4-15-28/h6-13,20,27H,2-5,14-19H2,1H3,(H2,26,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Nalpha-MeHA from human H3 receptor expressed in HEK293 cell membranes by scintillation counting method |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004520
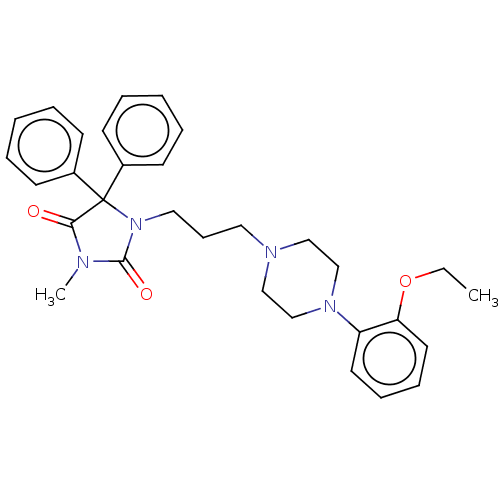
(CHEMBL3233401)Show SMILES CCOc1ccccc1N1CCN(CCCN2C(=O)N(C)C(=O)C2(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H36N4O3/c1-3-38-28-18-11-10-17-27(28)34-23-21-33(22-24-34)19-12-20-35-30(37)32(2)29(36)31(35,25-13-6-4-7-14-25)26-15-8-5-9-16-26/h4-11,13-18H,3,12,19-24H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in HEK293 cell membrane after 1 hr by Microbeta scintillation countin... |
Eur J Med Chem 78: 324-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.065
BindingDB Entry DOI: 10.7270/Q2J104Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor expressed in CHOK1 cells after 1 hr by microbeta scintillation counting method |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50569820
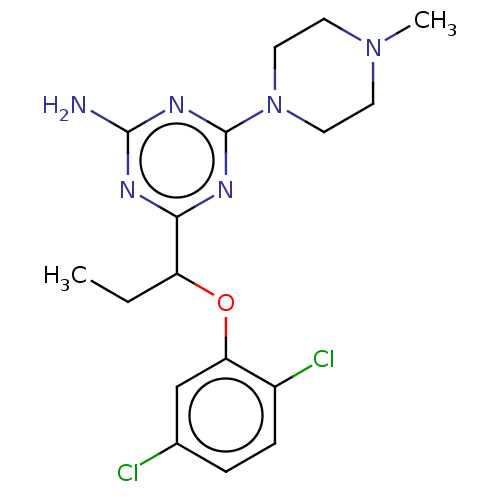
(CHEMBL4860275)Show SMILES CCC(Oc1cc(Cl)ccc1Cl)c1nc(N)nc(n1)N1CCN(C)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5-HT6 receptor expressed in HEK293 cells incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112529
BindingDB Entry DOI: 10.7270/Q2S46WR1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50569820

(CHEMBL4860275)Show SMILES CCC(Oc1cc(Cl)ccc1Cl)c1nc(N)nc(n1)N1CCN(C)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5-HT6 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112529
BindingDB Entry DOI: 10.7270/Q2S46WR1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data