 Found 238 hits with Last Name = 'hersperger' and Initial = 'r'
Found 238 hits with Last Name = 'hersperger' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546172

(CHEMBL4762397)Show SMILES CN(Cc1c[nH]c2ncnc(-c3cccc(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50085135
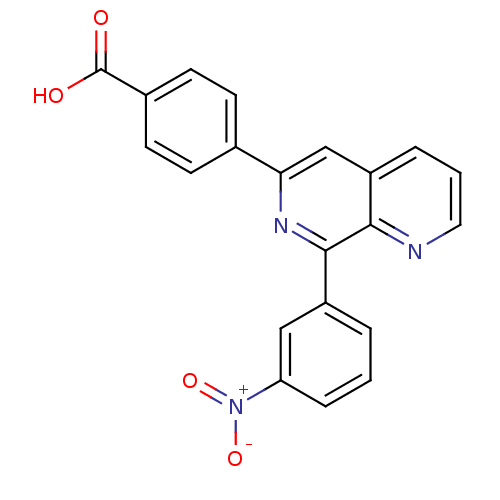
(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of [3H]rolipram binding in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546180
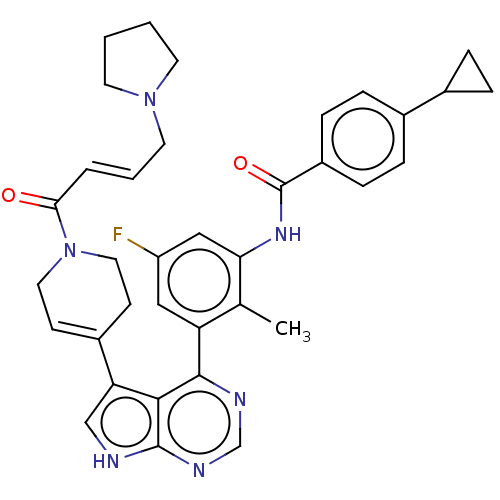
(CHEMBL4749522)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)\C=C\CN3CCCC3)c12 |t:31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50030448
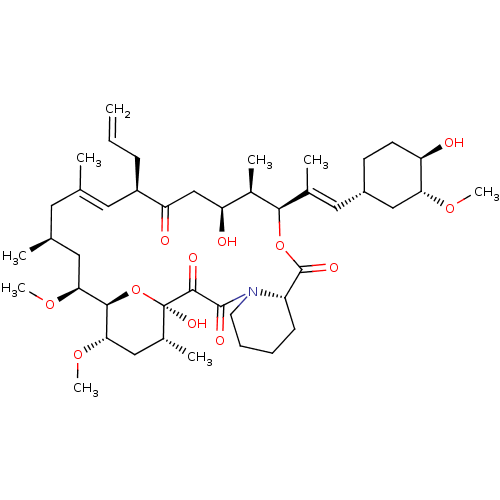
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BMX (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546179

(CHEMBL4739958)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(CC3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4D from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50108504
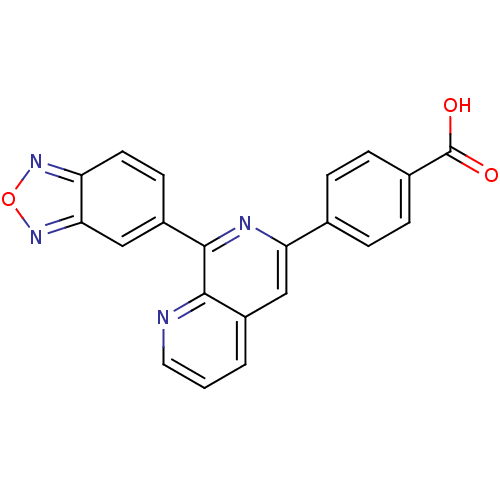
(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of [3H]rolipram binding in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546175

(CHEMBL4795673)Show SMILES CN(C(=O)Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546176

(CHEMBL4746262)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(c12)C1(O)CN(C1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50153090

(CHEMBL411735 | Macrolide derivative)Show SMILES COC1C[C@H](CC(C)[C@H]2OC(=O)C3CCCCN3C(=O)C(=O)[C@]3(O)CC([C@H](C[C@H]3C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)CC[C@H]1CC(=O)Nc1ccc(CCCC(=O)OC)cc1 |t:40| Show InChI InChI=1S/C58H88N2O13/c1-11-15-42-27-35(2)26-36(3)28-50(70-8)45-34-58(68,38(5)30-51(45)71-9)55(65)56(66)60-25-13-12-17-46(60)57(67)73-54(39(6)47(61)33-48(42)62)37(4)29-41-19-22-43(49(31-41)69-7)32-52(63)59-44-23-20-40(21-24-44)16-14-18-53(64)72-10/h11,20-21,23-24,27,36-39,41-43,45-47,49-51,54,61,68H,1,12-19,22,25-26,28-34H2,2-10H3,(H,59,63)/b35-27+/t36-,37?,38+,39+,41-,42+,43-,45?,46?,47-,49?,50-,51-,54+,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50108504

(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4D from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50153091
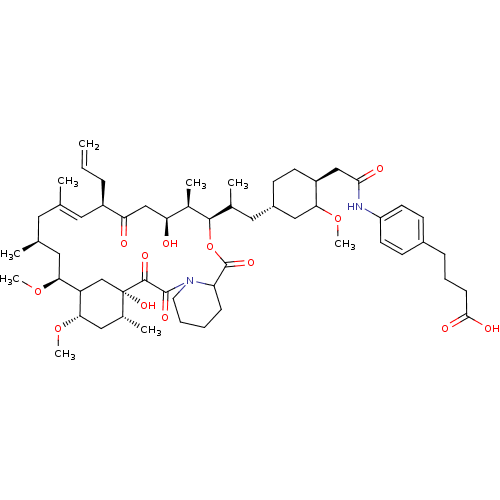
(CHEMBL265123 | Macrolide derivative)Show SMILES COC1C[C@H](CC(C)[C@H]2OC(=O)C3CCCCN3C(=O)C(=O)[C@]3(O)CC([C@H](C[C@H]3C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)CC[C@H]1CC(=O)Nc1ccc(CCCC(O)=O)cc1 |t:40| Show InChI InChI=1S/C57H86N2O13/c1-10-14-41-26-34(2)25-35(3)27-49(70-8)44-33-57(68,37(5)29-50(44)71-9)54(65)55(66)59-24-12-11-16-45(59)56(67)72-53(38(6)46(60)32-47(41)61)36(4)28-40-18-21-42(48(30-40)69-7)31-51(62)58-43-22-19-39(20-23-43)15-13-17-52(63)64/h10,19-20,22-23,26,35-38,40-42,44-46,48-50,53,60,68H,1,11-18,21,24-25,27-33H2,2-9H3,(H,58,62)(H,63,64)/b34-26+/t35-,36?,37+,38+,40-,41+,42-,44?,45?,46-,48?,49-,50-,53+,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085136
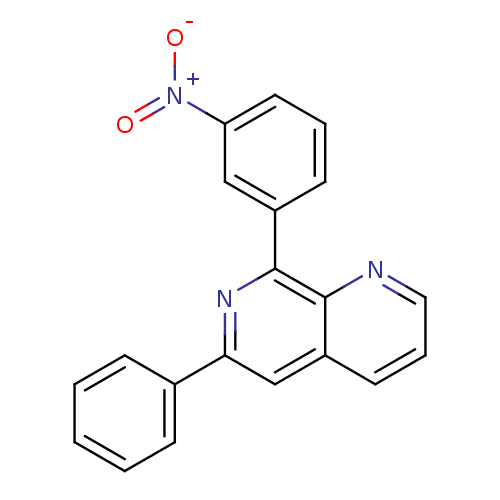
(8-(3-Nitro-phenyl)-6-phenyl-[1,7]naphthyridine | C...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(cc2cccnc12)-c1ccccc1 Show InChI InChI=1S/C20H13N3O2/c24-23(25)17-10-4-8-15(12-17)20-19-16(9-5-11-21-19)13-18(22-20)14-6-2-1-3-7-14/h1-13H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085138
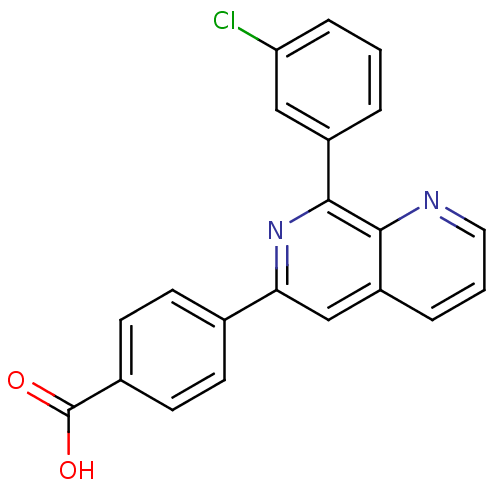
(4-[8-(3-Chloro-phenyl)-[1,7]naphthyridin-6-yl]-ben...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C21H13ClN2O2/c22-17-5-1-3-15(11-17)20-19-16(4-2-10-23-19)12-18(24-20)13-6-8-14(9-7-13)21(25)26/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399042

(CHEMBL2178812)Show SMILES Cc1cc(c(C)cc1Cl)S(=O)(=O)Nc1cccc(c1)-c1cc(C)c(C(=O)N[C@@H](CCN)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H30ClN3O5S/c1-15-13-24(16(2)12-22(15)28)37(35,36)31-21-7-5-6-19(14-21)20-10-17(3)25(18(4)11-20)26(32)30-23(8-9-29)27(33)34/h5-7,10-14,23,31H,8-9,29H2,1-4H3,(H,30,32)(H,33,34)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399045
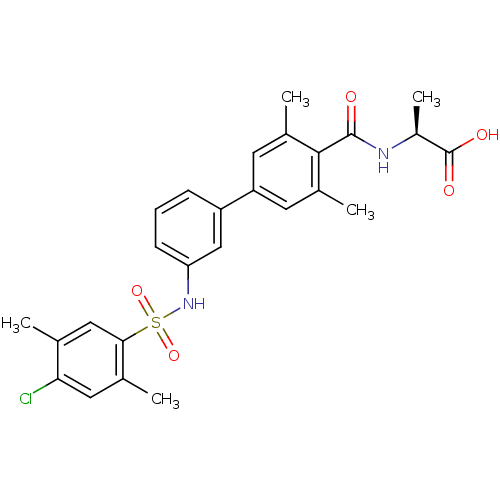
(CHEMBL2178809)Show SMILES C[C@H](NC(=O)c1c(C)cc(cc1C)-c1cccc(NS(=O)(=O)c2cc(C)c(Cl)cc2C)c1)C(O)=O |r| Show InChI InChI=1S/C26H27ClN2O5S/c1-14-12-23(15(2)11-22(14)27)35(33,34)29-21-8-6-7-19(13-21)20-9-16(3)24(17(4)10-20)25(30)28-18(5)26(31)32/h6-13,18,29H,1-5H3,(H,28,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465455
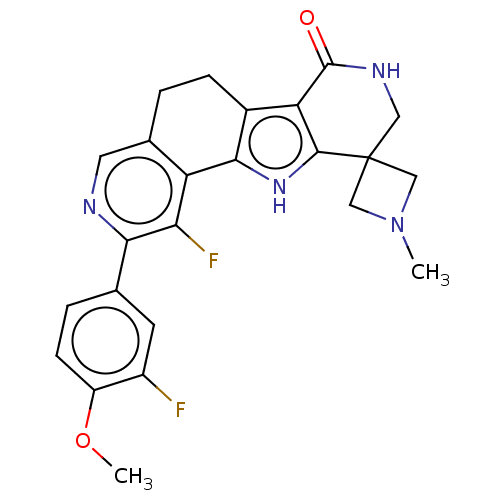
(CHEMBL4282514)Show SMILES COc1ccc(cc1F)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C24H22F2N4O2/c1-30-10-24(11-30)9-28-23(31)18-14-5-3-13-8-27-20(12-4-6-16(32-2)15(25)7-12)19(26)17(13)21(14)29-22(18)24/h4,6-8,29H,3,5,9-11H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465453

(CHEMBL4277929)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(c(F)c4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C23H20F2N4O/c1-29-10-23(11-29)9-27-22(30)17-15-6-5-13-8-26-19(12-3-2-4-14(24)7-12)18(25)16(13)20(15)28-21(17)23/h2-4,7-8,28H,5-6,9-11H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085146
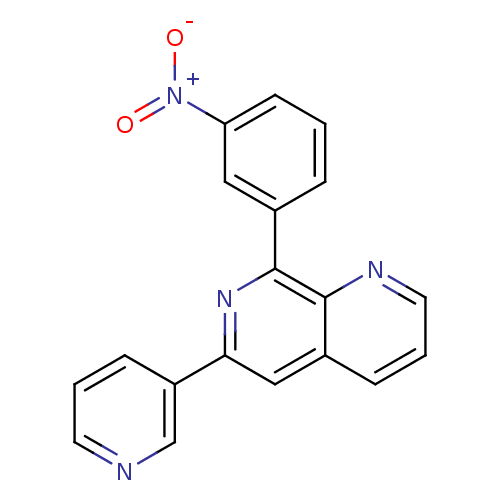
(8-(3-Nitro-phenyl)-6-pyridin-3-yl-[1,7]naphthyridi...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(cc2cccnc12)-c1cccnc1 Show InChI InChI=1S/C19H12N4O2/c24-23(25)16-7-1-4-13(10-16)19-18-14(5-3-9-21-18)11-17(22-19)15-6-2-8-20-12-15/h1-12H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465454

(CHEMBL4293121)Show SMILES COc1ccc(cn1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C23H22FN5O2/c1-29-10-23(11-29)9-27-22(30)17-14-5-3-12-7-26-19(13-4-6-15(31-2)25-8-13)18(24)16(12)20(14)28-21(17)23/h4,6-8,28H,3,5,9-11H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399039
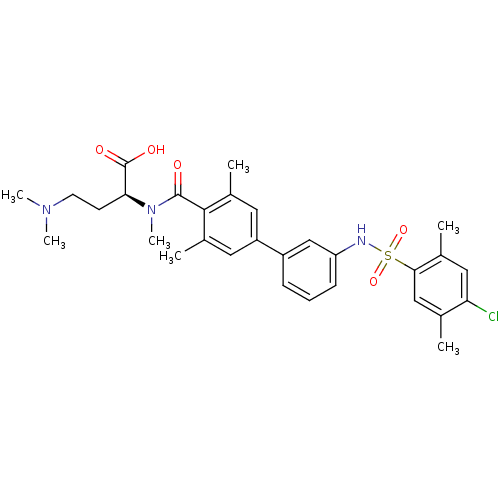
(CHEMBL2178814)Show SMILES CN(C)CC[C@H](N(C)C(=O)c1c(C)cc(cc1C)-c1cccc(NS(=O)(=O)c2cc(C)c(Cl)cc2C)c1)C(O)=O |r| Show InChI InChI=1S/C30H36ClN3O5S/c1-18-16-27(19(2)15-25(18)31)40(38,39)32-24-10-8-9-22(17-24)23-13-20(3)28(21(4)14-23)29(35)34(7)26(30(36)37)11-12-33(5)6/h8-10,13-17,26,32H,11-12H2,1-7H3,(H,36,37)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085139
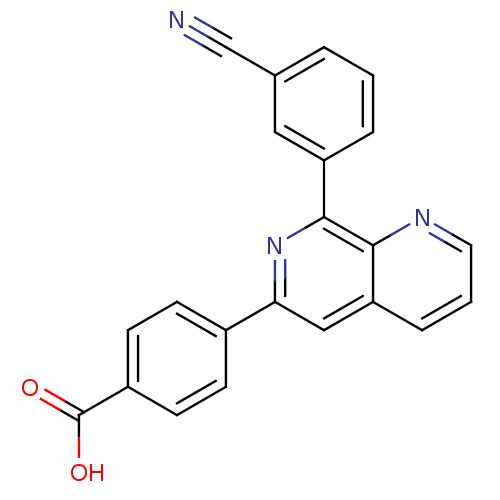
(4-[8-(3-Cyano-phenyl)-[1,7]naphthyridin-6-yl]-benz...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)C#N Show InChI InChI=1S/C22H13N3O2/c23-13-14-3-1-4-17(11-14)21-20-18(5-2-10-24-20)12-19(25-21)15-6-8-16(9-7-15)22(26)27/h1-12H,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by im... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546177
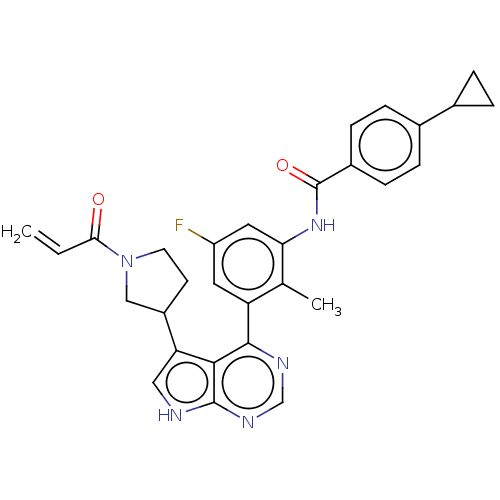
(CHEMBL4740933)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(C3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546181
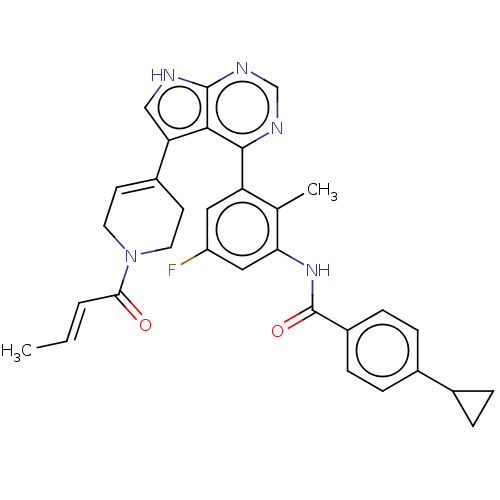
(CHEMBL4789435)Show SMILES C\C=C\C(=O)N1CCC(=CC1)c1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12 |c:8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399044
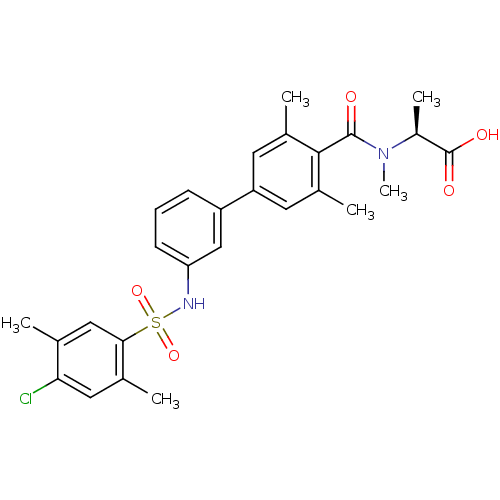
(CHEMBL2178810)Show SMILES C[C@H](N(C)C(=O)c1c(C)cc(cc1C)-c1cccc(NS(=O)(=O)c2cc(C)c(Cl)cc2C)c1)C(O)=O |r| Show InChI InChI=1S/C27H29ClN2O5S/c1-15-13-24(16(2)12-23(15)28)36(34,35)29-22-9-7-8-20(14-22)21-10-17(3)25(18(4)11-21)26(31)30(6)19(5)27(32)33/h7-14,19,29H,1-6H3,(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085140
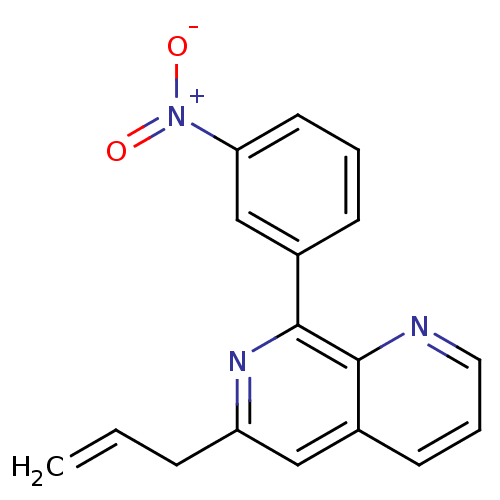
(6-Allyl-8-(3-nitro-phenyl)-[1,7]naphthyridine | CH...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(CC=C)cc2cccnc12 Show InChI InChI=1S/C17H13N3O2/c1-2-5-14-10-12-7-4-9-18-16(12)17(19-14)13-6-3-8-15(11-13)20(21)22/h2-4,6-11H,1,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERBB4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085137
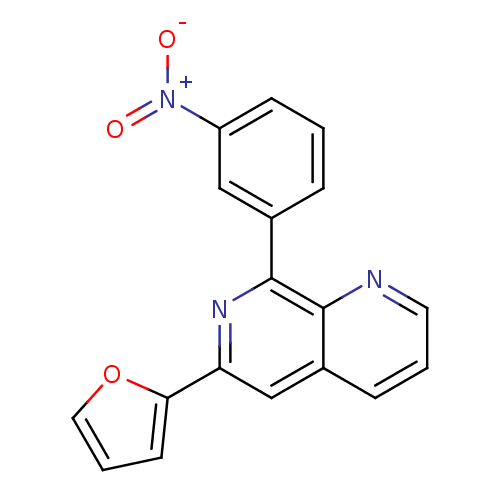
(6-Furan-2-yl-8-(3-nitro-phenyl)-[1,7]naphthyridine...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(cc2cccnc12)-c1ccco1 Show InChI InChI=1S/C18H11N3O3/c22-21(23)14-6-1-4-12(10-14)18-17-13(5-2-8-19-17)11-15(20-18)16-7-3-9-24-16/h1-11H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399043
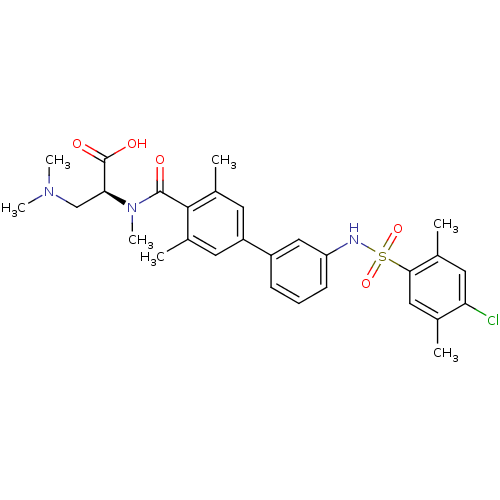
(CHEMBL2178811)Show SMILES CN(C)C[C@H](N(C)C(=O)c1c(C)cc(cc1C)-c1cccc(NS(=O)(=O)c2cc(C)c(Cl)cc2C)c1)C(O)=O |r| Show InChI InChI=1S/C29H34ClN3O5S/c1-17-14-26(18(2)13-24(17)30)39(37,38)31-23-10-8-9-21(15-23)22-11-19(3)27(20(4)12-22)28(34)33(7)25(29(35)36)16-32(5)6/h8-15,25,31H,16H2,1-7H3,(H,35,36)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085141
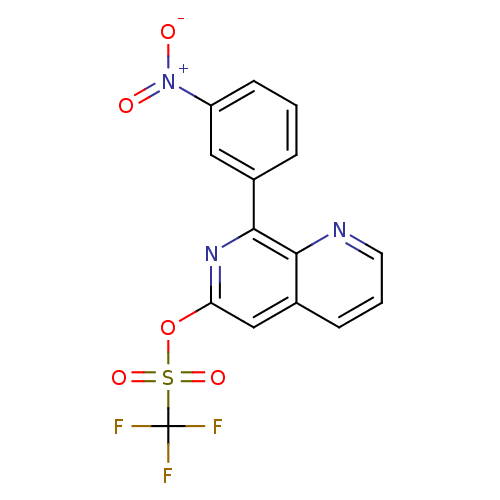
(CHEMBL351900 | Trifluoro-methanesulfonic acid 8-(3...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(OS(=O)(=O)C(F)(F)F)cc2cccnc12 Show InChI InChI=1S/C15H8F3N3O5S/c16-15(17,18)27(24,25)26-12-8-10-4-2-6-19-13(10)14(20-12)9-3-1-5-11(7-9)21(22)23/h1-8H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399041
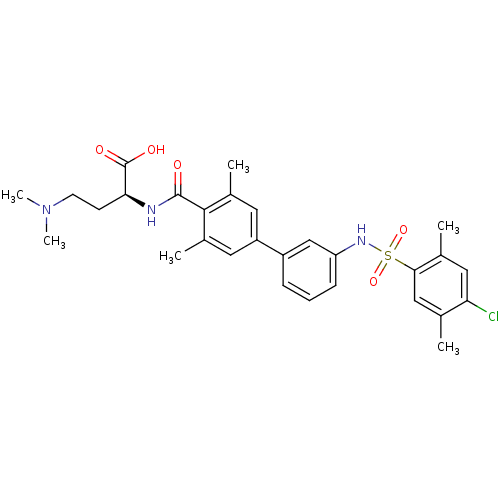
(CHEMBL2178813)Show SMILES CN(C)CC[C@H](NC(=O)c1c(C)cc(cc1C)-c1cccc(NS(=O)(=O)c2cc(C)c(Cl)cc2C)c1)C(O)=O |r| Show InChI InChI=1S/C29H34ClN3O5S/c1-17-15-26(18(2)14-24(17)30)39(37,38)32-23-9-7-8-21(16-23)22-12-19(3)27(20(4)13-22)28(34)31-25(29(35)36)10-11-33(5)6/h7-9,12-16,25,32H,10-11H2,1-6H3,(H,31,34)(H,35,36)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514646

(CHEMBL4566818)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1cc2c(ncnc2[nH]1)-c1cccc(NC(=O)c2ccc(cc2)C(C)(C)C)c1C Show InChI InChI=1S/C36H38N6O2/c1-23-28(7-6-8-30(23)40-34(43)25-13-15-27(16-14-25)36(2,3)4)32-29-21-31(39-33(29)38-22-37-32)24-9-11-26(12-10-24)35(44)42-19-17-41(5)18-20-42/h6-16,21-22H,17-20H2,1-5H3,(H,40,43)(H,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546175

(CHEMBL4795673)Show SMILES CN(C(=O)Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human whole blood assessed as reduction in polyclonal anti-IgM antibody/human IL4-stimulated CD69 cell surface expression on B c... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546179

(CHEMBL4739958)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(CC3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human whole blood assessed as reduction in polyclonal anti-IgM antibody/human IL4-stimulated CD69 cell surface expression on B c... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50399040

(CHEMBL2178815)Show SMILES CN(C)CC[C@@H](N(C)C(=O)c1c(C)cc(cc1C)-c1cccc(NS(=O)(=O)c2cc(C)c(Cl)cc2C)c1)C(O)=O |r| Show InChI InChI=1S/C30H36ClN3O5S/c1-18-16-27(19(2)15-25(18)31)40(38,39)32-24-10-8-9-22(17-24)23-13-20(3)28(21(4)14-23)29(35)34(7)26(30(36)37)11-12-33(5)6/h8-10,13-17,26,32H,11-12H2,1-7H3,(H,36,37)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay |
J Med Chem 55: 9722-34 (2012)
Article DOI: 10.1021/jm3009508
BindingDB Entry DOI: 10.7270/Q2K938PD |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50175686

((4-(4-(5-(4-hydroxybutyl)thiophen-2-yl)pyrimidin-2...)Show SMILES OCCCCc1ccc(s1)-c1ccnc(Nc2ccc(cc2)C(=O)N2CCC(CC2)N2CCCC2)n1 Show InChI InChI=1S/C28H35N5O2S/c34-20-4-1-5-24-10-11-26(36-24)25-12-15-29-28(31-25)30-22-8-6-21(7-9-22)27(35)33-18-13-23(14-19-33)32-16-2-3-17-32/h6-12,15,23,34H,1-5,13-14,16-20H2,(H,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against IKK2 |
Bioorg Med Chem Lett 16: 108-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.035
BindingDB Entry DOI: 10.7270/Q2BR8RQV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546174
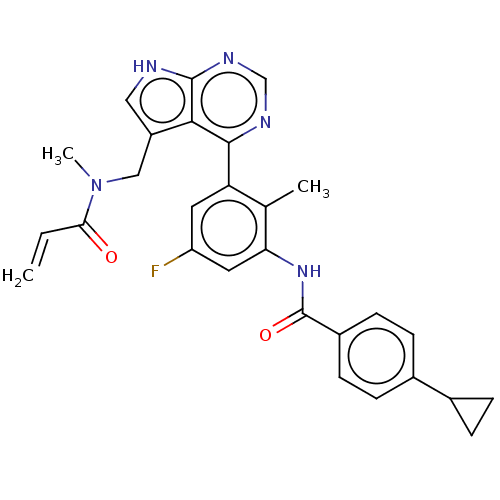
(CHEMBL4793026)Show SMILES CN(Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546180

(CHEMBL4749522)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)\C=C\CN3CCCC3)c12 |t:31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human whole blood assessed as reduction in polyclonal anti-IgM antibody/human IL4-stimulated CD69 cell surface expression on B c... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50175701
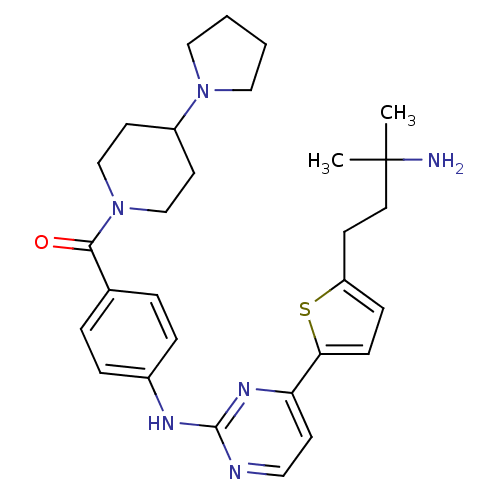
((4-(4-(5-(3-amino-3-methylbutyl)thiophen-2-yl)pyri...)Show SMILES CC(C)(N)CCc1ccc(s1)-c1ccnc(Nc2ccc(cc2)C(=O)N2CCC(CC2)N2CCCC2)n1 Show InChI InChI=1S/C29H38N6OS/c1-29(2,30)15-11-24-9-10-26(37-24)25-12-16-31-28(33-25)32-22-7-5-21(6-8-22)27(36)35-19-13-23(14-20-35)34-17-3-4-18-34/h5-10,12,16,23H,3-4,11,13-15,17-20,30H2,1-2H3,(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against IKK1 |
Bioorg Med Chem Lett 16: 108-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.035
BindingDB Entry DOI: 10.7270/Q2BR8RQV |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha/Inhibitor of nuclear factor kappa-B kinase subunit beta/NF-kappa-B essential modulator
(Homo sapiens (Human)) | BDBM50175690
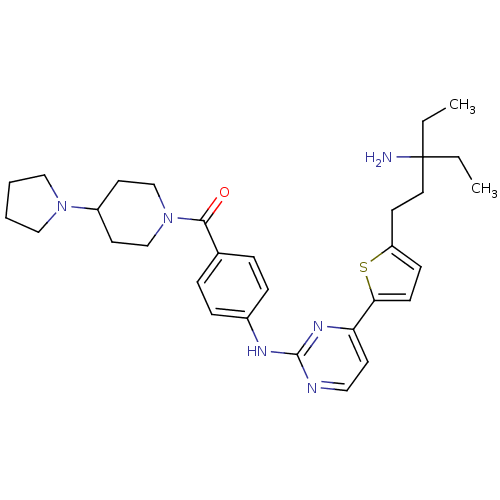
((4-(4-(5-(3-amino-3-ethylpentyl)thiophen-2-yl)pyri...)Show SMILES CCC(N)(CC)CCc1ccc(s1)-c1ccnc(Nc2ccc(cc2)C(=O)N2CCC(CC2)N2CCCC2)n1 Show InChI InChI=1S/C31H42N6OS/c1-3-31(32,4-2)17-13-26-11-12-28(39-26)27-14-18-33-30(35-27)34-24-9-7-23(8-10-24)29(38)37-21-15-25(16-22-37)36-19-5-6-20-36/h7-12,14,18,25H,3-6,13,15-17,19-22,32H2,1-2H3,(H,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against IKK complex isolated from HeLa cells |
Bioorg Med Chem Lett 16: 108-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.035
BindingDB Entry DOI: 10.7270/Q2BR8RQV |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50175690

((4-(4-(5-(3-amino-3-ethylpentyl)thiophen-2-yl)pyri...)Show SMILES CCC(N)(CC)CCc1ccc(s1)-c1ccnc(Nc2ccc(cc2)C(=O)N2CCC(CC2)N2CCCC2)n1 Show InChI InChI=1S/C31H42N6OS/c1-3-31(32,4-2)17-13-26-11-12-28(39-26)27-14-18-33-30(35-27)34-24-9-7-23(8-10-24)29(38)37-21-15-25(16-22-37)36-19-5-6-20-36/h7-12,14,18,25H,3-6,13,15-17,19-22,32H2,1-2H3,(H,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against IKK1 |
Bioorg Med Chem Lett 16: 108-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.035
BindingDB Entry DOI: 10.7270/Q2BR8RQV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human whole blood assessed as reduction in polyclonal anti-IgM antibody/human IL4-stimulated CD69 cell surface expression on B c... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50108504

(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4B from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha/Inhibitor of nuclear factor kappa-B kinase subunit beta/NF-kappa-B essential modulator
(Homo sapiens (Human)) | BDBM50175686

((4-(4-(5-(4-hydroxybutyl)thiophen-2-yl)pyrimidin-2...)Show SMILES OCCCCc1ccc(s1)-c1ccnc(Nc2ccc(cc2)C(=O)N2CCC(CC2)N2CCCC2)n1 Show InChI InChI=1S/C28H35N5O2S/c34-20-4-1-5-24-10-11-26(36-24)25-12-15-29-28(31-25)30-22-8-6-21(7-9-22)27(35)33-18-13-23(14-19-33)32-16-2-3-17-32/h6-12,15,23,34H,1-5,13-14,16-20H2,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against IKK complex isolated from HeLa cells |
Bioorg Med Chem Lett 16: 108-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.035
BindingDB Entry DOI: 10.7270/Q2BR8RQV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data