 Found 1995 hits with Last Name = 'hein' and Initial = 'a'
Found 1995 hits with Last Name = 'hein' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate by spectrophotometric method |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate by spectrophotometric method |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50324478
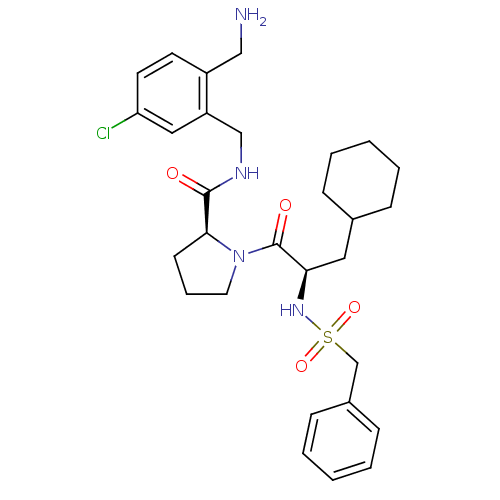
(Benzylsulfonyl-D-cyclohexylalanyl-proline-(2-amino...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C29H39ClN4O4S/c30-25-14-13-23(18-31)24(17-25)19-32-28(35)27-12-7-15-34(27)29(36)26(16-21-8-3-1-4-9-21)33-39(37,38)20-22-10-5-2-6-11-22/h2,5-6,10-11,13-14,17,21,26-27,33H,1,3-4,7-9,12,15-16,18-20,31H2,(H,32,35)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using Tos-Gly-Pro-Arg-AMC as substrate after 600 secs by fluorimetric method |
J Med Chem 55: 6094-110 (2012)
Article DOI: 10.1021/jm300337q
BindingDB Entry DOI: 10.7270/Q2930V7X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50505754

(CHEMBL4457051)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1 |r| Show InChI InChI=1S/C22H33ClN4O2/c23-18-9-8-16(13-24)17(12-18)14-26-21(28)20-7-4-10-27(20)22(29)19(25)11-15-5-2-1-3-6-15/h8-9,12,15,19-20H,1-7,10-11,13-14,24-25H2,(H,26,28)/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) using Tos-Gly-Pro-Arg-AMCA-TFA as a substrate incubated for 40 secs and measured every 15 secs interval for 2... |
J Med Chem 62: 9753-9771 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01196
BindingDB Entry DOI: 10.7270/Q2348PP6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532387
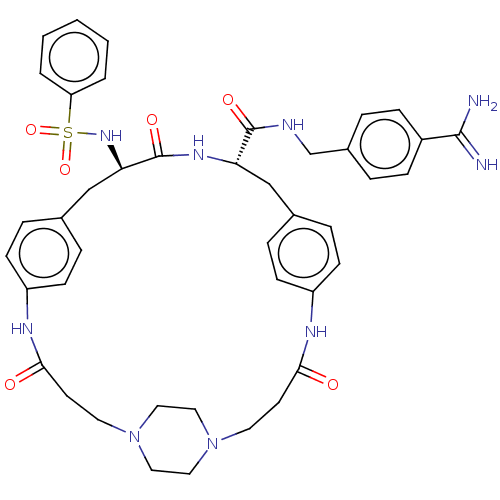
(CHEMBL4476141)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:49.47,wD:22.18,(24.52,-31.64,;25.32,-32.96,;24.57,-34.31,;26.87,-32.93,;28.27,-32.91,;27.9,-34.16,;27.86,-31.66,;25.46,-27.14,;26.25,-28.46,;25.5,-29.81,;27.8,-28.43,;29.21,-28.41,;28.84,-29.67,;28.8,-27.16,;25.54,-22.14,;26.33,-23.47,;25.58,-24.81,;27.88,-23.43,;29.28,-23.41,;28.92,-24.67,;28.88,-22.17,;8.51,-20.69,;8.47,-22.2,;8.42,-23.72,;7.44,-24.88,;5.94,-24.59,;4.95,-25.74,;5.46,-27.17,;4.71,-28.65,;5.45,-30.01,;4.64,-31.32,;6.99,-30.05,;7.73,-31.4,;9.27,-31.44,;10.07,-30.15,;11.59,-30.19,;12.3,-31.52,;11.51,-32.82,;9.99,-32.77,;13.84,-31.57,;14.66,-30.26,;16.2,-30.3,;16.93,-31.66,;16.96,-28.96,;15.17,-27.48,;15.54,-25.98,;14.43,-24.91,;12.94,-25.33,;12.39,-23.49,;12.44,-21.56,;12.48,-20.04,;11.1,-22.28,;9.81,-21.48,;9.85,-19.96,;13.72,-22.35,;13.68,-23.87,;15.06,-21.64,;16.35,-22.43,;17.69,-21.72,;18.98,-22.52,;20.32,-21.8,;20.36,-20.28,;19.06,-19.49,;17.73,-20.21,;21.7,-19.56,;23,-20.36,;21.74,-18.04,;12.56,-26.83,;13.68,-27.91,;6.96,-27.45,;7.94,-26.3,;7.17,-21.4,;5.81,-22.14,;6.57,-23.47,;5.04,-23.47,;4.5,-21.33,;4.54,-19.79,;3.23,-18.98,;1.87,-19.72,;1.83,-21.27,;3.15,-22.07,)| Show InChI InChI=1S/C42H49N9O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(54)36-26-29-8-14-33(15-9-29)46-38(52)18-20-50-22-24-51(25-23-50)21-19-39(53)47-34-16-10-30(11-17-34)27-37(42(55)48-36)49-58(56,57)35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h1-17,36-37,49H,18-28H2,(H3,43,44)(H,45,54)(H,46,52)(H,47,53)(H,48,55);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532387

(CHEMBL4476141)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:49.47,wD:22.18,(24.52,-31.64,;25.32,-32.96,;24.57,-34.31,;26.87,-32.93,;28.27,-32.91,;27.9,-34.16,;27.86,-31.66,;25.46,-27.14,;26.25,-28.46,;25.5,-29.81,;27.8,-28.43,;29.21,-28.41,;28.84,-29.67,;28.8,-27.16,;25.54,-22.14,;26.33,-23.47,;25.58,-24.81,;27.88,-23.43,;29.28,-23.41,;28.92,-24.67,;28.88,-22.17,;8.51,-20.69,;8.47,-22.2,;8.42,-23.72,;7.44,-24.88,;5.94,-24.59,;4.95,-25.74,;5.46,-27.17,;4.71,-28.65,;5.45,-30.01,;4.64,-31.32,;6.99,-30.05,;7.73,-31.4,;9.27,-31.44,;10.07,-30.15,;11.59,-30.19,;12.3,-31.52,;11.51,-32.82,;9.99,-32.77,;13.84,-31.57,;14.66,-30.26,;16.2,-30.3,;16.93,-31.66,;16.96,-28.96,;15.17,-27.48,;15.54,-25.98,;14.43,-24.91,;12.94,-25.33,;12.39,-23.49,;12.44,-21.56,;12.48,-20.04,;11.1,-22.28,;9.81,-21.48,;9.85,-19.96,;13.72,-22.35,;13.68,-23.87,;15.06,-21.64,;16.35,-22.43,;17.69,-21.72,;18.98,-22.52,;20.32,-21.8,;20.36,-20.28,;19.06,-19.49,;17.73,-20.21,;21.7,-19.56,;23,-20.36,;21.74,-18.04,;12.56,-26.83,;13.68,-27.91,;6.96,-27.45,;7.94,-26.3,;7.17,-21.4,;5.81,-22.14,;6.57,-23.47,;5.04,-23.47,;4.5,-21.33,;4.54,-19.79,;3.23,-18.98,;1.87,-19.72,;1.83,-21.27,;3.15,-22.07,)| Show InChI InChI=1S/C42H49N9O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(54)36-26-29-8-14-33(15-9-29)46-38(52)18-20-50-22-24-51(25-23-50)21-19-39(53)47-34-16-10-30(11-17-34)27-37(42(55)48-36)49-58(56,57)35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h1-17,36-37,49H,18-28H2,(H3,43,44)(H,45,54)(H,46,52)(H,47,53)(H,48,55);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM32657
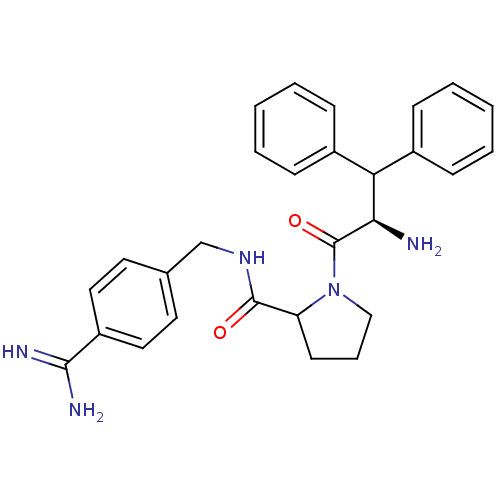
(benzamidine- based compound, 6)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCCC1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C28H31N5O2/c29-25(24(20-8-3-1-4-9-20)21-10-5-2-6-11-21)28(35)33-17-7-12-23(33)27(34)32-18-19-13-15-22(16-14-19)26(30)31/h1-6,8-11,13-16,23-25H,7,12,17-18,29H2,(H3,30,31)(H,32,34)/t23?,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Philipps-University Marburg
| Assay Description
Kinetic inhibition of human thrombin was determined photometrically at 405 nm using the chromogenic substrate Pefachrom tPa. Reactions were performed... |
J Mol Biol 391: 552-64 (2009)
Article DOI: 10.1016/j.jmb.2009.06.016
BindingDB Entry DOI: 10.7270/Q2RV0M1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50592222
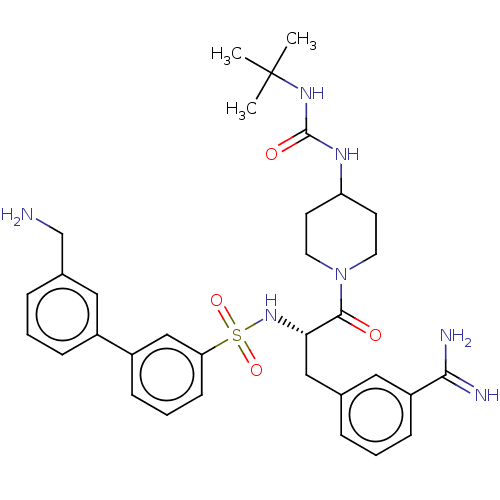
(CHEMBL5180135)Show SMILES CC(C)(C)NC(=O)NC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1cccc(CN)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114437
BindingDB Entry DOI: 10.7270/Q2KD22W8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50324477

(Benzylsulfonyl-D-cyclohexylalanyl-proline-(4-amidi...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](CC2CCCCC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C29H39N5O4S/c30-27(31)24-15-13-22(14-16-24)19-32-28(35)26-12-7-17-34(26)29(36)25(18-21-8-3-1-4-9-21)33-39(37,38)20-23-10-5-2-6-11-23/h2,5-6,10-11,13-16,21,25-26,33H,1,3-4,7-9,12,17-20H2,(H3,30,31)(H,32,35)/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using Tos-Gly-Pro-Arg-AMC as substrate after 600 secs by fluorimetric method |
J Med Chem 55: 6094-110 (2012)
Article DOI: 10.1021/jm300337q
BindingDB Entry DOI: 10.7270/Q2930V7X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50505753
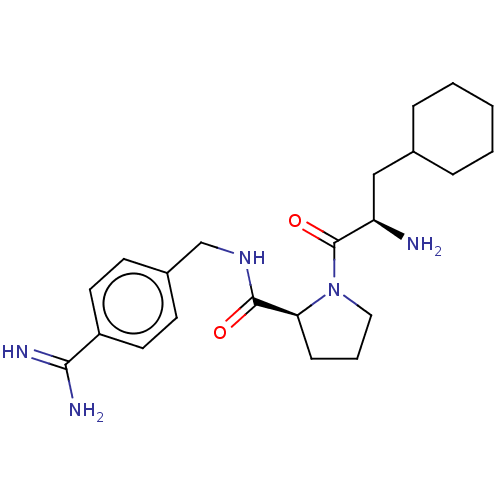
(CHEMBL4448453)Show SMILES N[C@H](CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C22H33N5O2/c23-18(13-15-5-2-1-3-6-15)22(29)27-12-4-7-19(27)21(28)26-14-16-8-10-17(11-9-16)20(24)25/h8-11,15,18-19H,1-7,12-14,23H2,(H3,24,25)(H,26,28)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) using Tos-Gly-Pro-Arg-AMCA-TFA as a substrate incubated for 40 secs and measured every 15 secs interval for 2... |
J Med Chem 62: 9753-9771 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01196
BindingDB Entry DOI: 10.7270/Q2348PP6 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062051

(1-Dimethylcarbamoyl-6-(4-fluoro-phenyl)-3-[4-(2-me...)Show SMILES COC(=O)c1cc(cc2n(cc(C(=O)c3ccc(Cn4c(C)nc5cnccc45)cc3)c12)C(=O)N(C)C)-c1ccc(F)cc1 Show InChI InChI=1S/C34H28FN5O4/c1-20-37-28-17-36-14-13-29(28)39(20)18-21-5-7-23(8-6-21)32(41)27-19-40(34(43)38(2)3)30-16-24(22-9-11-25(35)12-10-22)15-26(31(27)30)33(42)44-4/h5-17,19H,18H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
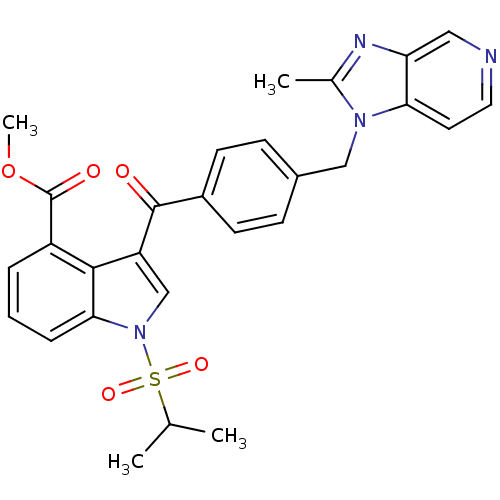
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062066
(3-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-ylmethyl)-b...)Show SMILES COC(=O)c1cccc2n(cc(C(=O)c3ccc(Cn4c(C)nc5cnccc45)cc3)c12)S(=O)(=O)C(C)C Show InChI InChI=1S/C28H26N4O5S/c1-17(2)38(35,36)32-16-22(26-21(28(34)37-4)6-5-7-25(26)32)27(33)20-10-8-19(9-11-20)15-31-18(3)30-23-14-29-13-12-24(23)31/h5-14,16-17H,15H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392028

(CHEMBL2152429)Show SMILES CC(C)C[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN |r| Show InChI InChI=1S/C26H35ClN4O4S/c1-18(2)13-23(30-36(34,35)17-19-7-4-3-5-8-19)26(33)31-12-6-9-24(31)25(32)29-16-21-14-22(27)11-10-20(21)15-28/h3-5,7-8,10-11,14,18,23-24,30H,6,9,12-13,15-17,28H2,1-2H3,(H,29,32)/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using Tos-Gly-Pro-Arg-AMC as substrate after 600 secs by fluorimetric method |
J Med Chem 55: 6094-110 (2012)
Article DOI: 10.1021/jm300337q
BindingDB Entry DOI: 10.7270/Q2930V7X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50324476

(Benzylsulfonyl-D-arginyl-proline-(2-aminomethyl-5-...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCCNC(N)=N)NS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C26H36ClN7O4S/c27-21-11-10-19(15-28)20(14-21)16-32-24(35)23-9-5-13-34(23)25(36)22(8-4-12-31-26(29)30)33-39(37,38)17-18-6-2-1-3-7-18/h1-3,6-7,10-11,14,22-23,33H,4-5,8-9,12-13,15-17,28H2,(H,32,35)(H4,29,30,31)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) using Tos-Gly-Pro-Arg-AMCA-TFA as a substrate incubated for 40 secs and measured every 15 secs interval for 2... |
J Med Chem 62: 9753-9771 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01196
BindingDB Entry DOI: 10.7270/Q2348PP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50286027
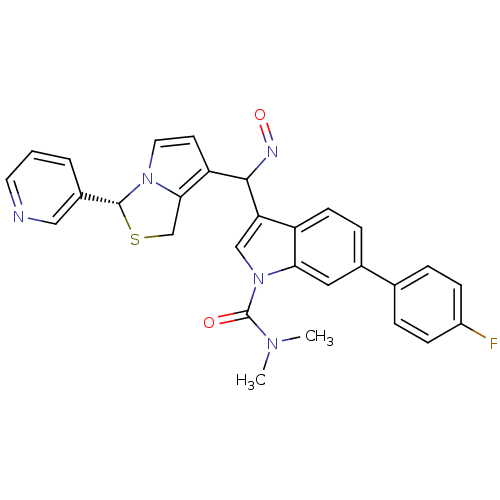
(6-(4-Fluoro-phenyl)-3-[[(Z)-hydroxyimino]-((R)-3-p...)Show SMILES CN(C)C(=O)n1cc(C(N=O)c2ccn3[C@H](SCc23)c2cccnc2)c2ccc(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C29H24FN5O2S/c1-33(2)29(36)35-16-24(22-10-7-19(14-25(22)35)18-5-8-21(30)9-6-18)27(32-37)23-11-13-34-26(23)17-38-28(34)20-4-3-12-31-15-20/h3-16,27-28H,17H2,1-2H3/t27?,28-/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for platelet activating factor receptor using [3H]-PAF in rabbit platelet membranes |
Bioorg Med Chem Lett 5: 2913-2918 (1995)
Article DOI: 10.1016/0960-894X(95)00511-Q
BindingDB Entry DOI: 10.7270/Q2NC6157 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50048485
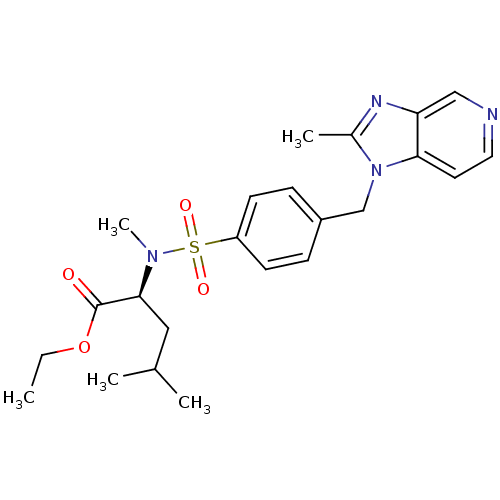
((S)-4-Methyl-2-{methyl-[4-(2-methyl-imidazo[4,5-c]...)Show SMILES CCOC(=O)[C@H](CC(C)C)N(C)S(=O)(=O)c1ccc(Cn2c(C)nc3cnccc23)cc1 Show InChI InChI=1S/C23H30N4O4S/c1-6-31-23(28)22(13-16(2)3)26(5)32(29,30)19-9-7-18(8-10-19)15-27-17(4)25-20-14-24-12-11-21(20)27/h7-12,14,16,22H,6,13,15H2,1-5H3/t22-/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity against PAF receptor in rabbit platelet |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50048485

((S)-4-Methyl-2-{methyl-[4-(2-methyl-imidazo[4,5-c]...)Show SMILES CCOC(=O)[C@H](CC(C)C)N(C)S(=O)(=O)c1ccc(Cn2c(C)nc3cnccc23)cc1 Show InChI InChI=1S/C23H30N4O4S/c1-6-31-23(28)22(13-16(2)3)26(5)32(29,30)19-9-7-18(8-10-19)15-27-17(4)25-20-14-24-12-11-21(20)27/h7-12,14,16,22H,6,13,15H2,1-5H3/t22-/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity against PAF receptor in rabbit platelet |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394
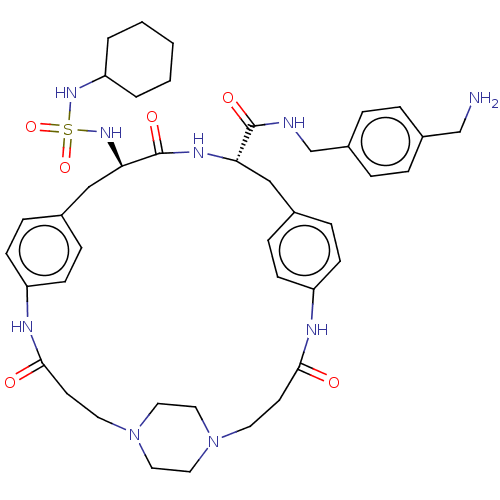
(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394

(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532391
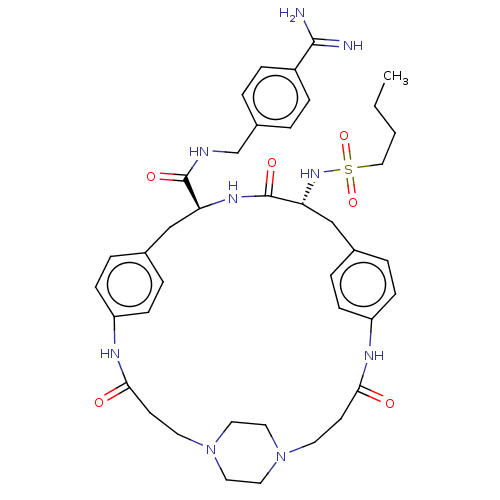
(CHEMBL4591922)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)CCCC |r,wU:49.47,wD:22.18,(25.66,-27.36,;26.46,-28.69,;25.7,-30.05,;28.02,-28.66,;29.44,-28.63,;29.07,-29.9,;29.03,-27.38,;25.74,-22.32,;26.54,-23.65,;25.78,-25.01,;28.1,-23.62,;29.52,-23.6,;29.15,-24.86,;29.11,-22.34,;26.34,-32.07,;27.14,-33.4,;26.39,-34.77,;28.71,-33.37,;30.12,-33.35,;29.75,-34.62,;29.71,-32.1,;11.88,-20.61,;11.84,-22.13,;11.79,-23.66,;10.8,-24.83,;9.29,-24.54,;8.29,-25.7,;8.8,-27.15,;8.05,-28.63,;8.79,-30,;7.98,-31.33,;10.35,-30.04,;11.09,-31.41,;12.65,-31.45,;13.45,-30.14,;14.98,-30.18,;15.7,-31.53,;14.9,-32.83,;13.37,-32.78,;17.26,-31.58,;18.07,-30.25,;19.63,-30.3,;20.37,-31.67,;20.4,-28.94,;18.59,-27.45,;18.96,-25.94,;17.84,-24.86,;16.34,-25.29,;15.79,-23.43,;15.84,-21.49,;15.88,-19.96,;14.49,-22.21,;13.18,-21.41,;13.23,-19.88,;17.13,-22.29,;17.09,-23.82,;18.48,-21.57,;19.78,-22.37,;21.13,-21.64,;22.43,-22.46,;23.78,-21.73,;23.83,-20.2,;22.52,-19.4,;21.17,-20.13,;25.17,-19.48,;26.48,-20.27,;25.22,-17.93,;15.97,-26.8,;17.09,-27.89,;10.32,-27.42,;11.3,-26.26,;10.53,-21.33,;9.16,-22.07,;9.93,-23.41,;8.38,-23.41,;7.84,-21.26,;6.47,-22,;5.14,-21.18,;3.77,-21.92,)| Show InChI InChI=1S/C40H53N9O6S.3C2HF3O2/c1-2-3-24-56(54,55)47-35-26-29-8-14-33(15-9-29)45-37(51)17-19-49-22-20-48(21-23-49)18-16-36(50)44-32-12-6-28(7-13-32)25-34(46-40(35)53)39(52)43-27-30-4-10-31(11-5-30)38(41)42;3*3-2(4,5)1(6)7/h4-15,34-35,47H,2-3,16-27H2,1H3,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532391

(CHEMBL4591922)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)CCCC |r,wU:49.47,wD:22.18,(25.66,-27.36,;26.46,-28.69,;25.7,-30.05,;28.02,-28.66,;29.44,-28.63,;29.07,-29.9,;29.03,-27.38,;25.74,-22.32,;26.54,-23.65,;25.78,-25.01,;28.1,-23.62,;29.52,-23.6,;29.15,-24.86,;29.11,-22.34,;26.34,-32.07,;27.14,-33.4,;26.39,-34.77,;28.71,-33.37,;30.12,-33.35,;29.75,-34.62,;29.71,-32.1,;11.88,-20.61,;11.84,-22.13,;11.79,-23.66,;10.8,-24.83,;9.29,-24.54,;8.29,-25.7,;8.8,-27.15,;8.05,-28.63,;8.79,-30,;7.98,-31.33,;10.35,-30.04,;11.09,-31.41,;12.65,-31.45,;13.45,-30.14,;14.98,-30.18,;15.7,-31.53,;14.9,-32.83,;13.37,-32.78,;17.26,-31.58,;18.07,-30.25,;19.63,-30.3,;20.37,-31.67,;20.4,-28.94,;18.59,-27.45,;18.96,-25.94,;17.84,-24.86,;16.34,-25.29,;15.79,-23.43,;15.84,-21.49,;15.88,-19.96,;14.49,-22.21,;13.18,-21.41,;13.23,-19.88,;17.13,-22.29,;17.09,-23.82,;18.48,-21.57,;19.78,-22.37,;21.13,-21.64,;22.43,-22.46,;23.78,-21.73,;23.83,-20.2,;22.52,-19.4,;21.17,-20.13,;25.17,-19.48,;26.48,-20.27,;25.22,-17.93,;15.97,-26.8,;17.09,-27.89,;10.32,-27.42,;11.3,-26.26,;10.53,-21.33,;9.16,-22.07,;9.93,-23.41,;8.38,-23.41,;7.84,-21.26,;6.47,-22,;5.14,-21.18,;3.77,-21.92,)| Show InChI InChI=1S/C40H53N9O6S.3C2HF3O2/c1-2-3-24-56(54,55)47-35-26-29-8-14-33(15-9-29)45-37(51)17-19-49-22-20-48(21-23-49)18-16-36(50)44-32-12-6-28(7-13-32)25-34(46-40(35)53)39(52)43-27-30-4-10-31(11-5-30)38(41)42;3*3-2(4,5)1(6)7/h4-15,34-35,47H,2-3,16-27H2,1H3,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated Protein C (unknown origin) |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated Protein C (unknown origin) |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50244204
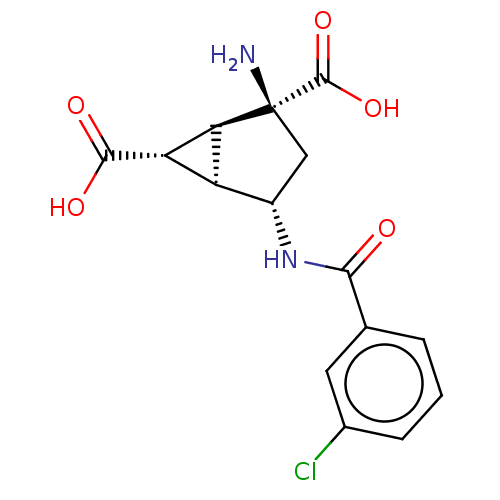
(CHEMBL4071962)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2NC(=O)c1cccc(Cl)c1)C(O)=O |r| Show InChI InChI=1S/C15H15ClN2O5/c16-7-3-1-2-6(4-7)12(19)18-8-5-15(17,14(22)23)11-9(8)10(11)13(20)21/h1-4,8-11H,5,17H2,(H,18,19)(H,20,21)(H,22,23)/t8-,9-,10-,11-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting |
J Med Chem 61: 2303-2328 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01481
BindingDB Entry DOI: 10.7270/Q2W95CM9 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532383

(CHEMBL4571212)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:47.45,wD:22.18,(25.74,-27.44,;26.54,-28.78,;25.78,-30.14,;28.11,-28.75,;29.53,-28.72,;29.16,-29.99,;29.12,-27.47,;25.82,-22.39,;26.62,-23.73,;25.86,-25.09,;28.19,-23.69,;29.61,-23.67,;29.24,-24.94,;29.2,-22.41,;26.43,-32.17,;27.23,-33.51,;26.47,-34.87,;28.8,-33.48,;30.22,-33.45,;29.85,-34.72,;29.81,-32.2,;11.04,-20.93,;11.04,-22.46,;11.04,-23.99,;9.95,-25.08,;8.46,-24.66,;7.37,-25.74,;7.76,-27.22,;6.89,-28.65,;7.55,-30.89,;6.11,-31.45,;8.74,-31.86,;10.28,-31.86,;11.04,-30.52,;12.58,-30.52,;13.34,-31.85,;12.58,-33.18,;11.04,-33.17,;14.87,-31.85,;16.1,-30.93,;17.51,-31.53,;16.53,-29.23,;16.31,-27.73,;14.8,-27.35,;14.36,-25.85,;15.45,-24.72,;14.85,-23.32,;15.03,-21.69,;15.03,-20.15,;13.7,-22.46,;12.37,-21.69,;12.37,-20.15,;16.36,-22.46,;16.36,-23.99,;17.69,-21.69,;19.02,-22.46,;20.35,-21.69,;21.68,-22.47,;23.01,-21.7,;23.01,-20.17,;21.67,-19.4,;20.34,-20.17,;24.34,-19.4,;25.67,-20.16,;24.33,-17.85,;16.97,-25.1,;17.41,-26.59,;9.25,-27.63,;10.33,-26.55,;9.71,-21.69,;9.71,-20.13,;10.11,-18.61,;11.22,-19.72,;8.35,-19.35,;8.36,-17.8,;7.01,-17.02,;5.65,-17.8,;5.66,-19.37,;7.01,-20.14,)| Show InChI InChI=1S/C40H45N9O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(52)34-22-27-8-14-31(15-9-27)44-36(50)25-48-18-20-49(21-19-48)26-37(51)45-32-16-10-28(11-17-32)23-35(40(53)46-34)47-56(54,55)33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,34-35,47H,18-26H2,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062069
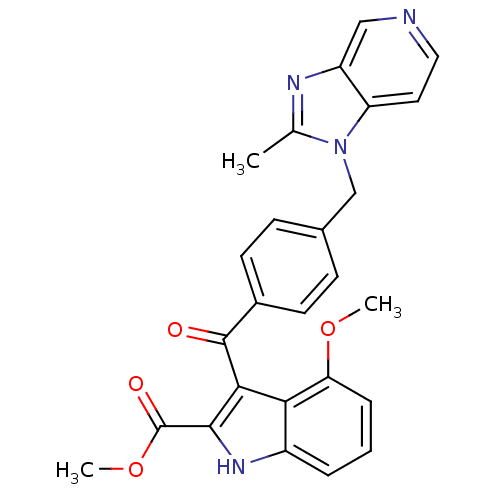
(4-Methoxy-3-[4-(2-methyl-imidazo[4,5-c]pyridin-1-y...)Show SMILES COC(=O)c1[nH]c2cccc(OC)c2c1C(=O)c1ccc(Cn2c(C)nc3cnccc23)cc1 Show InChI InChI=1S/C26H22N4O4/c1-15-28-19-13-27-12-11-20(19)30(15)14-16-7-9-17(10-8-16)25(31)23-22-18(5-4-6-21(22)33-2)29-24(23)26(32)34-3/h4-13,29H,14H2,1-3H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532383

(CHEMBL4571212)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:47.45,wD:22.18,(25.74,-27.44,;26.54,-28.78,;25.78,-30.14,;28.11,-28.75,;29.53,-28.72,;29.16,-29.99,;29.12,-27.47,;25.82,-22.39,;26.62,-23.73,;25.86,-25.09,;28.19,-23.69,;29.61,-23.67,;29.24,-24.94,;29.2,-22.41,;26.43,-32.17,;27.23,-33.51,;26.47,-34.87,;28.8,-33.48,;30.22,-33.45,;29.85,-34.72,;29.81,-32.2,;11.04,-20.93,;11.04,-22.46,;11.04,-23.99,;9.95,-25.08,;8.46,-24.66,;7.37,-25.74,;7.76,-27.22,;6.89,-28.65,;7.55,-30.89,;6.11,-31.45,;8.74,-31.86,;10.28,-31.86,;11.04,-30.52,;12.58,-30.52,;13.34,-31.85,;12.58,-33.18,;11.04,-33.17,;14.87,-31.85,;16.1,-30.93,;17.51,-31.53,;16.53,-29.23,;16.31,-27.73,;14.8,-27.35,;14.36,-25.85,;15.45,-24.72,;14.85,-23.32,;15.03,-21.69,;15.03,-20.15,;13.7,-22.46,;12.37,-21.69,;12.37,-20.15,;16.36,-22.46,;16.36,-23.99,;17.69,-21.69,;19.02,-22.46,;20.35,-21.69,;21.68,-22.47,;23.01,-21.7,;23.01,-20.17,;21.67,-19.4,;20.34,-20.17,;24.34,-19.4,;25.67,-20.16,;24.33,-17.85,;16.97,-25.1,;17.41,-26.59,;9.25,-27.63,;10.33,-26.55,;9.71,-21.69,;9.71,-20.13,;10.11,-18.61,;11.22,-19.72,;8.35,-19.35,;8.36,-17.8,;7.01,-17.02,;5.65,-17.8,;5.66,-19.37,;7.01,-20.14,)| Show InChI InChI=1S/C40H45N9O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(52)34-22-27-8-14-31(15-9-27)44-36(50)25-48-18-20-49(21-19-48)26-37(51)45-32-16-10-28(11-17-32)23-35(40(53)46-34)47-56(54,55)33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,34-35,47H,18-26H2,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394

(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394

(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384
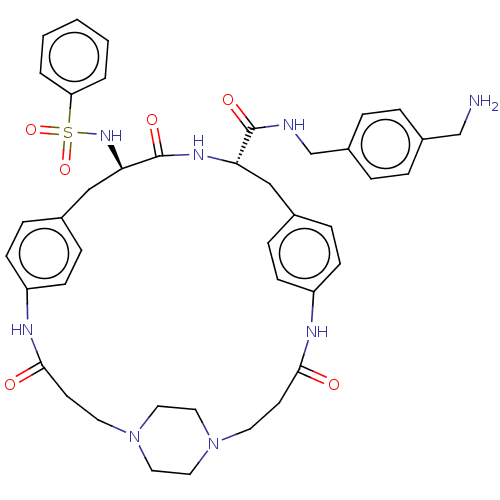
(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384

(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384

(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384

(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062101
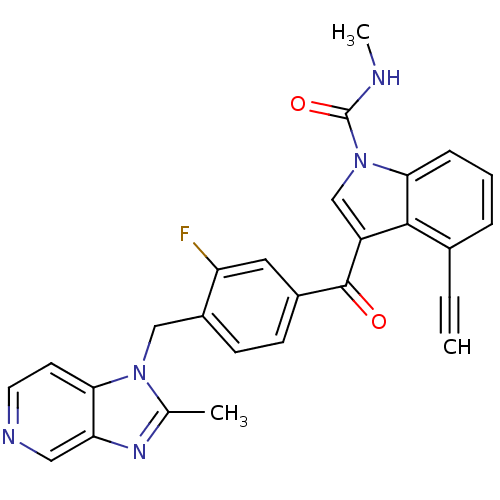
(4-Ethynyl-3-[3-fluoro-4-(2-methyl-imidazo[4,5-c]py...)Show SMILES CNC(=O)n1cc(C(=O)c2ccc(Cn3c(C)nc4cnccc34)c(F)c2)c2c(cccc12)C#C Show InChI InChI=1S/C27H20FN5O2/c1-4-17-6-5-7-24-25(17)20(15-33(24)27(35)29-3)26(34)18-8-9-19(21(28)12-18)14-32-16(2)31-22-13-30-11-10-23(22)32/h1,5-13,15H,14H2,2-3H3,(H,29,35) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392025
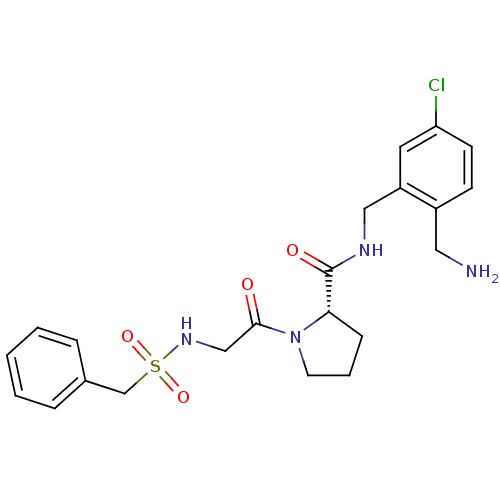
(CHEMBL2152426)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)CNS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C22H27ClN4O4S/c23-19-9-8-17(12-24)18(11-19)13-25-22(29)20-7-4-10-27(20)21(28)14-26-32(30,31)15-16-5-2-1-3-6-16/h1-3,5-6,8-9,11,20,26H,4,7,10,12-15,24H2,(H,25,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) using Tos-Gly-Pro-Arg-AMCA-TFA as a substrate incubated for 40 secs and measured every 15 secs interval for 2... |
J Med Chem 62: 9753-9771 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01196
BindingDB Entry DOI: 10.7270/Q2348PP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062056
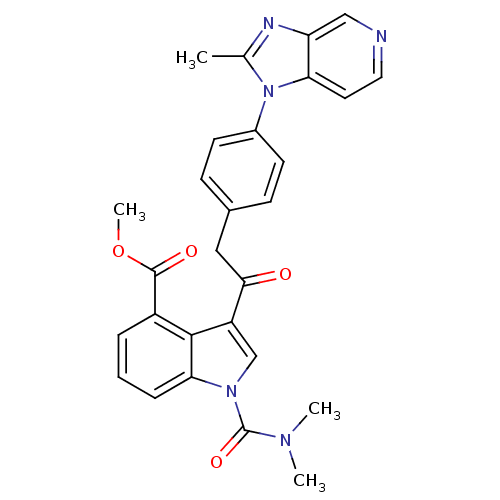
(1-Dimethylcarbamoyl-3-{2-[4-(2-methyl-imidazo[4,5-...)Show SMILES COC(=O)c1cccc2n(cc(C(=O)Cc3ccc(cc3)-n3c(C)nc4cnccc34)c12)C(=O)N(C)C Show InChI InChI=1S/C28H25N5O4/c1-17-30-22-15-29-13-12-23(22)33(17)19-10-8-18(9-11-19)14-25(34)21-16-32(28(36)31(2)3)24-7-5-6-20(26(21)24)27(35)37-4/h5-13,15-16H,14H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062061

(1-(3,3-Dimethyl-2-oxo-butyl)-3-[4-(2-methyl-imidaz...)Show SMILES COC(=O)c1cccc2n(CC(=O)C(C)(C)C)cc(C(=O)c3ccc(Cn4c(C)nc5cnccc45)cc3)c12 Show InChI InChI=1S/C31H30N4O4/c1-19-33-24-15-32-14-13-25(24)35(19)16-20-9-11-21(12-10-20)29(37)23-17-34(18-27(36)31(2,3)4)26-8-6-7-22(28(23)26)30(38)39-5/h6-15,17H,16,18H2,1-5H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50285986

(2-[6-(4-Fluoro-phenyl)-3-(3-pyridin-3-yl-1H-pyrrol...)Show SMILES CN(C)C(=O)Cn1cc(C(=O)c2ccn3C(SCc23)c2cccnc2)c2ccc(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C30H25FN4O2S/c1-33(2)28(36)17-34-16-25(23-10-7-20(14-26(23)34)19-5-8-22(31)9-6-19)29(37)24-11-13-35-27(24)18-38-30(35)21-4-3-12-32-15-21/h3-16,30H,17-18H2,1-2H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for antagonistic activity to displace [3H]-PAF from rabbit platelet membrane PAF receptors |
Bioorg Med Chem Lett 5: 2903-2908 (1995)
Article DOI: 10.1016/0960-894X(95)00509-R
BindingDB Entry DOI: 10.7270/Q2WW7HN1 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50592224
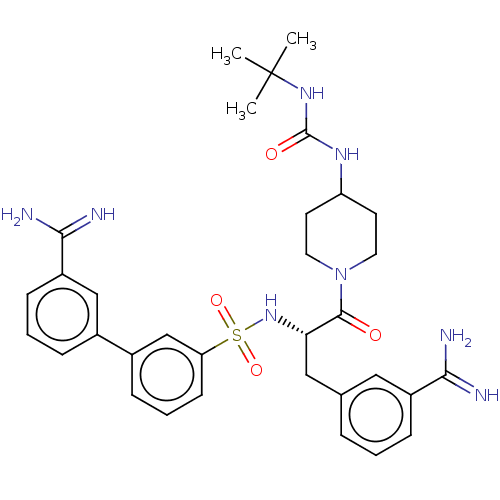
(CHEMBL5193920)Show SMILES CC(C)(C)NC(=O)NC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1cccc(c1)C(N)=N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114437
BindingDB Entry DOI: 10.7270/Q2KD22W8 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532406

(CHEMBL4545331)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NC(=O)OCc1ccccc1 |r,wU:49.47,wD:22.18,(28.26,-22.28,;29.05,-23.61,;28.3,-24.96,;30.6,-23.57,;32.01,-23.55,;31.64,-24.81,;31.6,-22.31,;22.31,-21.79,;23.1,-23.12,;22.35,-24.46,;24.65,-23.08,;26.06,-23.06,;25.69,-24.32,;25.65,-21.82,;23.44,-28.1,;24.23,-29.42,;23.48,-30.77,;25.78,-29.39,;27.19,-29.37,;26.82,-30.63,;26.78,-28.12,;8.28,-19.98,;8.23,-21.49,;8.19,-23.01,;7.2,-24.17,;5.7,-23.88,;4.72,-25.03,;5.22,-26.46,;4.48,-27.94,;5.22,-29.3,;4.41,-30.61,;6.76,-29.34,;7.5,-30.69,;9.04,-30.74,;9.83,-29.44,;11.35,-29.48,;12.07,-30.81,;11.27,-32.11,;9.76,-32.06,;13.61,-30.86,;14.42,-29.55,;15.97,-29.59,;16.7,-30.95,;16.73,-28.25,;14.94,-26.77,;15.3,-25.27,;14.19,-24.2,;12.71,-24.63,;12.16,-22.78,;12.2,-20.85,;12.25,-19.33,;10.87,-21.57,;9.57,-20.77,;9.62,-19.25,;13.49,-21.65,;13.44,-23.17,;14.83,-20.93,;16.12,-21.72,;17.46,-21.01,;18.75,-21.81,;20.09,-21.09,;20.13,-19.57,;18.83,-18.78,;17.5,-19.5,;21.47,-18.86,;22.76,-19.65,;21.51,-17.33,;12.33,-26.12,;13.44,-27.2,;6.73,-26.74,;7.7,-25.59,;6.94,-20.7,;5.58,-21.43,;5.54,-22.97,;4.27,-20.62,;4.31,-19.08,;2.99,-18.27,;3.03,-16.73,;1.72,-15.92,;.36,-16.66,;.32,-18.21,;1.64,-19.01,)| Show InChI InChI=1S/C44H51N9O6.3C2HF3O2/c45-41(46)34-12-6-32(7-13-34)28-47-42(56)37-26-30-8-14-35(15-9-30)48-39(54)18-20-52-22-24-53(25-23-52)21-19-40(55)49-36-16-10-31(11-17-36)27-38(43(57)50-37)51-44(58)59-29-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,37-38H,18-29H2,(H3,45,46)(H,47,56)(H,48,54)(H,49,55)(H,50,57)(H,51,58);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532406

(CHEMBL4545331)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NC(=O)OCc1ccccc1 |r,wU:49.47,wD:22.18,(28.26,-22.28,;29.05,-23.61,;28.3,-24.96,;30.6,-23.57,;32.01,-23.55,;31.64,-24.81,;31.6,-22.31,;22.31,-21.79,;23.1,-23.12,;22.35,-24.46,;24.65,-23.08,;26.06,-23.06,;25.69,-24.32,;25.65,-21.82,;23.44,-28.1,;24.23,-29.42,;23.48,-30.77,;25.78,-29.39,;27.19,-29.37,;26.82,-30.63,;26.78,-28.12,;8.28,-19.98,;8.23,-21.49,;8.19,-23.01,;7.2,-24.17,;5.7,-23.88,;4.72,-25.03,;5.22,-26.46,;4.48,-27.94,;5.22,-29.3,;4.41,-30.61,;6.76,-29.34,;7.5,-30.69,;9.04,-30.74,;9.83,-29.44,;11.35,-29.48,;12.07,-30.81,;11.27,-32.11,;9.76,-32.06,;13.61,-30.86,;14.42,-29.55,;15.97,-29.59,;16.7,-30.95,;16.73,-28.25,;14.94,-26.77,;15.3,-25.27,;14.19,-24.2,;12.71,-24.63,;12.16,-22.78,;12.2,-20.85,;12.25,-19.33,;10.87,-21.57,;9.57,-20.77,;9.62,-19.25,;13.49,-21.65,;13.44,-23.17,;14.83,-20.93,;16.12,-21.72,;17.46,-21.01,;18.75,-21.81,;20.09,-21.09,;20.13,-19.57,;18.83,-18.78,;17.5,-19.5,;21.47,-18.86,;22.76,-19.65,;21.51,-17.33,;12.33,-26.12,;13.44,-27.2,;6.73,-26.74,;7.7,-25.59,;6.94,-20.7,;5.58,-21.43,;5.54,-22.97,;4.27,-20.62,;4.31,-19.08,;2.99,-18.27,;3.03,-16.73,;1.72,-15.92,;.36,-16.66,;.32,-18.21,;1.64,-19.01,)| Show InChI InChI=1S/C44H51N9O6.3C2HF3O2/c45-41(46)34-12-6-32(7-13-34)28-47-42(56)37-26-30-8-14-35(15-9-30)48-39(54)18-20-52-22-24-53(25-23-52)21-19-40(55)49-36-16-10-31(11-17-36)27-38(43(57)50-37)51-44(58)59-29-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,37-38H,18-29H2,(H3,45,46)(H,47,56)(H,48,54)(H,49,55)(H,50,57)(H,51,58);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532401

(CHEMBL4515955)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NC(=O)OC)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(cc1)C(N)=N |r,wU:22.18,wD:49.47,(22.95,-24.3,;23.75,-25.64,;23,-27,;25.32,-25.61,;26.74,-25.59,;26.37,-26.85,;26.33,-24.33,;27.58,-20.32,;28.38,-21.66,;27.63,-23.02,;29.95,-21.62,;31.37,-21.6,;31,-22.87,;30.96,-20.34,;22.33,-19.99,;23.13,-21.33,;22.37,-22.69,;24.7,-21.29,;26.11,-21.27,;25.75,-22.54,;25.7,-20.01,;14.55,-14.2,;14.5,-15.74,;14.46,-17.69,;15.01,-19.55,;16.51,-19.12,;17.64,-20.2,;17.26,-21.71,;19.07,-23.21,;18.3,-24.57,;19.04,-25.94,;16.74,-24.52,;15.93,-25.85,;14.37,-25.8,;13.57,-27.11,;12.04,-27.06,;11.31,-25.72,;12.11,-24.41,;13.65,-24.45,;9.75,-25.68,;9.01,-24.31,;7.45,-24.27,;6.63,-25.6,;6.7,-22.9,;7.46,-21.41,;6.94,-19.96,;7.94,-18.8,;9.45,-19.09,;10.45,-17.92,;10.5,-16.39,;10.54,-14.86,;9.19,-15.58,;7.82,-16.32,;7.78,-17.88,;6.49,-15.51,;6.53,-13.95,;11.85,-15.66,;11.89,-14.13,;13.15,-16.46,;9.96,-20.52,;8.97,-21.69,;15.76,-22.15,;14.63,-21.06,;15.8,-16.54,;15.76,-18.08,;17.15,-15.82,;18.46,-16.62,;19.81,-15.9,;21.11,-16.71,;22.46,-15.98,;22.51,-14.45,;21.2,-13.65,;19.85,-14.38,;23.86,-13.72,;25.17,-14.52,;23.9,-12.18,)| Show InChI InChI=1S/C38H47N9O6.3C2HF3O2/c1-53-38(52)45-32-23-26-6-12-30(13-7-26)43-34(49)15-17-47-20-18-46(19-21-47)16-14-33(48)42-29-10-4-25(5-11-29)22-31(44-37(32)51)36(50)41-24-27-2-8-28(9-3-27)35(39)40;3*3-2(4,5)1(6)7/h2-13,31-32H,14-24H2,1H3,(H3,39,40)(H,41,50)(H,42,48)(H,43,49)(H,44,51)(H,45,52);3*(H,6,7)/t31-,32+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data























































