 Found 54 hits with Last Name = 'hikita' and Initial = 'k'
Found 54 hits with Last Name = 'hikita' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor IX
(Rattus norvegicus) | BDBM50240469
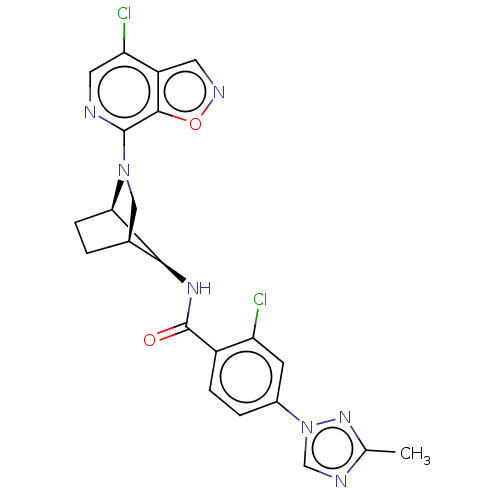
(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat coagulation factor 9a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240487
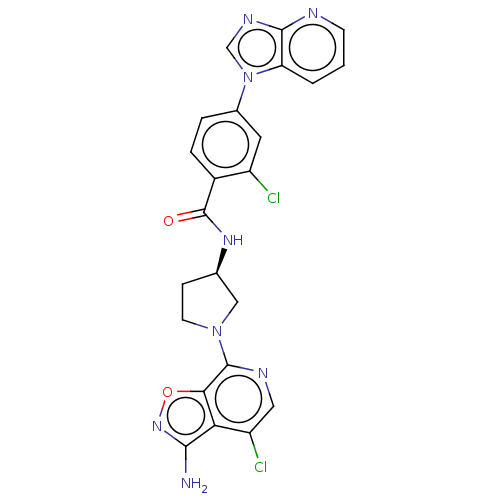
(CHEMBL4066380 | US9969724, Example 32)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnc2ncccc12 |r| Show InChI InChI=1S/C23H18Cl2N8O2/c24-15-8-13(33-11-29-21-17(33)2-1-6-27-21)3-4-14(15)23(34)30-12-5-7-32(10-12)22-19-18(16(25)9-28-22)20(26)31-35-19/h1-4,6,8-9,11-12H,5,7,10H2,(H2,26,31)(H,30,34)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240470
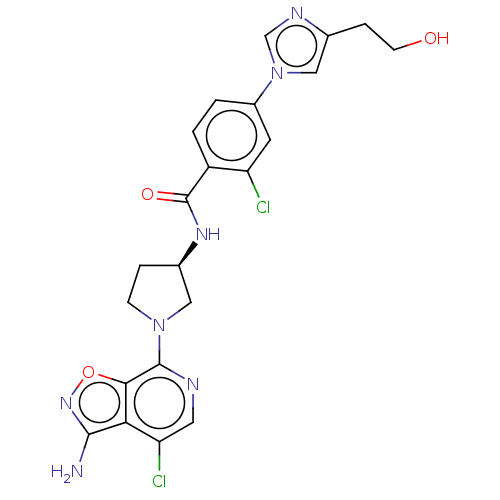
(CHEMBL4092982 | US9969724, Example 27)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnc(CCO)c1 |r| Show InChI InChI=1S/C22H21Cl2N7O3/c23-16-7-14(31-9-12(4-6-32)27-11-31)1-2-15(16)22(33)28-13-3-5-30(10-13)21-19-18(17(24)8-26-21)20(25)29-34-19/h1-2,7-9,11,13,32H,3-6,10H2,(H2,25,29)(H,28,33)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240471

(CHEMBL4069082 | US9969724, Example 45)Show SMILES Cn1ncn(-c2ccc(C(=O)N[C@@H]3CCN(C3)c3ncc(Cl)c4c(N)noc34)c(Cl)c2)c1=O |r| Show InChI InChI=1S/C20H18Cl2N8O3/c1-28-20(32)30(9-25-28)11-2-3-12(13(21)6-11)19(31)26-10-4-5-29(8-10)18-16-15(14(22)7-24-18)17(23)27-33-16/h2-3,6-7,9-10H,4-5,8H2,1H3,(H2,23,27)(H,26,31)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240473
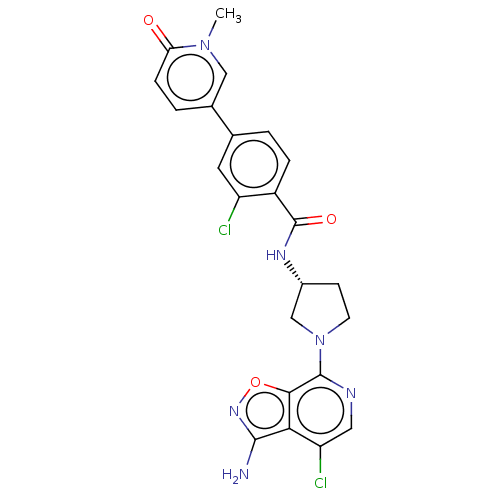
(CHEMBL4095415)Show SMILES Cn1cc(ccc1=O)-c1ccc(C(=O)N[C@@H]2CCN(C2)c2ncc(Cl)c3c(N)noc23)c(Cl)c1 |r| Show InChI InChI=1S/C23H20Cl2N6O3/c1-30-10-13(3-5-18(30)32)12-2-4-15(16(24)8-12)23(33)28-14-6-7-31(11-14)22-20-19(17(25)9-27-22)21(26)29-34-20/h2-5,8-10,14H,6-7,11H2,1H3,(H2,26,29)(H,28,33)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.5 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240472
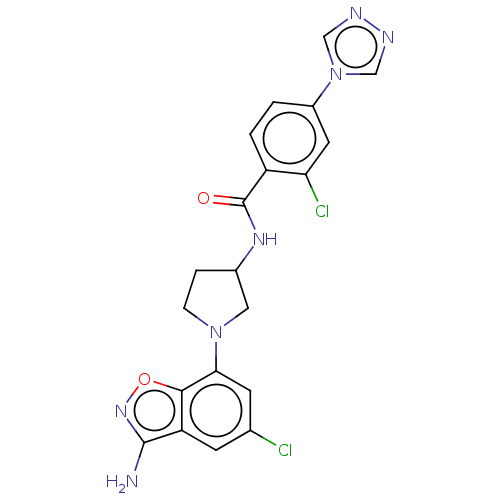
(CHEMBL4090664)Show SMILES Nc1noc2c(cc(Cl)cc12)N1CCC(C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 Show InChI InChI=1S/C20H17Cl2N7O2/c21-11-5-15-18(31-27-19(15)23)17(6-11)28-4-3-12(8-28)26-20(30)14-2-1-13(7-16(14)22)29-9-24-25-10-29/h1-2,5-7,9-10,12H,3-4,8H2,(H2,23,27)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240489

(CHEMBL4101793 | US9969724, Example 20)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-c1cncnc1 |r| Show InChI InChI=1S/C21H17Cl2N7O2/c22-15-5-11(12-6-25-10-26-7-12)1-2-14(15)21(31)28-13-3-4-30(9-13)20-18-17(16(23)8-27-20)19(24)29-32-18/h1-2,5-8,10,13H,3-4,9H2,(H2,24,29)(H,28,31)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240474
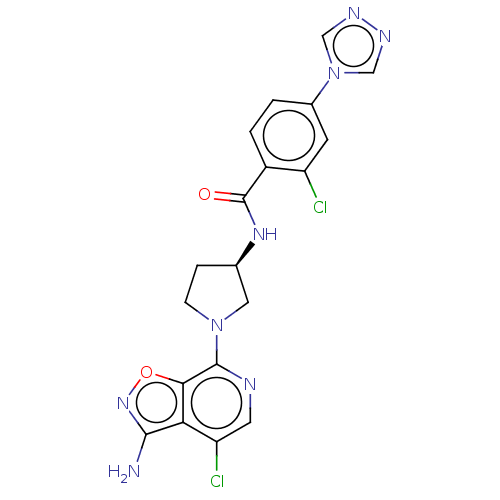
(CHEMBL4082857)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C19H16Cl2N8O2/c20-13-5-11(29-8-24-25-9-29)1-2-12(13)19(30)26-10-3-4-28(7-10)18-16-15(14(21)6-23-18)17(22)27-31-16/h1-2,5-6,8-10H,3-4,7H2,(H2,22,27)(H,26,30)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240475

(CHEMBL4080361 | US9969724, Example 9)Show SMILES Nc1noc2c(ccc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H17Cl2N7O2/c21-14-3-4-16(18-17(14)19(23)27-31-18)28-6-5-11(8-28)26-20(30)13-2-1-12(7-15(13)22)29-9-24-25-10-29/h1-4,7,9-11H,5-6,8H2,(H2,23,27)(H,26,30)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240476

(CHEMBL4087720 | US9969724, Example 29)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnc2ccccc12 |r| Show InChI InChI=1S/C24H19Cl2N7O2/c25-16-9-14(33-12-29-18-3-1-2-4-19(18)33)5-6-15(16)24(34)30-13-7-8-32(11-13)23-21-20(17(26)10-28-23)22(27)31-35-21/h1-6,9-10,12-13H,7-8,11H2,(H2,27,31)(H,30,34)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240491

(CHEMBL4065316 | US9969724, Example 11)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@H]2CCN(C2)c2ncc(Cl)c3c(N)noc23)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl2N8O2/c1-10-25-9-30(27-10)12-2-3-13(14(21)6-12)20(31)26-11-4-5-29(8-11)19-17-16(15(22)7-24-19)18(23)28-32-17/h2-3,6-7,9,11H,4-5,8H2,1H3,(H2,23,28)(H,26,31)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240477
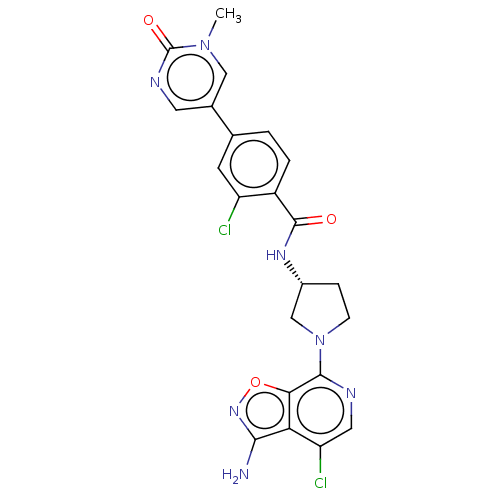
(CHEMBL4074983 | US9969724, Example 44)Show SMILES Cn1cc(cnc1=O)-c1ccc(C(=O)N[C@@H]2CCN(C2)c2ncc(Cl)c3c(N)noc23)c(Cl)c1 |r| Show InChI InChI=1S/C22H19Cl2N7O3/c1-30-9-12(7-27-22(30)33)11-2-3-14(15(23)6-11)21(32)28-13-4-5-31(10-13)20-18-17(16(24)8-26-20)19(25)29-34-18/h2-3,6-9,13H,4-5,10H2,1H3,(H2,25,29)(H,28,32)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240478
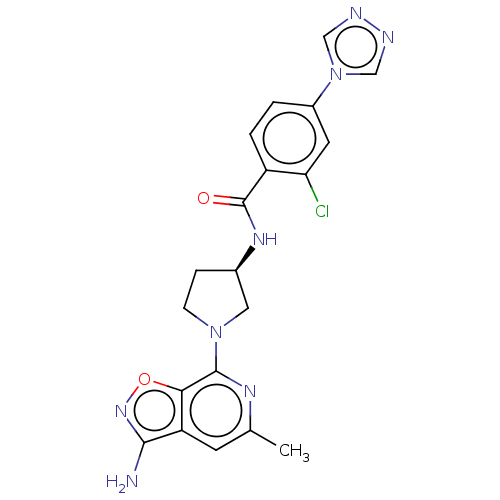
(CHEMBL4083789)Show SMILES Cc1cc2c(N)noc2c(n1)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H19ClN8O2/c1-11-6-15-17(31-27-18(15)22)19(25-11)28-5-4-12(8-28)26-20(30)14-3-2-13(7-16(14)21)29-9-23-24-10-29/h2-3,6-7,9-10,12H,4-5,8H2,1H3,(H2,22,27)(H,26,30)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240493
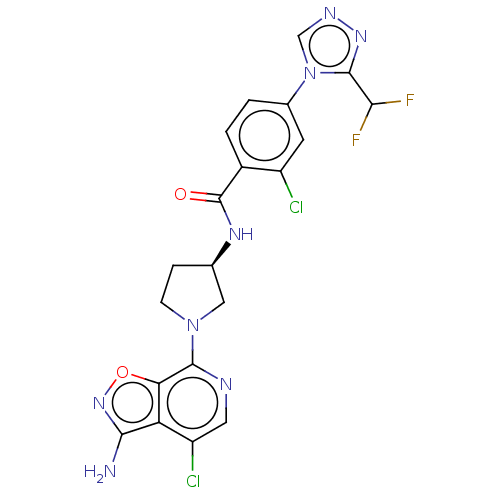
(CHEMBL4085239 | US9969724, Example 28)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1C(F)F |r| Show InChI InChI=1S/C20H16Cl2F2N8O2/c21-12-5-10(32-8-27-29-19(32)16(23)24)1-2-11(12)20(33)28-9-3-4-31(7-9)18-15-14(13(22)6-26-18)17(25)30-34-15/h1-2,5-6,8-9,16H,3-4,7H2,(H2,25,30)(H,28,33)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240479

(CHEMBL4072364 | US9969724, Example 14)Show SMILES Nc1noc2c(cc(Cl)c(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H16Cl3N7O2/c21-13-5-11(30-8-25-26-9-30)1-2-12(13)20(31)27-10-3-4-29(7-10)15-6-14(22)17(23)16-18(15)32-28-19(16)24/h1-2,5-6,8-10H,3-4,7H2,(H2,24,28)(H,27,31)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240482
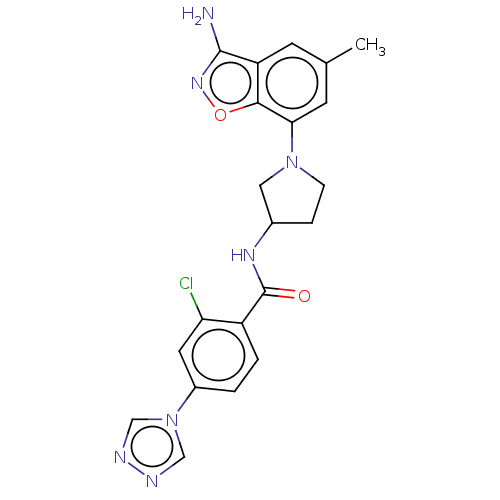
(CHEMBL4069648)Show SMILES Cc1cc(N2CCC(C2)NC(=O)c2ccc(cc2Cl)-n2cnnc2)c2onc(N)c2c1 Show InChI InChI=1S/C21H20ClN7O2/c1-12-6-16-19(31-27-20(16)23)18(7-12)28-5-4-13(9-28)26-21(30)15-3-2-14(8-17(15)22)29-10-24-25-11-29/h2-3,6-8,10-11,13H,4-5,9H2,1H3,(H2,23,27)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240483
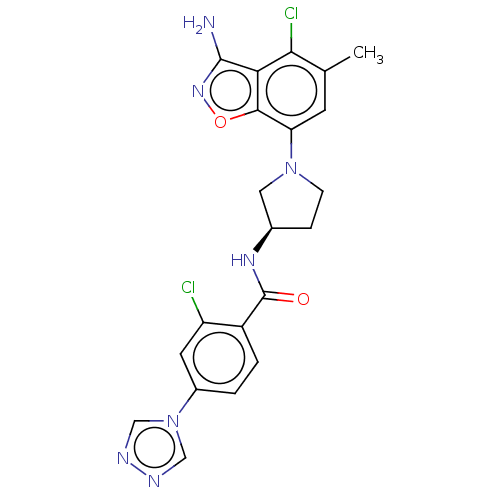
(CHEMBL4099310 | US9969724, Example 12)Show SMILES Cc1cc(N2CC[C@H](C2)NC(=O)c2ccc(cc2Cl)-n2cnnc2)c2onc(N)c2c1Cl |r| Show InChI InChI=1S/C21H19Cl2N7O2/c1-11-6-16(19-17(18(11)23)20(24)28-32-19)29-5-4-12(8-29)27-21(31)14-3-2-13(7-15(14)22)30-9-25-26-10-30/h2-3,6-7,9-10,12H,4-5,8H2,1H3,(H2,24,28)(H,27,31)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240488
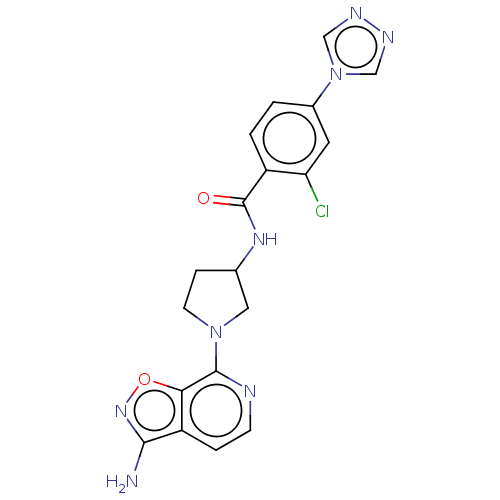
(CHEMBL4060976 | US9969724, Example 5)Show SMILES Nc1noc2c(nccc12)N1CCC(C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 Show InChI InChI=1S/C19H17ClN8O2/c20-15-7-12(28-9-23-24-10-28)1-2-13(15)19(29)25-11-4-6-27(8-11)18-16-14(3-5-22-18)17(21)26-30-16/h1-3,5,7,9-11H,4,6,8H2,(H2,21,26)(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240484
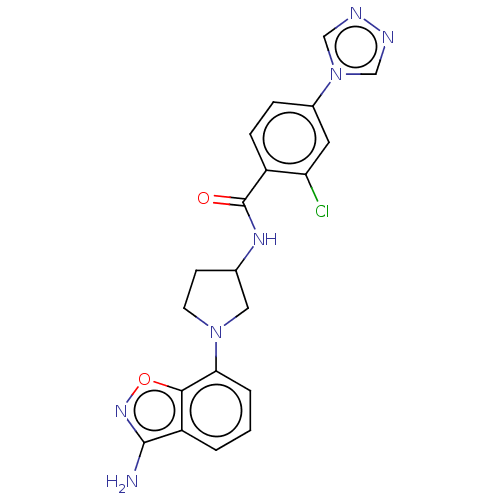
(CHEMBL4085322 | US9969724, Example 1)Show SMILES Nc1noc2c(cccc12)N1CCC(C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 Show InChI InChI=1S/C20H18ClN7O2/c21-16-8-13(28-10-23-24-11-28)4-5-14(16)20(29)25-12-6-7-27(9-12)17-3-1-2-15-18(17)30-26-19(15)22/h1-5,8,10-12H,6-7,9H2,(H2,22,26)(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240486
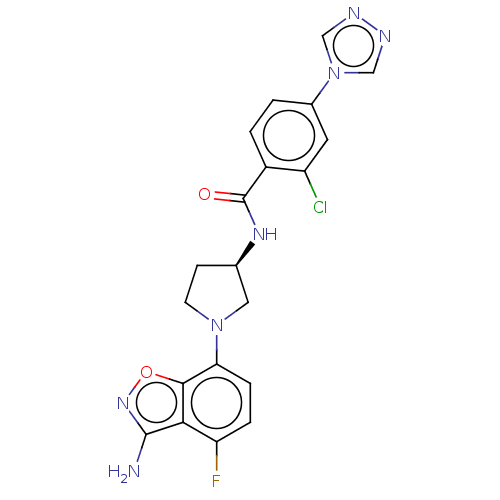
(CHEMBL4098387 | US9969724, Example 13)Show SMILES Nc1noc2c(ccc(F)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H17ClFN7O2/c21-14-7-12(29-9-24-25-10-29)1-2-13(14)20(30)26-11-5-6-28(8-11)16-4-3-15(22)17-18(16)31-27-19(17)23/h1-4,7,9-11H,5-6,8H2,(H2,23,27)(H,26,30)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240492
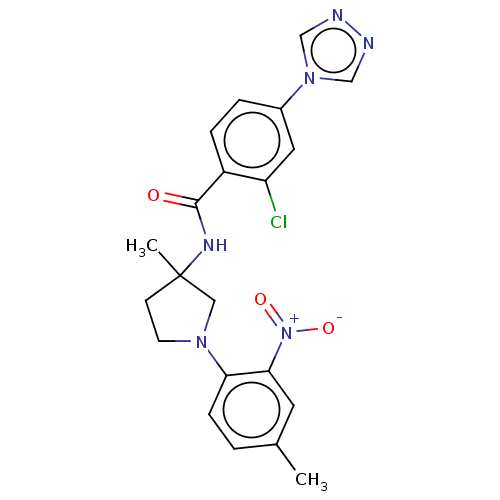
(CHEMBL4071351)Show SMILES Cc1ccc(N2CCC(C)(C2)NC(=O)c2ccc(cc2Cl)-n2cnnc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H21ClN6O3/c1-14-3-6-18(19(9-14)28(30)31)26-8-7-21(2,11-26)25-20(29)16-5-4-15(10-17(16)22)27-12-23-24-13-27/h3-6,9-10,12-13H,7-8,11H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50240490
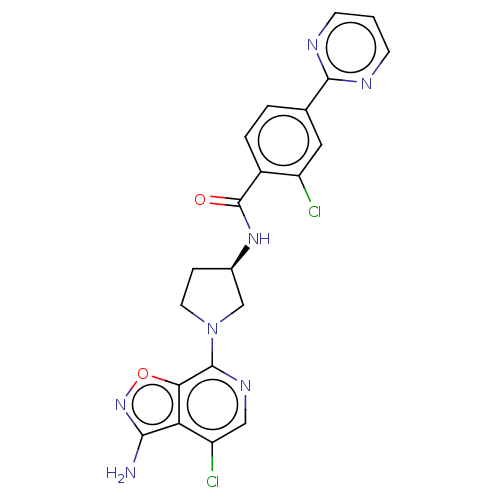
(CHEMBL4103170)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-c1ncccn1 |r| Show InChI InChI=1S/C21H17Cl2N7O2/c22-14-8-11(19-25-5-1-6-26-19)2-3-13(14)21(31)28-12-4-7-30(10-12)20-17-16(15(23)9-27-20)18(24)29-32-17/h1-3,5-6,8-9,12H,4,7,10H2,(H2,24,29)(H,28,31)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using SPECTROFLUOR F9a as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Whole-cell voltage clamp electrophysiology method |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in liver microsomes (unknown origin) assessed as formation of acetaminophen from phenacetin in presence of NADPH by LC-MS/MS ana... |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in liver microsomes (unknown origin) assessed as formation of 4-hydroxydiclofenac from diclofenac in presence of NADPH by LC-MS/... |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in liver microsomes (unknown origin) assessed as formation of 4-hydroxymephenytoin from S-mephenytoin in presence of NADPH by L... |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in liver microsomes (unknown origin) assessed as formation of 1-hydroxybufuralol from bufuralol in presence of NADPH by LC-MS/MS... |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in liver microsomes (unknown origin) assessed as formation of 1-hydroxymidazolam from midazolam in presence of NADPH by LC-MS/MS... |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240473

(CHEMBL4095415)Show SMILES Cn1cc(ccc1=O)-c1ccc(C(=O)N[C@@H]2CCN(C2)c2ncc(Cl)c3c(N)noc23)c(Cl)c1 |r| Show InChI InChI=1S/C23H20Cl2N6O3/c1-30-10-13(3-5-18(30)32)12-2-4-15(16(24)8-12)23(33)28-14-6-7-31(11-14)22-20-19(17(25)9-27-22)21(26)29-34-20/h2-5,8-10,14H,6-7,11H2,1H3,(H2,26,29)(H,28,33)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240483

(CHEMBL4099310 | US9969724, Example 12)Show SMILES Cc1cc(N2CC[C@H](C2)NC(=O)c2ccc(cc2Cl)-n2cnnc2)c2onc(N)c2c1Cl |r| Show InChI InChI=1S/C21H19Cl2N7O2/c1-11-6-16(19-17(18(11)23)20(24)28-32-19)29-5-4-12(8-29)27-21(31)14-3-2-13(7-15(14)22)30-9-25-26-10-30/h2-3,6-7,9-10,12H,4-5,8H2,1H3,(H2,24,28)(H,27,31)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.5 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240487

(CHEMBL4066380 | US9969724, Example 32)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnc2ncccc12 |r| Show InChI InChI=1S/C23H18Cl2N8O2/c24-15-8-13(33-11-29-21-17(33)2-1-6-27-21)3-4-14(15)23(34)30-12-5-7-32(10-12)22-19-18(16(25)9-28-22)20(26)31-35-19/h1-4,6,8-9,11-12H,5,7,10H2,(H2,26,31)(H,30,34)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240477

(CHEMBL4074983 | US9969724, Example 44)Show SMILES Cn1cc(cnc1=O)-c1ccc(C(=O)N[C@@H]2CCN(C2)c2ncc(Cl)c3c(N)noc23)c(Cl)c1 |r| Show InChI InChI=1S/C22H19Cl2N7O3/c1-30-9-12(7-27-22(30)33)11-2-3-14(15(23)6-11)21(32)28-13-4-5-31(10-13)20-18-17(16(24)8-26-20)19(25)29-34-18/h2-3,6-9,13H,4-5,10H2,1H3,(H2,25,29)(H,28,32)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240479

(CHEMBL4072364 | US9969724, Example 14)Show SMILES Nc1noc2c(cc(Cl)c(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H16Cl3N7O2/c21-13-5-11(30-8-25-26-9-30)1-2-12(13)20(31)27-10-3-4-29(7-10)15-6-14(22)17(23)16-18(15)32-28-19(16)24/h1-2,5-6,8-10H,3-4,7H2,(H2,24,28)(H,27,31)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240475

(CHEMBL4080361 | US9969724, Example 9)Show SMILES Nc1noc2c(ccc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H17Cl2N7O2/c21-14-3-4-16(18-17(14)19(23)27-31-18)28-6-5-11(8-28)26-20(30)13-2-1-12(7-15(13)22)29-9-24-25-10-29/h1-4,7,9-11H,5-6,8H2,(H2,23,27)(H,26,30)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240469

(CHEMBL4096341)Show SMILES [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| Show InChI InChI=1S/C22H19Cl2N7O2/c1-11-26-10-31(29-11)13-3-4-14(16(23)6-13)22(32)28-19-12-2-5-18(19)30(9-12)21-20-15(7-27-33-20)17(24)8-25-21/h3-4,6-8,10,12,18-19H,2,5,9H2,1H3,(H,28,32)/t12-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240474

(CHEMBL4082857)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C19H16Cl2N8O2/c20-13-5-11(29-8-24-25-9-29)1-2-12(13)19(30)26-10-3-4-28(7-10)18-16-15(14(21)6-23-18)17(22)27-31-16/h1-2,5-6,8-10H,3-4,7H2,(H2,22,27)(H,26,30)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240476

(CHEMBL4087720 | US9969724, Example 29)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnc2ccccc12 |r| Show InChI InChI=1S/C24H19Cl2N7O2/c25-16-9-14(33-12-29-18-3-1-2-4-19(18)33)5-6-15(16)24(34)30-13-7-8-32(11-13)23-21-20(17(26)10-28-23)22(27)31-35-21/h1-6,9-10,12-13H,7-8,11H2,(H2,27,31)(H,30,34)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240492

(CHEMBL4071351)Show SMILES Cc1ccc(N2CCC(C)(C2)NC(=O)c2ccc(cc2Cl)-n2cnnc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H21ClN6O3/c1-14-3-6-18(19(9-14)28(30)31)26-8-7-21(2,11-26)25-20(29)16-5-4-15(10-17(16)22)27-12-23-24-13-27/h3-6,9-10,12-13H,7-8,11H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240491

(CHEMBL4065316 | US9969724, Example 11)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@H]2CCN(C2)c2ncc(Cl)c3c(N)noc23)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl2N8O2/c1-10-25-9-30(27-10)12-2-3-13(14(21)6-12)20(31)26-11-4-5-29(8-11)19-17-16(15(22)7-24-19)18(23)28-32-17/h2-3,6-7,9,11H,4-5,8H2,1H3,(H2,23,28)(H,26,31)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240490

(CHEMBL4103170)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-c1ncccn1 |r| Show InChI InChI=1S/C21H17Cl2N7O2/c22-14-8-11(19-25-5-1-6-26-19)2-3-13(14)21(31)28-12-4-7-30(10-12)20-17-16(15(23)9-27-20)18(24)29-32-17/h1-3,5-6,8-9,12H,4,7,10H2,(H2,24,29)(H,28,31)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240489

(CHEMBL4101793 | US9969724, Example 20)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-c1cncnc1 |r| Show InChI InChI=1S/C21H17Cl2N7O2/c22-15-5-11(12-6-25-10-26-7-12)1-2-14(15)21(31)28-13-3-4-30(9-13)20-18-17(16(23)8-27-20)19(24)29-32-18/h1-2,5-8,10,13H,3-4,9H2,(H2,24,29)(H,28,31)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240488

(CHEMBL4060976 | US9969724, Example 5)Show SMILES Nc1noc2c(nccc12)N1CCC(C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 Show InChI InChI=1S/C19H17ClN8O2/c20-15-7-12(28-9-23-24-10-28)1-2-13(15)19(29)25-11-4-6-27(8-11)18-16-14(3-5-22-18)17(21)26-30-16/h1-3,5,7,9-11H,4,6,8H2,(H2,21,26)(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240486

(CHEMBL4098387 | US9969724, Example 13)Show SMILES Nc1noc2c(ccc(F)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H17ClFN7O2/c21-14-7-12(29-9-24-25-10-29)1-2-13(14)20(30)26-11-5-6-28(8-11)16-4-3-15(22)17-18(16)31-27-19(17)23/h1-4,7,9-11H,5-6,8H2,(H2,23,27)(H,26,30)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240472

(CHEMBL4090664)Show SMILES Nc1noc2c(cc(Cl)cc12)N1CCC(C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 Show InChI InChI=1S/C20H17Cl2N7O2/c21-11-5-15-18(31-27-19(15)23)17(6-11)28-4-3-12(8-28)26-20(30)14-2-1-13(7-16(14)22)29-9-24-25-10-29/h1-2,5-7,9-10,12H,3-4,8H2,(H2,23,27)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240484

(CHEMBL4085322 | US9969724, Example 1)Show SMILES Nc1noc2c(cccc12)N1CCC(C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 Show InChI InChI=1S/C20H18ClN7O2/c21-16-8-13(28-10-23-24-11-28)4-5-14(16)20(29)25-12-6-7-27(9-12)17-3-1-2-15-18(17)30-26-19(15)22/h1-5,8,10-12H,6-7,9H2,(H2,22,26)(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240482

(CHEMBL4069648)Show SMILES Cc1cc(N2CCC(C2)NC(=O)c2ccc(cc2Cl)-n2cnnc2)c2onc(N)c2c1 Show InChI InChI=1S/C21H20ClN7O2/c1-12-6-16-19(31-27-20(16)23)18(7-12)28-5-4-13(9-28)26-21(30)15-3-2-14(8-17(15)22)29-10-24-25-11-29/h2-3,6-8,10-11,13H,4-5,9H2,1H3,(H2,23,27)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240478

(CHEMBL4083789)Show SMILES Cc1cc2c(N)noc2c(n1)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1 |r| Show InChI InChI=1S/C20H19ClN8O2/c1-11-6-15-17(31-27-18(15)22)19(25-11)28-5-4-12(8-28)26-20(30)14-3-2-13(7-16(14)21)29-9-23-24-10-29/h2-3,6-7,9-10,12H,4-5,8H2,1H3,(H2,22,27)(H,26,30)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240493

(CHEMBL4085239 | US9969724, Example 28)Show SMILES Nc1noc2c(ncc(Cl)c12)N1CC[C@H](C1)NC(=O)c1ccc(cc1Cl)-n1cnnc1C(F)F |r| Show InChI InChI=1S/C20H16Cl2F2N8O2/c21-12-5-10(32-8-27-29-19(32)16(23)24)1-2-11(12)20(33)28-9-3-4-31(7-9)18-15-14(13(22)6-26-18)17(25)30-34-15/h1-2,5-6,8-9,16H,3-4,7H2,(H2,25,30)(H,28,33)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50240471

(CHEMBL4069082 | US9969724, Example 45)Show SMILES Cn1ncn(-c2ccc(C(=O)N[C@@H]3CCN(C3)c3ncc(Cl)c4c(N)noc34)c(Cl)c2)c1=O |r| Show InChI InChI=1S/C20H18Cl2N8O3/c1-28-20(32)30(9-25-28)11-2-3-12(13(21)6-11)19(31)26-10-4-5-29(8-10)18-16-15(14(22)7-24-18)17(23)27-33-16/h2-3,6-7,9-10H,4-5,8H2,1H3,(H2,23,27)(H,26,31)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mochida Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a |
Bioorg Med Chem Lett 27: 2622-2628 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.002
BindingDB Entry DOI: 10.7270/Q28054RH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data