

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50401764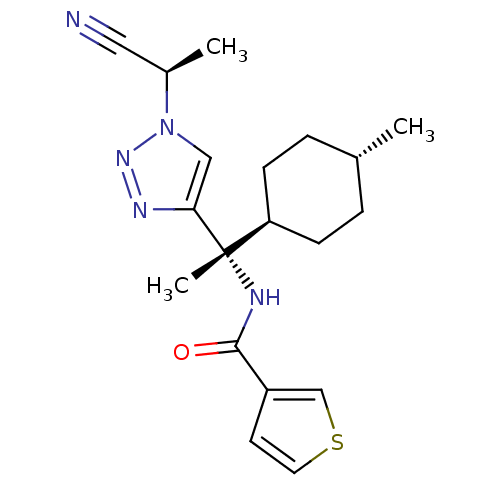 (CHEMBL2207564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401763 (CHEMBL2207565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126726 (US8785489, 5-methyl-4-[2-(3-methyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126725 (US8785489, 4-{[({5-methyl-4-[2-(3-methyl-1,2,4-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126724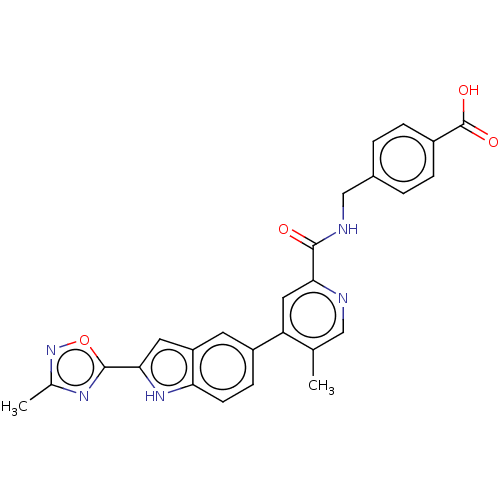 (US8785489, 4-{[({5-methyl-4-[2-(3-methyl-1,2,4-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126723 (US8785489, 4-{[({4-[2-(3-methyl-1,2,4-oxadiazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126722 (US8785489, 4-{[({1-methyl-5-[2-(3-methyl-1,2,4-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401766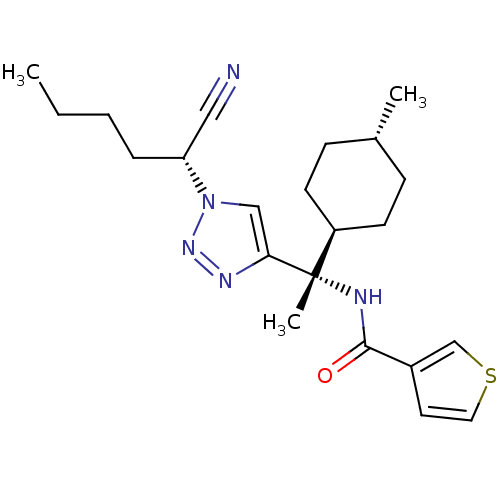 (CHEMBL2207562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921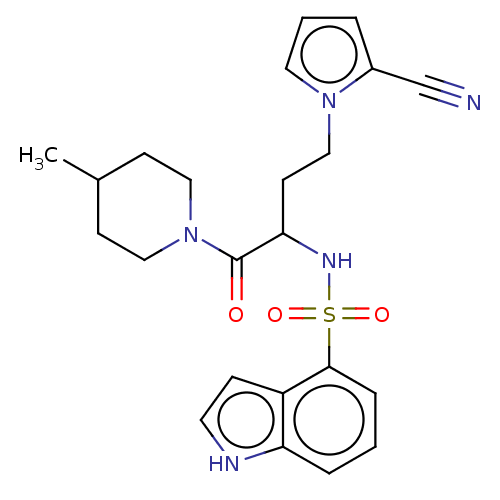 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in CHOK1 cells coexpressing aequorin/Galphaq assessed as inhibition of CCL27-dependent calcium flux in p... | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126728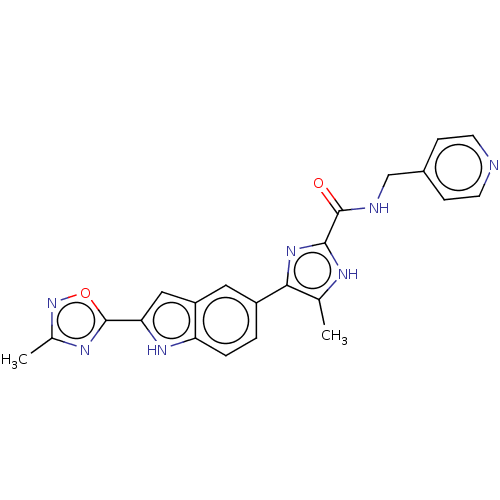 (US8785489, 5-methyl-4-[2-(3-methyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126727 (US8785489, 5-methyl-4-[2-(3-methyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19502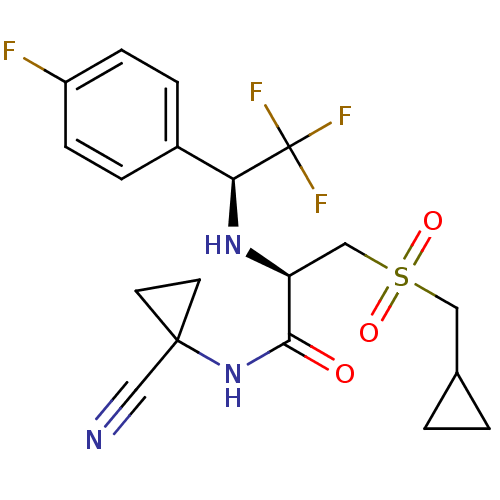 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401814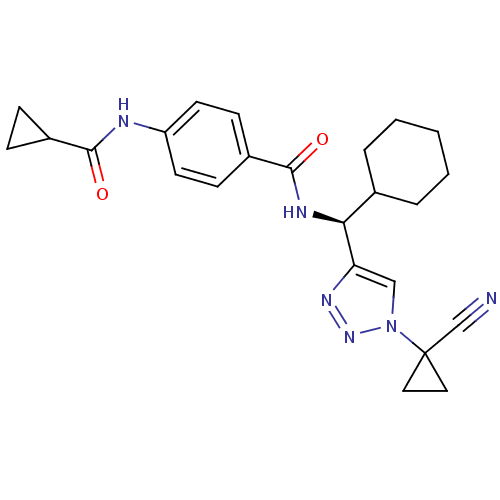 (CHEMBL2207591) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401765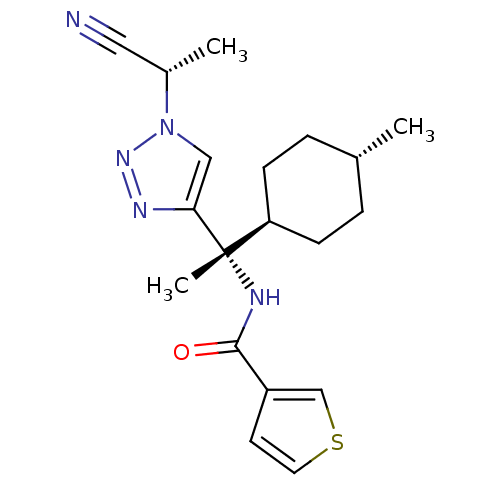 (CHEMBL2207563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126730 (US8785489, 5-methyl-4-[2-(3-methyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126729 (US8785489, 5-methyl-4-[2-(3-methyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401834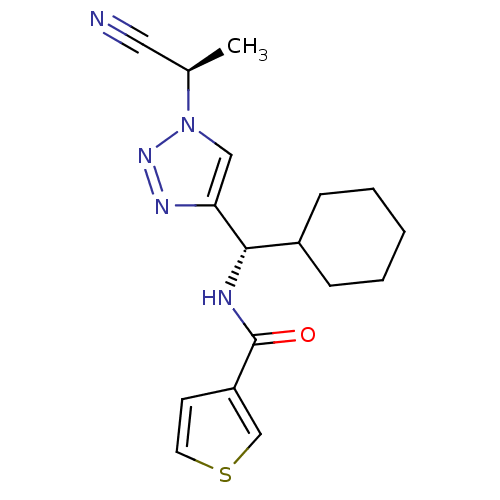 (CHEMBL2207571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401835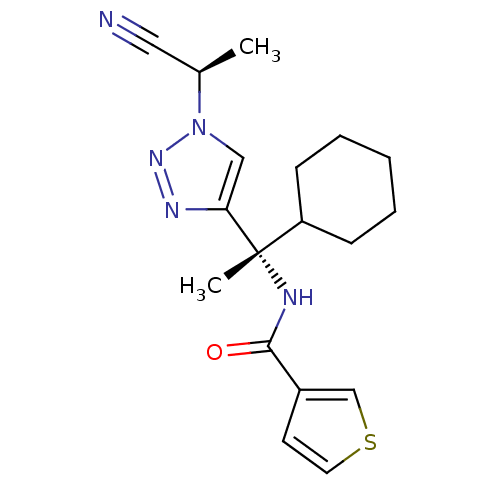 (CHEMBL2207570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126731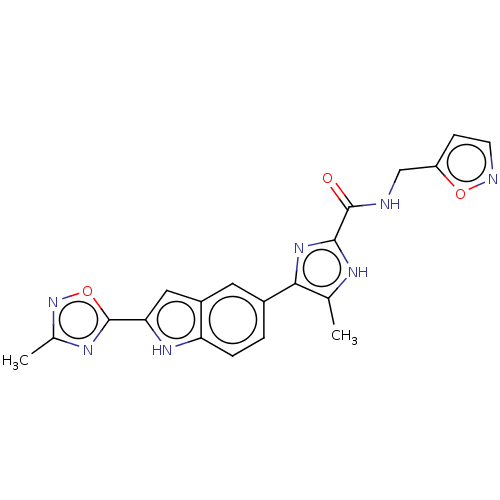 (US8785489, N-(isoxazol-5-ylmethyl)-5-methyl-4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401761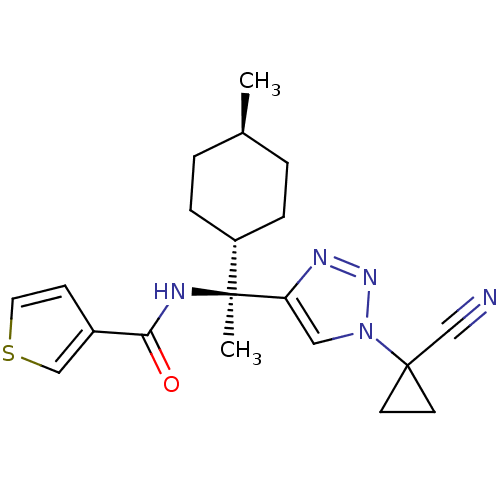 (CHEMBL2207567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxycytidine kinase (Homo sapiens (Human)) | BDBM50311570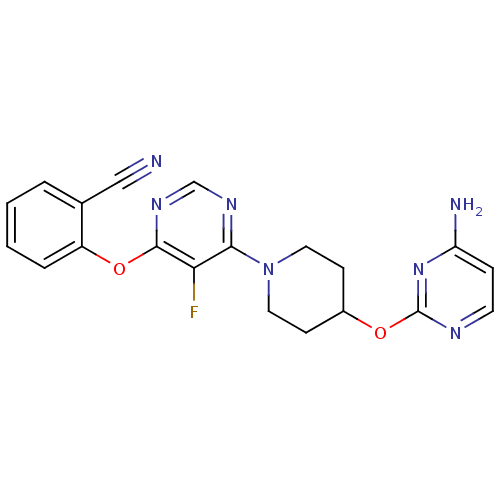 (2-(6-(4-(4-aminopyrimidin-2-yloxy)piperidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human dCK | Bioorg Med Chem Lett 19: 6780-3 (2009) Article DOI: 10.1016/j.bmcl.2009.09.082 BindingDB Entry DOI: 10.7270/Q2HM58K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126732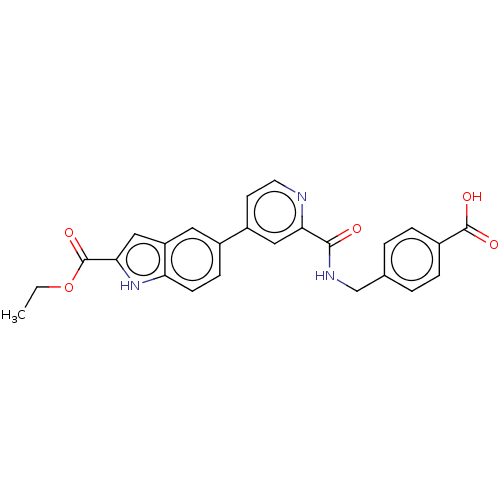 (US8785489, 4-{[({4-[2-(ethoxycarbonyl)-1H-indol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126734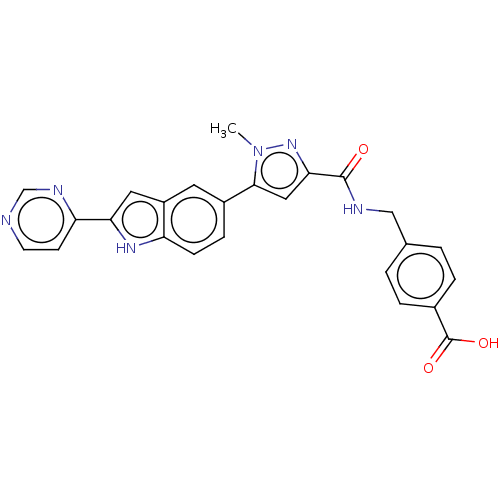 (US8785489, 4-[({[1-methyl-5-(2-pyrimidin-4-yl-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM50361618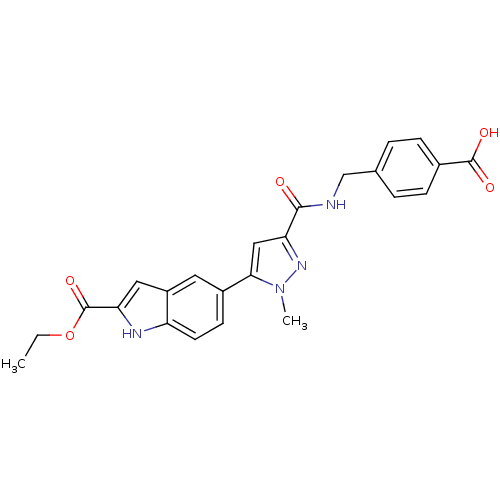 (CHEMBL1939876 | US8785489, 4-{[({5-[2-(ethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126733 (US8785489, 4-[({[1-methyl-5-(2-pyridin-4-yl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126736 (US8785489, 5-methyl-4-[2-(3-methyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126735 (US8785489, 5-methyl-N-[(5-methylisoxazol-3-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401770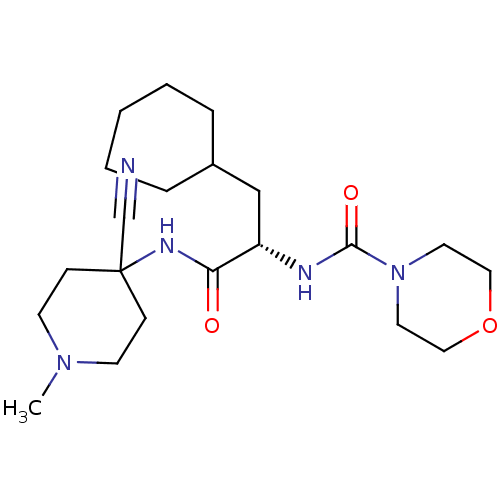 (CHEMBL1236882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S-mediated antigen presentation in B/T hybridoma cells assessed as IL-2 level | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Mus musculus (Mouse)) | BDBM126737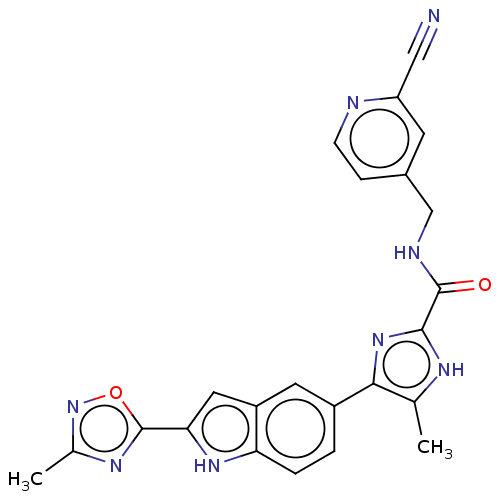 (US8785489, N-[(2-cyanopyridin-4-yl)methyl]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The EnzoLyte 520 Generic MMP Assay Kit (AnaSpec Inc.) can detect the activity of several MMPs including MMP-1, 2, 3, 7, 8, 9, 13, and 14. This kit us... | US Patent US8785489 (2014) BindingDB Entry DOI: 10.7270/Q24B300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118757 (US8674113, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118851 (US8674113, 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401762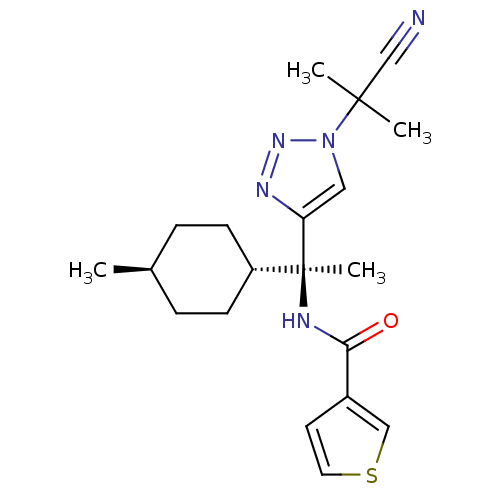 (CHEMBL2207566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118850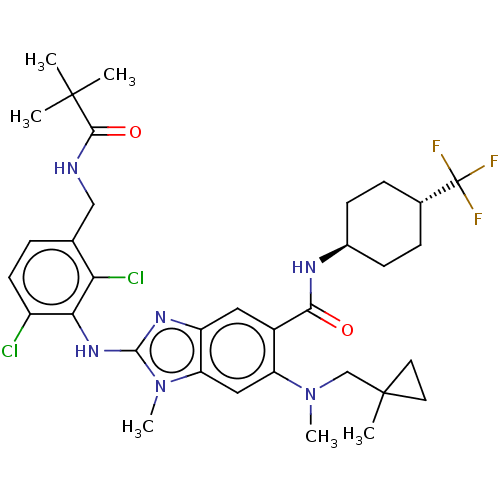 (US8674113, 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118846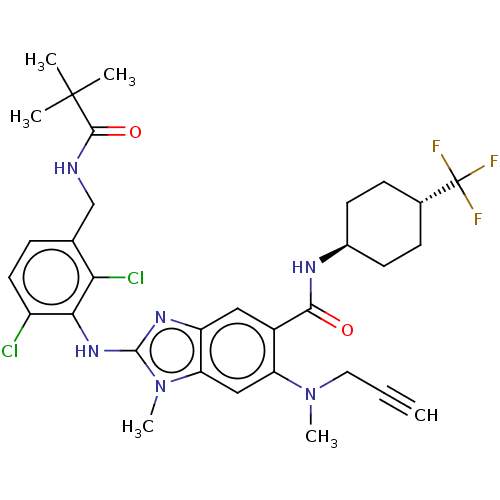 (US8674113, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118842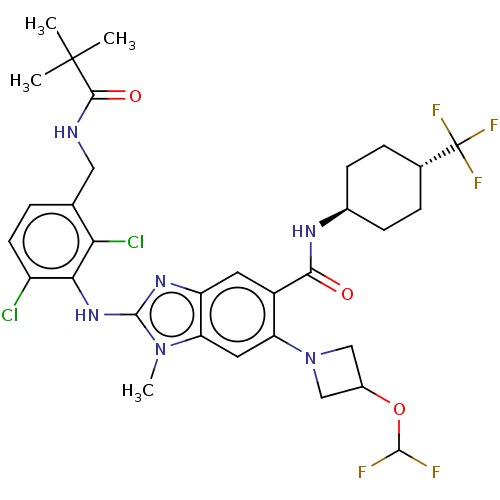 (US8674113, 99) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118819 (US8674113, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118818 (US8674113, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118814 (US8674113, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in mouse BA/F3 cells assessed as inhibition of human CCL27-dependent chemotaxis | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in CHOK1 cells coexpressing aequorin/Galphaq assessed as inhibition of human CCL27-dependent calcium flu... | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 10 (Homo sapiens (Human)) | BDBM50198921 (CHEMBL3889627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human CCR10 expressed in CHOK1 cells coexpressing aequorin/Galphaq assessed as inhibition of human CCL28-dependent calcium flu... | Bioorg Med Chem Lett 26: 5277-5283 (2016) Article DOI: 10.1016/j.bmcl.2016.09.047 BindingDB Entry DOI: 10.7270/Q2KD20WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361618 (CHEMBL1939876 | US8785489, 4-{[({5-[2-(ethoxycarbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human MMP13 expressed in Escherichia coli after 30 mins by fluorimetry | J Med Chem 54: 8174-87 (2011) Article DOI: 10.1021/jm201129m BindingDB Entry DOI: 10.7270/Q2C53M9C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118803 (US8674113, 60 | US8674113, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401818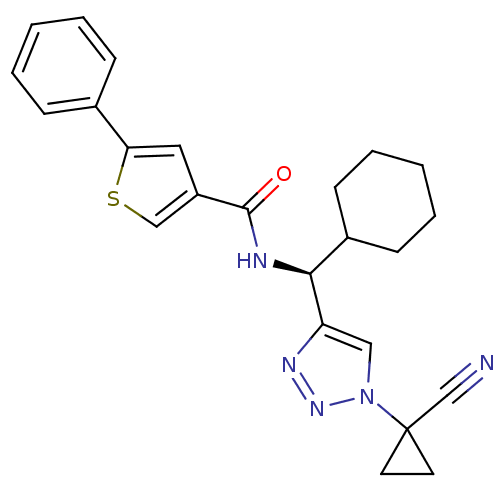 (CHEMBL2207587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxycytidine kinase (Homo sapiens (Human)) | BDBM50311564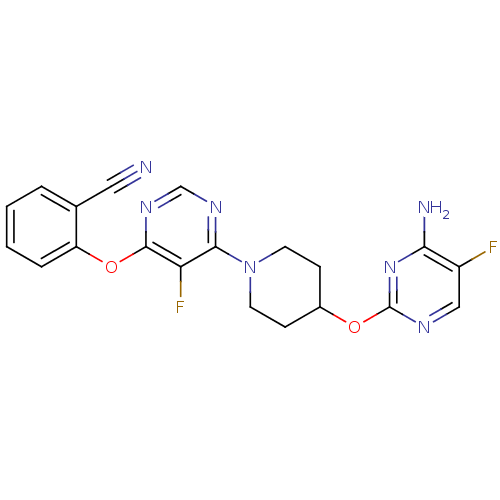 (2-(6-(4-(4-amino-5-fluoropyrimidin-2-yloxy)piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human dCK | Bioorg Med Chem Lett 19: 6780-3 (2009) Article DOI: 10.1016/j.bmcl.2009.09.082 BindingDB Entry DOI: 10.7270/Q2HM58K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxycytidine kinase (Homo sapiens (Human)) | BDBM50311568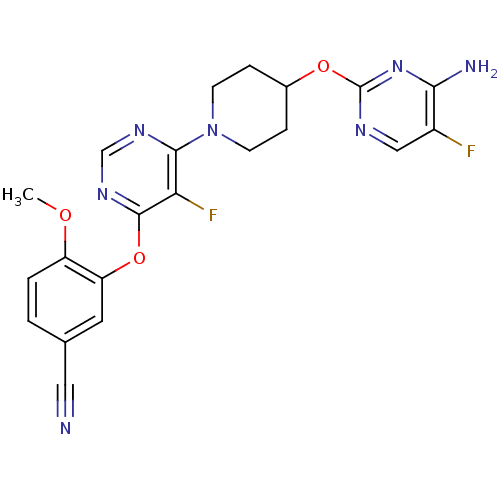 (3-(6-(4-(4-amino-5-fluoropyrimidin-2-yloxy)piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human dCK | Bioorg Med Chem Lett 19: 6780-3 (2009) Article DOI: 10.1016/j.bmcl.2009.09.082 BindingDB Entry DOI: 10.7270/Q2HM58K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118800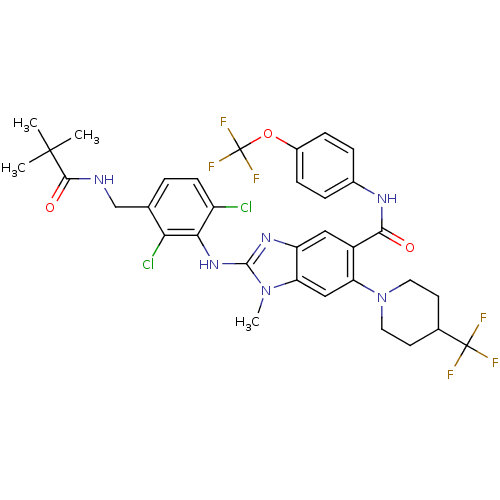 (US8674113, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118799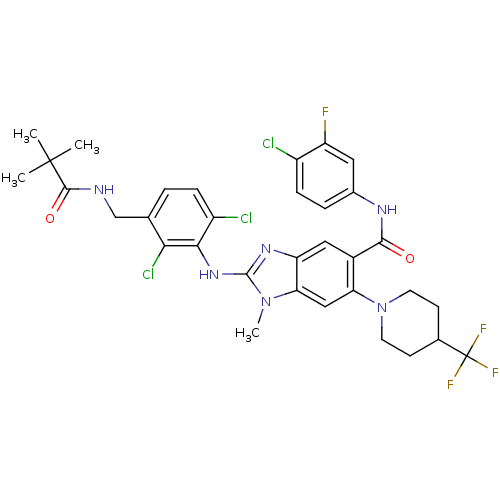 (US8674113, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118782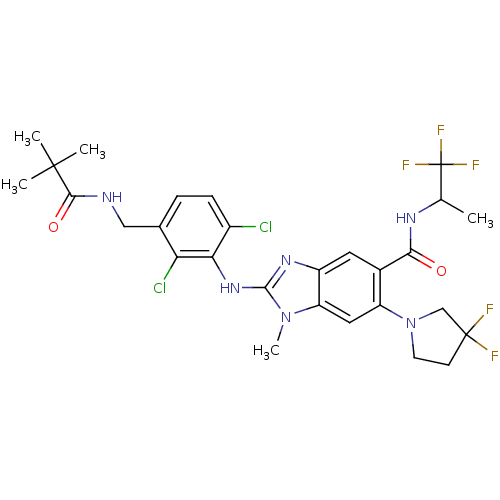 (US8674113, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM118808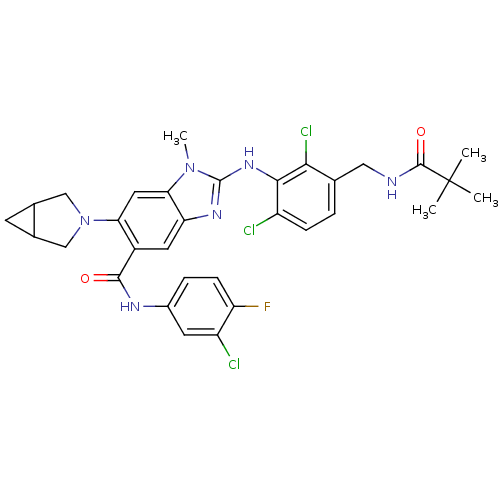 (US8674113, 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The aim of this assay is to determine the affinity of a test compound for the mPGES-1 enzyme. 47 μl of recombinant human mPGES-1 (0.5 μg p... | US Patent US8674113 (2014) BindingDB Entry DOI: 10.7270/Q2708035 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 742 total ) | Next | Last >> |