

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucagon receptor (Homo sapiens (Human)) | BDBM50122102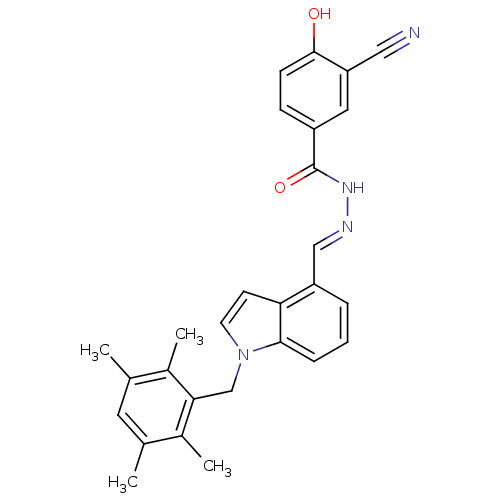 (3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human glucagon receptor | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057796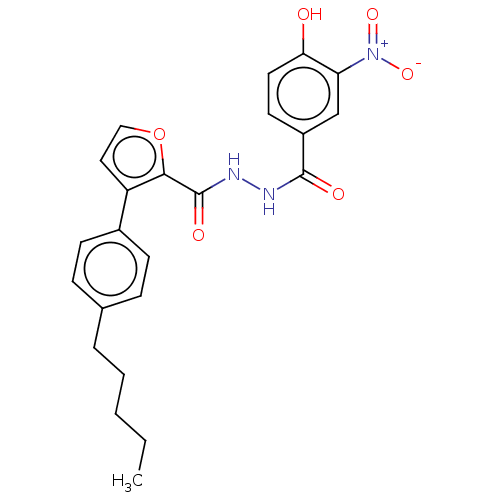 (CHEMBL3326188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of glucagon-induced glucagon receptor-mediated cAMP production in rat hepatocytes after 15 mins by cAMP dynamic2 assay | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057824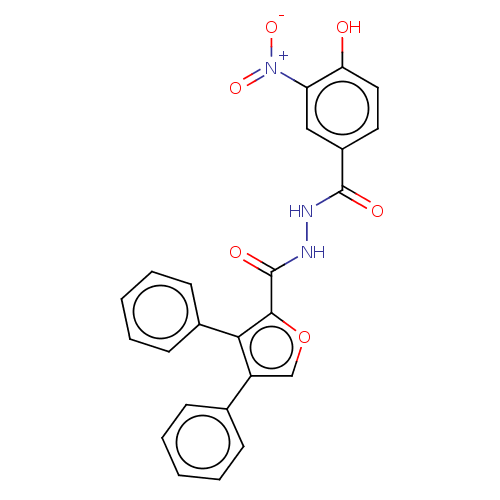 (CHEMBL3326175) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057820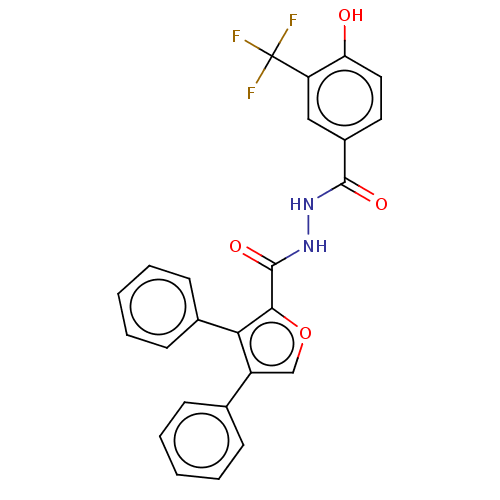 (CHEMBL3326174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057813 (CHEMBL3326173) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50057796 (CHEMBL3326188) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of glucagon-induced glucagon receptor-mediated cAMP production in human hepatocytes after 15 mins by cAMP dynamic2 assay | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057806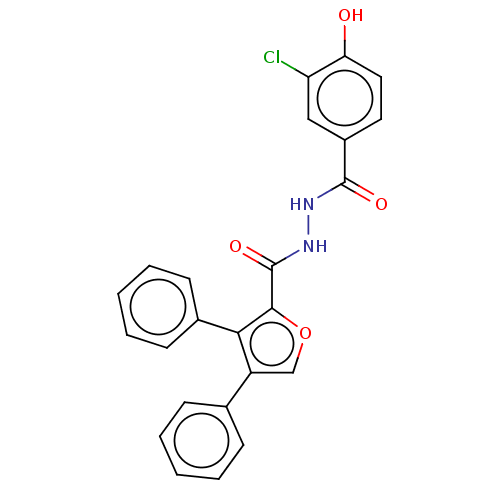 (CHEMBL3326172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057798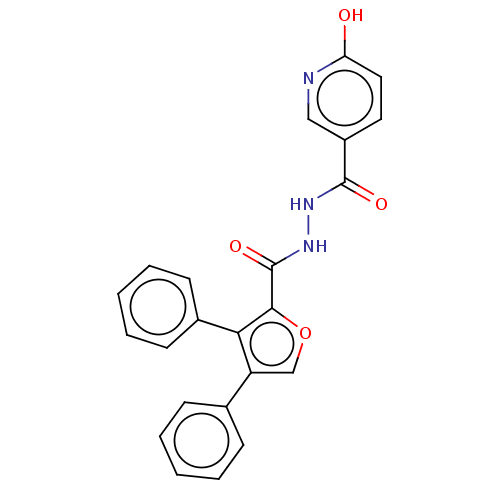 (CHEMBL3326170) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50057796 (CHEMBL3326188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50057796 (CHEMBL3326188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of GLP-1R (unknown origin) | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057800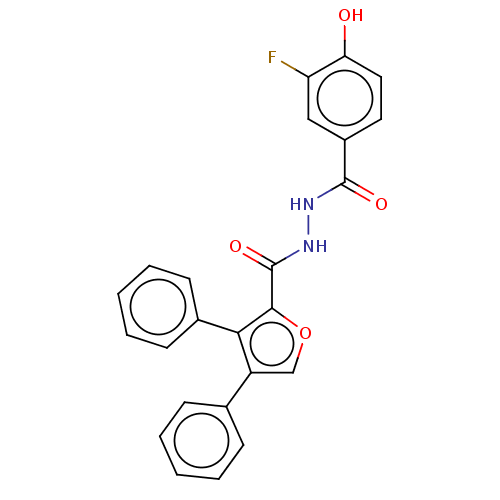 (CHEMBL3326171) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057864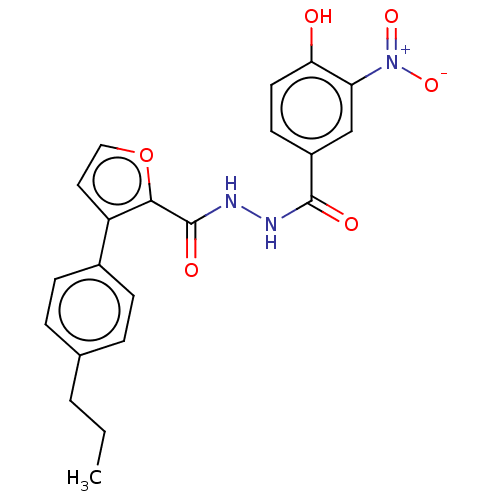 (CHEMBL3325456) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057817 (CHEMBL3326187) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057797 (CHEMBL3326165) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057825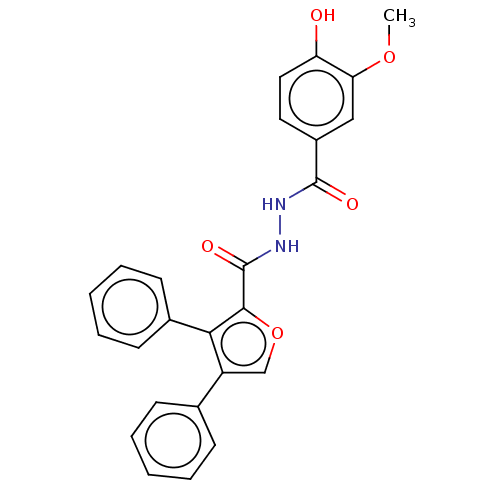 (CHEMBL3326176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057796 (CHEMBL3326188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057831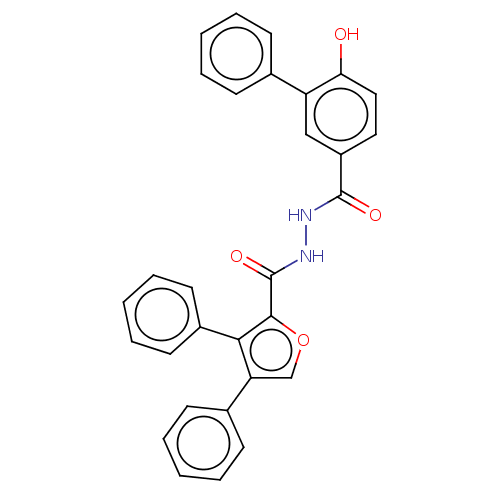 (CHEMBL3326177) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307527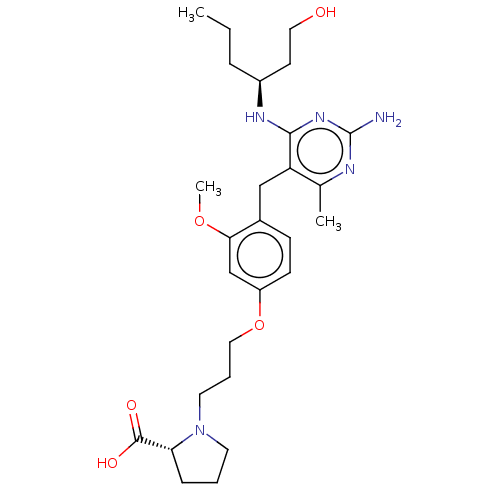 ((R)-1-(3-(4-((2-amino-4-((S)-1-hydroxyhexan-3-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307528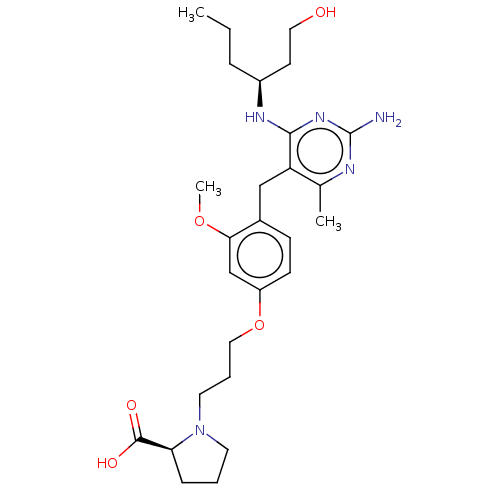 ((S)-1-(3-(4-((2-amino-4-((S)-1-hydroxyhexan-3-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307526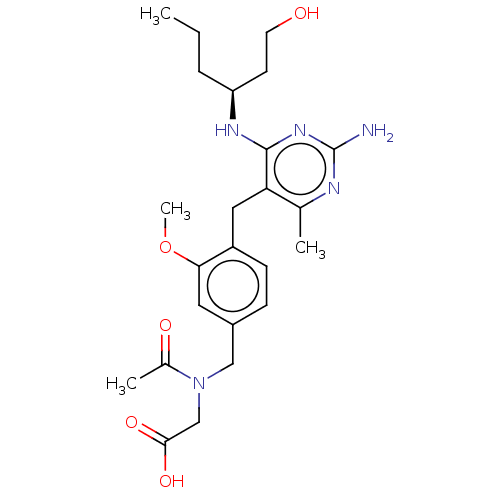 ((S)-2-(N-(4-((2-amino-4-(1-hydroxyhexan-3-ylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307525 ((S)-3-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307524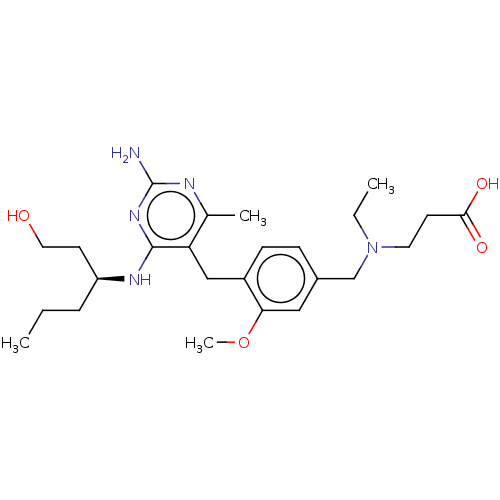 ((S)-3-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307523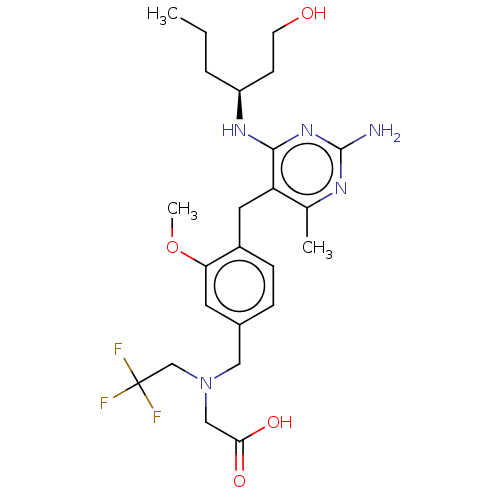 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307522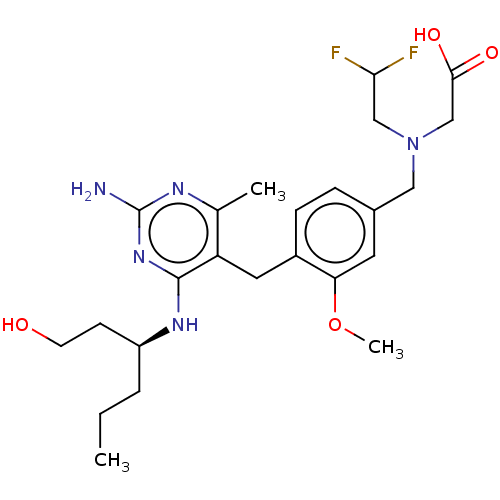 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307521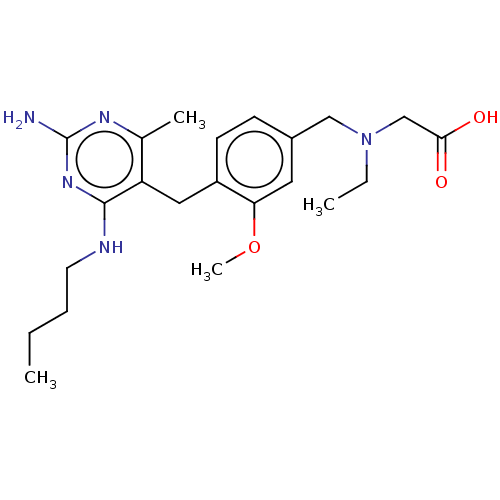 (2-((4-((2-amino-4-(butylamino)-6-methylpyrimidin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307520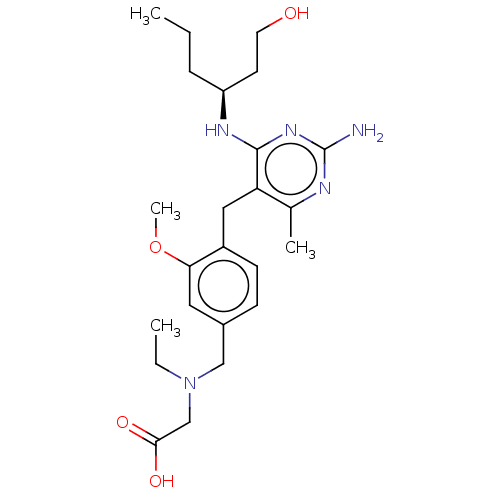 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description The hERG potassium current was measured in a hERG-stably-expressing Chinese hamster ovary K1 (CHO) cells. The experiments were performed using an aut... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50058003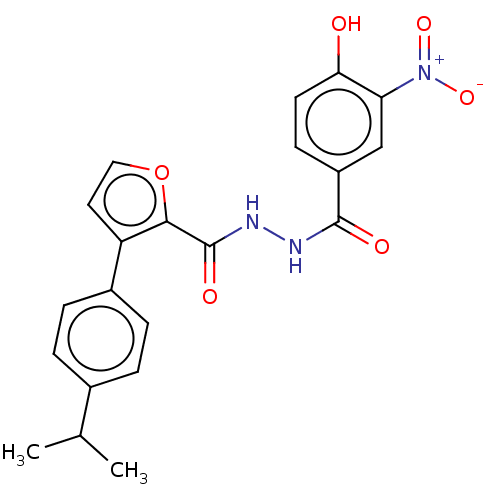 (CHEMBL3326186) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057859 (CHEMBL3326181) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307524 ((S)-3-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Cell CultureThe hERG-expressing Chinese hamster ovary K1 (CHO) cells described by (Persson, Carlsson, Duker, & Jacobson, 2005) were grown to semi-con... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307520 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Cell CultureThe hERG-expressing Chinese hamster ovary K1 (CHO) cells described by (Persson, Carlsson, Duker, & Jacobson, 2005) were grown to semi-con... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307525 ((S)-3-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Cell CultureThe hERG-expressing Chinese hamster ovary K1 (CHO) cells described by (Persson, Carlsson, Duker, & Jacobson, 2005) were grown to semi-con... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM307522 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Cell CultureThe hERG-expressing Chinese hamster ovary K1 (CHO) cells described by (Persson, Carlsson, Duker, & Jacobson, 2005) were grown to semi-con... | US Patent US10562861 (2020) BindingDB Entry DOI: 10.7270/Q22F7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057863 (CHEMBL3326185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057861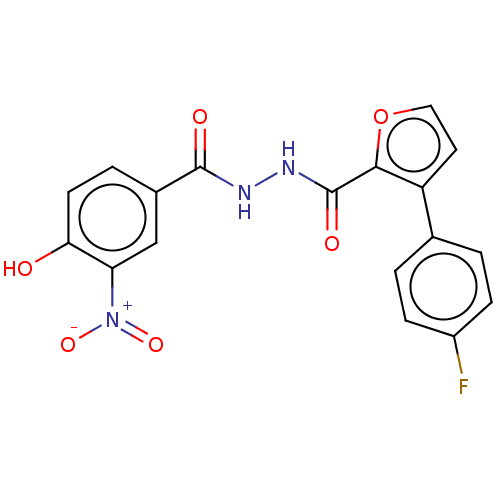 (CHEMBL3326183) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50260354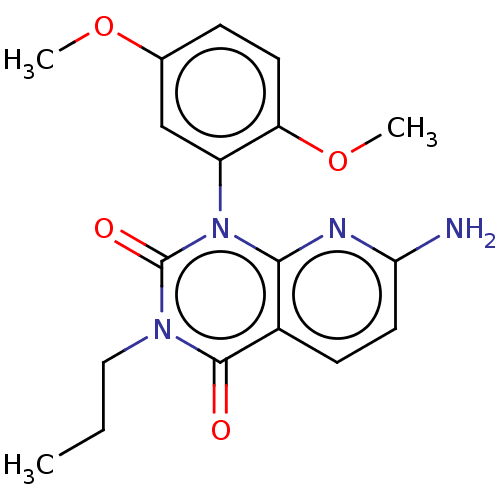 (CHEMBL4082465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human full length recombinant PDE4A1A using FAM-cAMP as substrate after 30 mins by fluorescence based spectrophotometric method | Bioorg Med Chem 25: 4506-4511 (2017) Article DOI: 10.1016/j.bmc.2017.06.042 BindingDB Entry DOI: 10.7270/Q2J105M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50260354 (CHEMBL4082465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human full length recombinant PDE4A1A using FAM-cAMP as substrate after 30 mins by fluorescence based spectrophotometric method | Bioorg Med Chem 25: 4506-4511 (2017) Article DOI: 10.1016/j.bmc.2017.06.042 BindingDB Entry DOI: 10.7270/Q2J105M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057834 (CHEMBL3326178) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057860 (CHEMBL3326182) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057849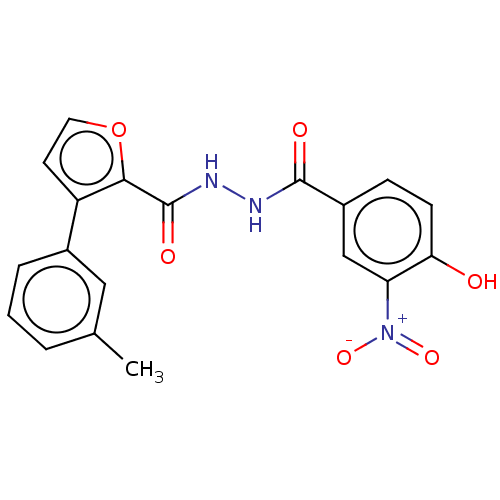 (CHEMBL3326180) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057836 (CHEMBL3326179) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057862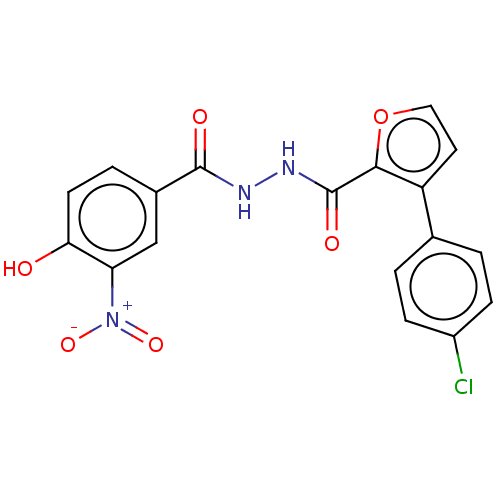 (CHEMBL3326184) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307520 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307521 (2-((4-((2-amino-4-(butylamino)-6-methylpyrimidin-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307522 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307523 ((S)-2-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307525 ((S)-3-((4-((2-amino-4-(1-hydroxyhexan-3-ylamino)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307526 ((S)-2-(N-(4-((2-amino-4-(1-hydroxyhexan-3-ylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307527 ((R)-1-(3-(4-((2-amino-4-((S)-1-hydroxyhexan-3-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307528 ((S)-1-(3-(4-((2-amino-4-((S)-1-hydroxyhexan-3-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM307529 ((S)-3-((3-(4-((2-amino-4-(1-hydroxyhexan-3-ylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. US Patent | Assay Description Recombinant human TLR7 was stably expressed in a HEK293 cell line already stably expressing the pNiFty2-SEAP reporter plasmid; integration of the rep... | US Patent US10150743 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |