 Found 308 hits with Last Name = 'hingorani' and Initial = 'gp'
Found 308 hits with Last Name = 'hingorani' and Initial = 'gp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
GTPase KRas
(Homo sapiens (Human)) | BDBM50539763
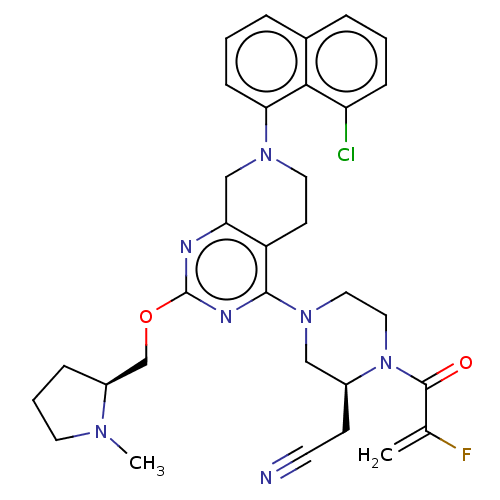
(Adagrasib | Mrtx-849 | Mrtx849)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C(F)=C)c1cccc2cccc(Cl)c12 Show InChI InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant KRAS G12C mutant (unknown origin) assessed as rate of inactivation by LC-MS analysis |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539762

(CHEMBL4632935)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C=C)c1cccc2cccc(C)c12 |r| Show InChI InChI=1S/C33H39N7O2/c1-4-30(41)40-19-18-39(20-25(40)13-15-34)32-27-14-17-38(29-12-6-10-24-9-5-8-23(2)31(24)29)21-28(27)35-33(36-32)42-22-26-11-7-16-37(26)3/h4-6,8-10,12,25-26H,1,7,11,13-14,16-22H2,2-3H3/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539761

(CHEMBL4648852)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C=C)c1cccc2cccc(Cl)c12 |r| Show InChI InChI=1S/C32H36ClN7O2/c1-3-29(41)40-18-17-39(19-23(40)12-14-34)31-25-13-16-38(28-11-5-8-22-7-4-10-26(33)30(22)28)20-27(25)35-32(36-31)42-21-24-9-6-15-37(24)2/h3-5,7-8,10-11,23-24H,1,6,9,12-13,15-21H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539765
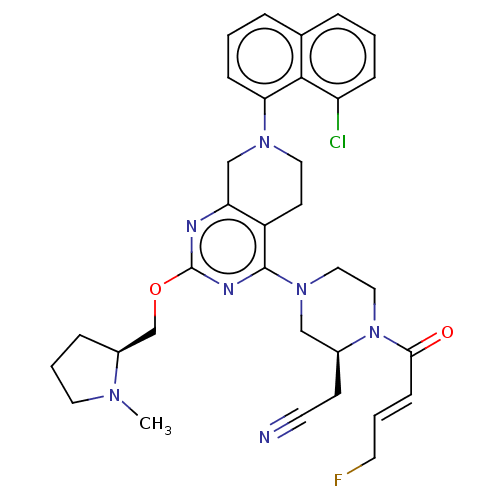
(CHEMBL4640636)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)\C=C\CF)c1cccc2cccc(Cl)c12 |r| Show InChI InChI=1S/C33H37ClFN7O2/c1-39-16-5-8-25(39)22-44-33-37-28-21-40(29-10-3-7-23-6-2-9-27(34)31(23)29)17-13-26(28)32(38-33)41-18-19-42(24(20-41)12-15-36)30(43)11-4-14-35/h2-4,6-7,9-11,24-25H,5,8,12-14,16-22H2,1H3/b11-4+/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738
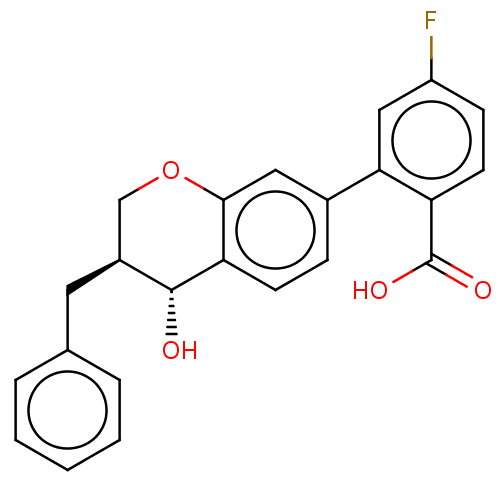
(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215740

(CHEMBL48906)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C29H23FO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215740

(CHEMBL48906)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C29H23FO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539760
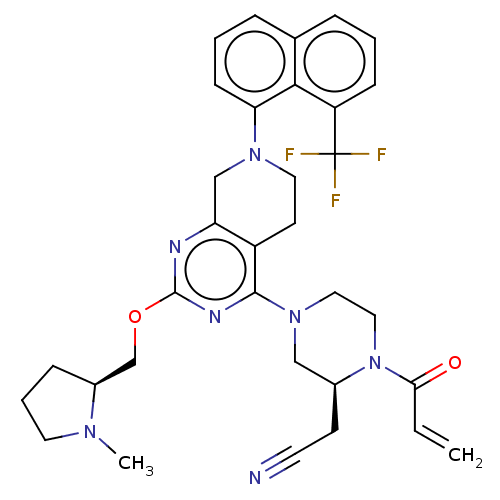
(CHEMBL4636611)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C=C)c1cccc2cccc(c12)C(F)(F)F |r| Show InChI InChI=1S/C33H36F3N7O2/c1-3-29(44)43-18-17-42(19-23(43)12-14-37)31-25-13-16-41(20-27(25)38-32(39-31)45-21-24-9-6-15-40(24)2)28-11-5-8-22-7-4-10-26(30(22)28)33(34,35)36/h3-5,7-8,10-11,23-24H,1,6,9,12-13,15-21H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539763

(Adagrasib | Mrtx-849 | Mrtx849)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C(F)=C)c1cccc2cccc(Cl)c12 Show InChI InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human MIAPaCa2 cells assessed as reduction in ERK phosphorylation incubated for 24 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215855
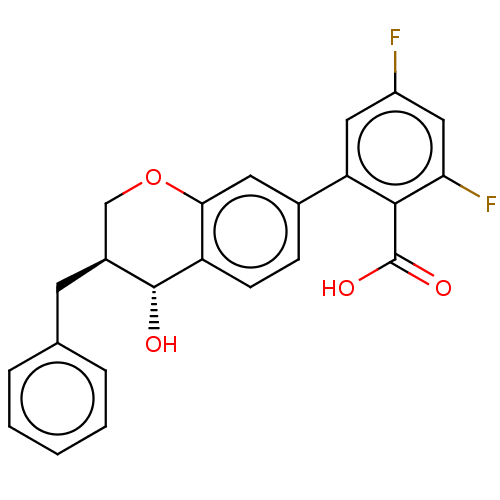
(CHEMBL51467)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)cc(F)c1C(O)=O Show InChI InChI=1S/C23H18F2O4/c24-16-10-18(21(23(27)28)19(25)11-16)14-6-7-17-20(9-14)29-12-15(22(17)26)8-13-4-2-1-3-5-13/h1-7,9-11,15,22,26H,8,12H2,(H,27,28)/t15-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50037218
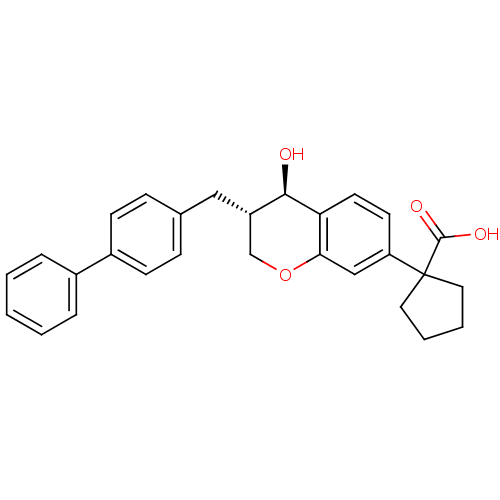
(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced CD11b up-regulation on isolated human neutrophils in whole blood |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539759
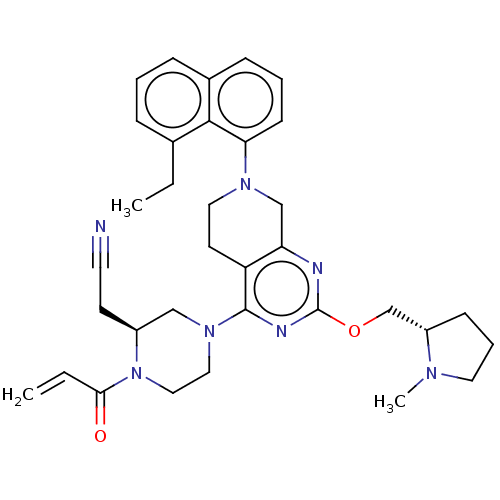
(CHEMBL4646899)Show SMILES CCc1cccc2cccc(N3CCc4c(C3)nc(OC[C@@H]3CCCN3C)nc4N3CCN([C@@H](CC#N)C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C34H41N7O2/c1-4-24-9-6-10-25-11-7-13-30(32(24)25)39-18-15-28-29(22-39)36-34(43-23-27-12-8-17-38(27)3)37-33(28)40-19-20-41(31(42)5-2)26(21-40)14-16-35/h5-7,9-11,13,26-27H,2,4,8,12,14-15,17-23H2,1,3H3/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215853

(CHEMBL417364)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C30H23F3O4/c31-30(32,33)23-11-13-24(29(35)36)26(16-23)21-10-12-25-27(15-21)37-17-22(28(25)34)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,34H,14,17H2,(H,35,36)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215853

(CHEMBL417364)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C30H23F3O4/c31-30(32,33)23-11-13-24(29(35)36)26(16-23)21-10-12-25-27(15-21)37-17-22(28(25)34)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,34H,14,17H2,(H,35,36)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit [3H]LTB4 binding to LTB4 receptors on guinea pig spleen membranes |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215854

(CHEMBL301829)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(ccc1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)17-7-9-18(23(29)30)20(12-17)15-6-8-19-21(11-15)31-13-16(22(19)28)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,28H,10,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50037218

(1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)C1(CCCC1)C(O)=O Show InChI InChI=1S/C28H28O4/c29-26-22(16-19-8-10-21(11-9-19)20-6-2-1-3-7-20)18-32-25-17-23(12-13-24(25)26)28(27(30)31)14-4-5-15-28/h1-3,6-13,17,22,26,29H,4-5,14-16,18H2,(H,30,31)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539766
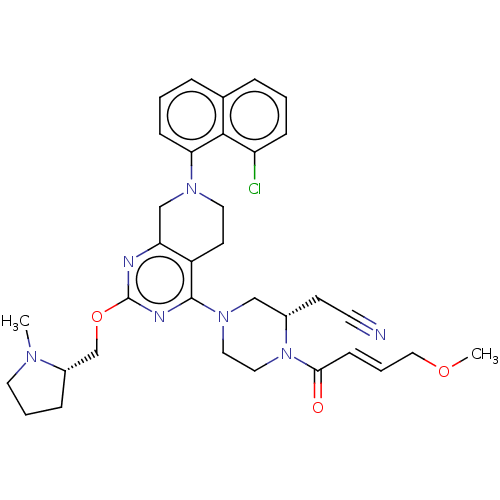
(CHEMBL4645376)Show SMILES COC\C=C\C(=O)N1CCN(C[C@@H]1CC#N)c1nc(OC[C@@H]2CCCN2C)nc2CN(CCc12)c1cccc2cccc(Cl)c12 |r| Show InChI InChI=1S/C34H40ClN7O3/c1-39-16-5-9-26(39)23-45-34-37-29-22-40(30-11-4-8-24-7-3-10-28(35)32(24)30)17-14-27(29)33(38-34)41-18-19-42(25(21-41)13-15-36)31(43)12-6-20-44-2/h3-4,6-8,10-12,25-26H,5,9,13-14,16-23H2,1-2H3/b12-6+/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215739

(CHEMBL52374)Show SMILES OCc1ccc(cc1-c1ccc2[C@H](O)[C@@H](Cc3ccccc3)COc2c1)C(F)(F)F Show InChI InChI=1S/C24H21F3O3/c25-24(26,27)19-8-6-17(13-28)21(12-19)16-7-9-20-22(11-16)30-14-18(23(20)29)10-15-4-2-1-3-5-15/h1-9,11-12,18,23,28-29H,10,13-14H2/t18-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539754
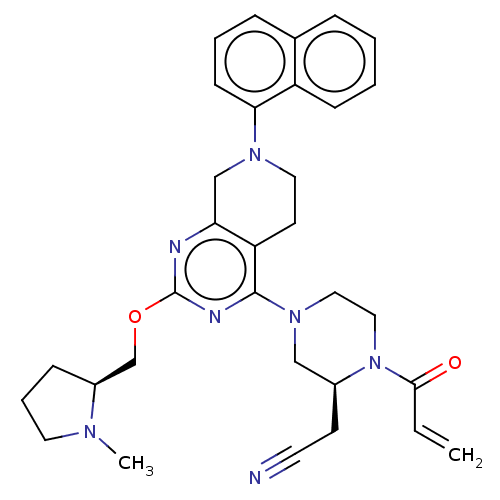
(CHEMBL4648671)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C=C)c1cccc2ccccc12 |r| Show InChI InChI=1S/C32H37N7O2/c1-3-30(40)39-19-18-38(20-24(39)13-15-33)31-27-14-17-37(29-12-6-9-23-8-4-5-11-26(23)29)21-28(27)34-32(35-31)41-22-25-10-7-16-36(25)2/h3-6,8-9,11-12,24-25H,1,7,10,13-14,16-22H2,2H3/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215738

(CHEMBL52675)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215741
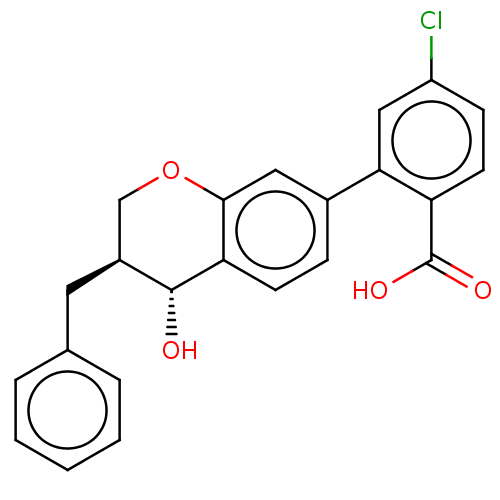
(CHEMBL418264)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C23H19ClO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539763

(Adagrasib | Mrtx-849 | Mrtx849)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C(F)=C)c1cccc2cccc(Cl)c12 Show InChI InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539753
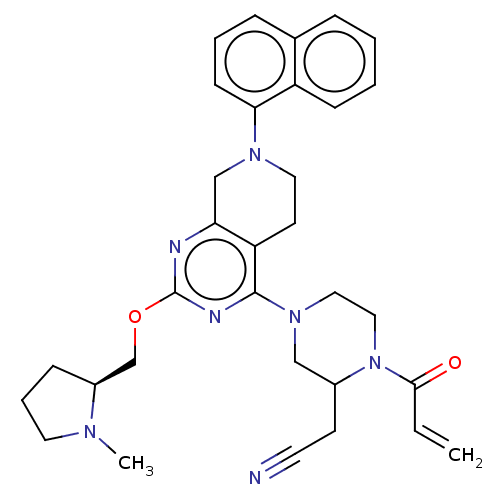
(CHEMBL4648056)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN(C(CC#N)C1)C(=O)C=C)c1cccc2ccccc12 |r| Show InChI InChI=1S/C32H37N7O2/c1-3-30(40)39-19-18-38(20-24(39)13-15-33)31-27-14-17-37(29-12-6-9-23-8-4-5-11-26(23)29)21-28(27)34-32(35-31)41-22-25-10-7-16-36(25)2/h3-6,8-9,11-12,24-25H,1,7,10,13-14,16-22H2,2H3/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215868

(CHEMBL51535)Show SMILES NS(=O)(=O)c1ccc(F)cc1-c1ccc2[C@H](O)[C@@H](Cc3ccccc3)COc2c1 Show InChI InChI=1S/C22H20FNO4S/c23-17-7-9-21(29(24,26)27)19(12-17)15-6-8-18-20(11-15)28-13-16(22(18)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H2,24,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215859
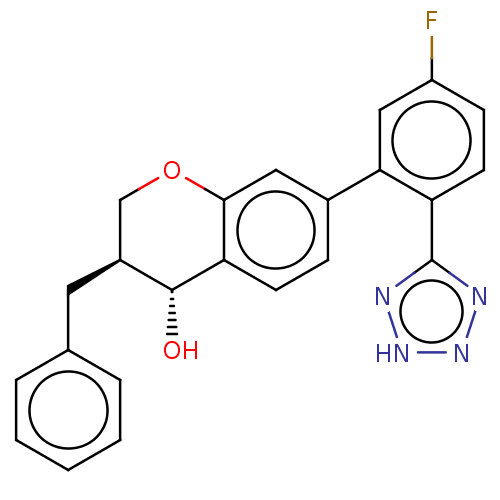
(CHEMBL51775)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1-c1nn[nH]n1 Show InChI InChI=1S/C23H19FN4O2/c24-17-7-9-18(23-25-27-28-26-23)20(12-17)15-6-8-19-21(11-15)30-13-16(22(19)29)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,29H,10,13H2,(H,25,26,27,28)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215861

(CHEMBL298724)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(F)c1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-19-8-4-7-17(21(19)23(26)27)15-9-10-18-20(12-15)28-13-16(22(18)25)11-14-5-2-1-3-6-14/h1-10,12,16,22,25H,11,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539757
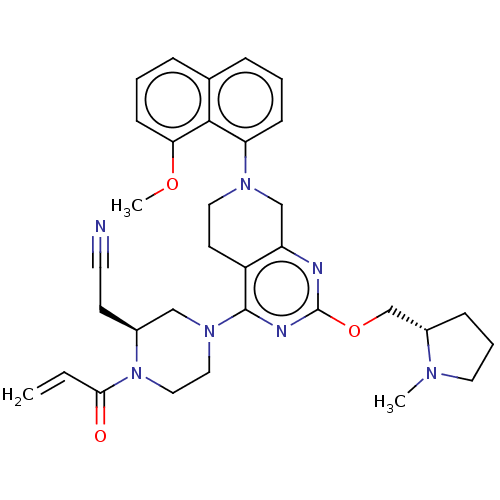
(CHEMBL4648124)Show SMILES COc1cccc2cccc(N3CCc4c(C3)nc(OC[C@@H]3CCCN3C)nc4N3CCN([C@@H](CC#N)C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C33H39N7O3/c1-4-30(41)40-19-18-39(20-24(40)13-15-34)32-26-14-17-38(28-11-5-8-23-9-6-12-29(42-3)31(23)28)21-27(26)35-33(36-32)43-22-25-10-7-16-37(25)2/h4-6,8-9,11-12,24-25H,1,7,10,13-14,16-22H2,2-3H3/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 3 hrs by in-cell western method |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215743
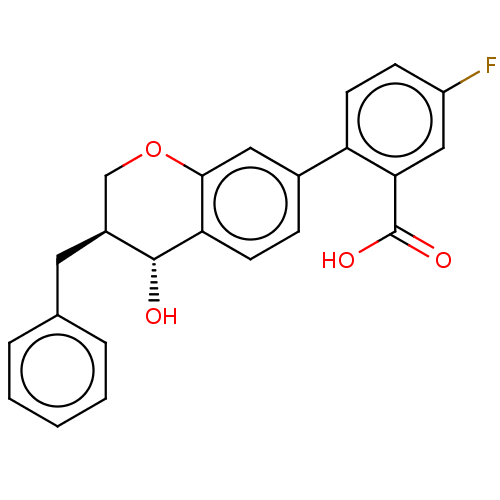
(CHEMBL51219)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1ccc(F)cc1C(O)=O Show InChI InChI=1S/C23H19FO4/c24-17-7-9-18(20(12-17)23(26)27)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215744
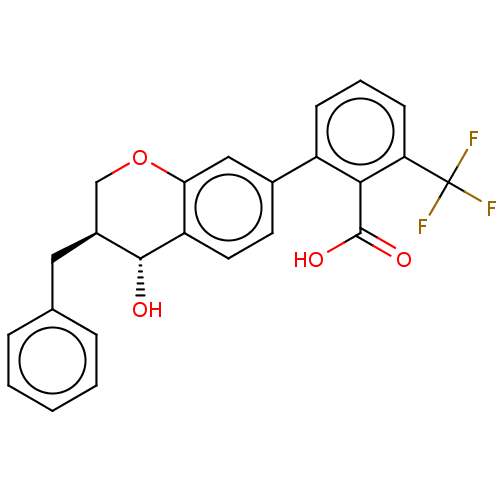
(CHEMBL299150)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cccc(c1C(O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3O4/c25-24(26,27)19-8-4-7-17(21(19)23(29)30)15-9-10-18-20(12-15)31-13-16(22(18)28)11-14-5-2-1-3-6-14/h1-10,12,16,22,28H,11,13H2,(H,29,30)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B/1 receptor
(RAT) | BDBM50045239
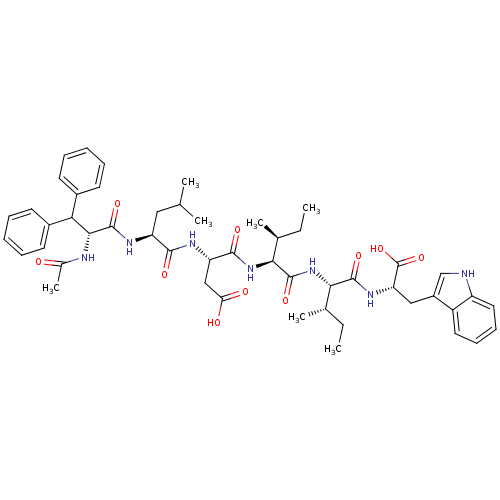
(Ac-D-Dip-Leu-Asp-Ile-Ile-Trp | Ac-D-Dip-Leu-Asp-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Endothelin receptor from rat heart ventricle |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215737

(CHEMBL50425)Show SMILES O[C@@H]1[C@@H](Cc2ccc(cc2)-c2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C29H23ClO4/c30-23-11-13-24(29(32)33)26(16-23)21-10-12-25-27(15-21)34-17-22(28(25)31)14-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h1-13,15-16,22,28,31H,14,17H2,(H,32,33)/t22-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215741

(CHEMBL418264)Show SMILES O[C@@H]1[C@@H](Cc2ccccc2)COc2cc(ccc12)-c1cc(Cl)ccc1C(O)=O Show InChI InChI=1S/C23H19ClO4/c24-17-7-9-18(23(26)27)20(12-17)15-6-8-19-21(11-15)28-13-16(22(19)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215742

(CHEMBL51492)Show SMILES O[C@@H]1[C@@H](CCc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C24H21FO4/c25-18-9-11-19(24(27)28)21(13-18)16-8-10-20-22(12-16)29-14-17(23(20)26)7-6-15-4-2-1-3-5-15/h1-5,8-13,17,23,26H,6-7,14H2,(H,27,28)/t17-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215742

(CHEMBL51492)Show SMILES O[C@@H]1[C@@H](CCc2ccccc2)COc2cc(ccc12)-c1cc(F)ccc1C(O)=O Show InChI InChI=1S/C24H21FO4/c25-18-9-11-19(24(27)28)21(13-18)16-8-10-20-22(12-16)29-14-17(23(20)26)7-6-15-4-2-1-3-5-15/h1-5,8-13,17,23,26H,6-7,14H2,(H,27,28)/t17-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50045240
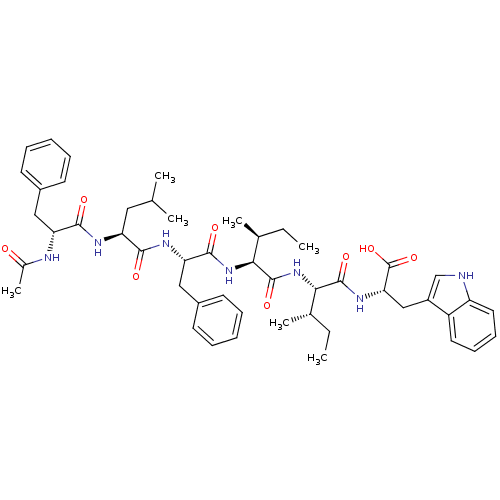
((S)-2-[(S)-2-((2S,5S)-2-{(S)-2-[(S)-2-((R)-2-Acety...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C49H65N7O8/c1-8-30(5)42(47(61)54-41(49(63)64)27-35-28-50-37-23-17-16-22-36(35)37)56-48(62)43(31(6)9-2)55-46(60)40(26-34-20-14-11-15-21-34)53-44(58)38(24-29(3)4)52-45(59)39(51-32(7)57)25-33-18-12-10-13-19-33/h10-23,28-31,38-43,50H,8-9,24-27H2,1-7H3,(H,51,57)(H,52,59)(H,53,58)(H,54,61)(H,55,60)(H,56,62)(H,63,64)/t30-,31-,38-,39+,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50215868

(CHEMBL51535)Show SMILES NS(=O)(=O)c1ccc(F)cc1-c1ccc2[C@H](O)[C@@H](Cc3ccccc3)COc2c1 Show InChI InChI=1S/C22H20FNO4S/c23-17-7-9-21(29(24,26)27)19(12-17)15-6-8-18-20(11-15)28-13-16(22(18)25)10-14-4-2-1-3-5-14/h1-9,11-12,16,22,25H,10,13H2,(H2,24,26,27)/t16-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. |
Bioorg Med Chem Lett 8: 1781-6 (1998)
BindingDB Entry DOI: 10.7270/Q2RR21F1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data