

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541792 (CHEMBL4635011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541786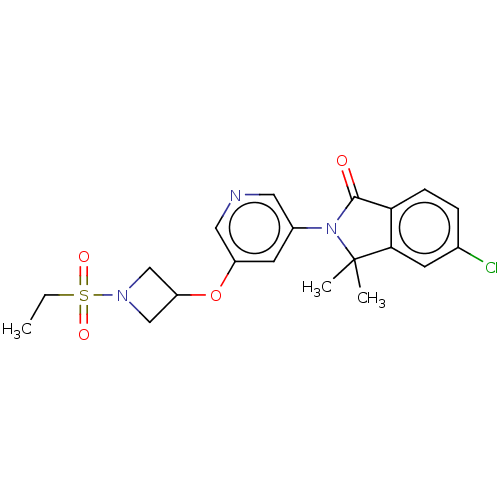 (CHEMBL4647543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in HEK293 cell assessed as reduction in 18-hyroxycorticosterone by LC/MS-MS method | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432632 (CHEMBL2347211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432614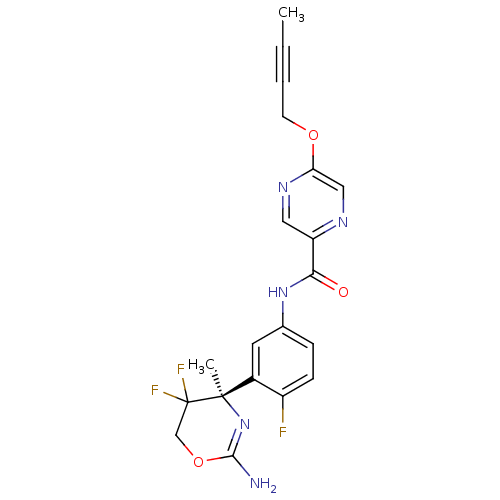 (CHEMBL2347198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432615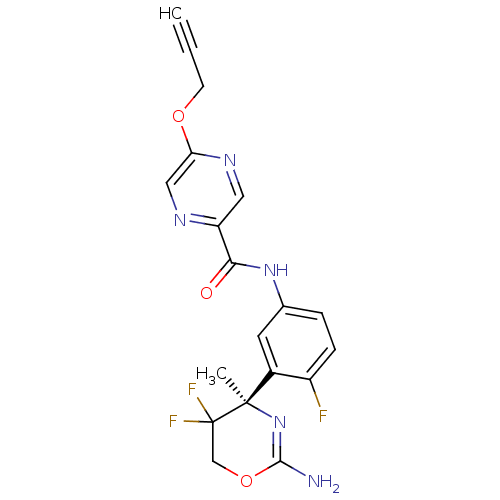 (CHEMBL2347197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432608 (CHEMBL2347204 | US8754075, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541786 (CHEMBL4647543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in HEK293 cell assessed as reduction in aldosterone by LC/MS-MS method | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM279728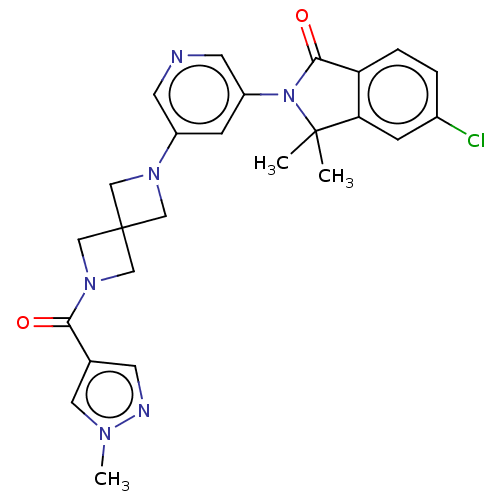 (5-Chloro-3,3-dimethyl-2-[5-[2-(1-methylpyrazole-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541785 (CHEMBL4635517) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432601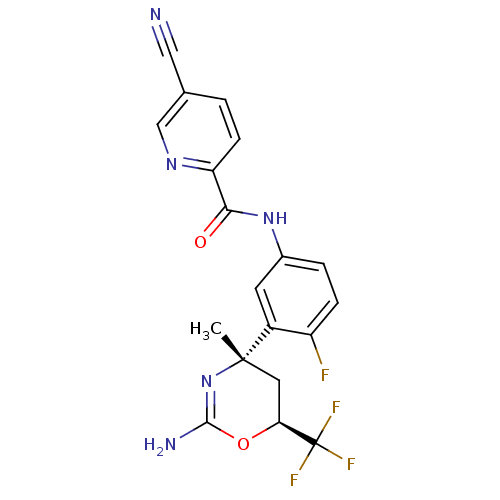 (CHEMBL2347208 | US9296734, 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541783 (CHEMBL4642895) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541791 (CHEMBL4642138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541764 (CHEMBL4645158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541786 (CHEMBL4647543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541789 (CHEMBL4646980) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432631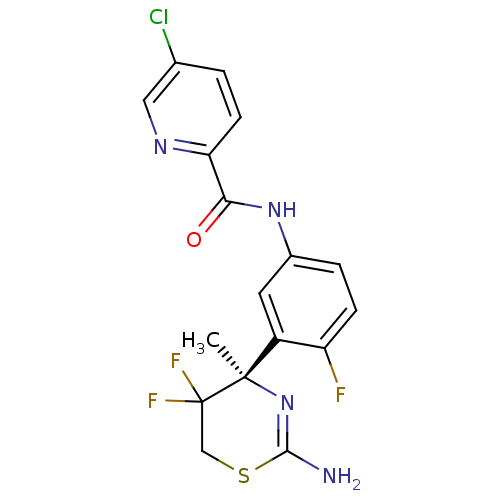 (CHEMBL2347212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541790 (CHEMBL4632945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541782 (CHEMBL4644311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432614 (CHEMBL2347198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541768 (CHEMBL4647877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541788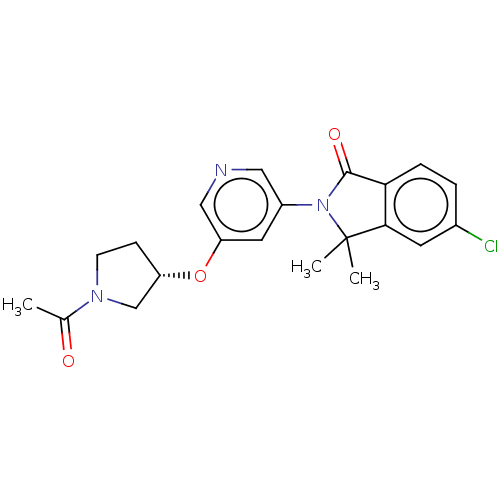 (CHEMBL4638889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541765 (CHEMBL4639314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541779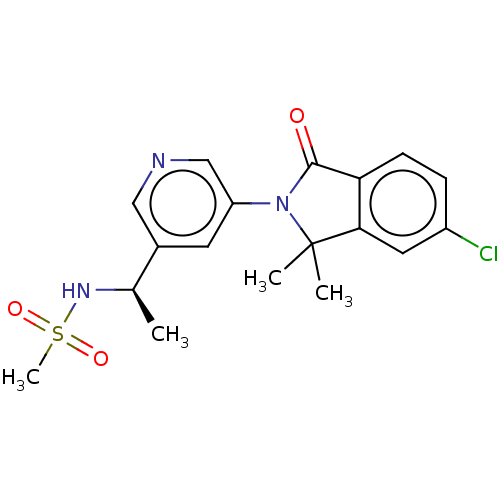 (CHEMBL4632486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541784 (CHEMBL4634550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541781 (CHEMBL4643528) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50432632 (CHEMBL2347211) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE2 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432606 (CHEMBL2347206 | US8999980, I-53) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432599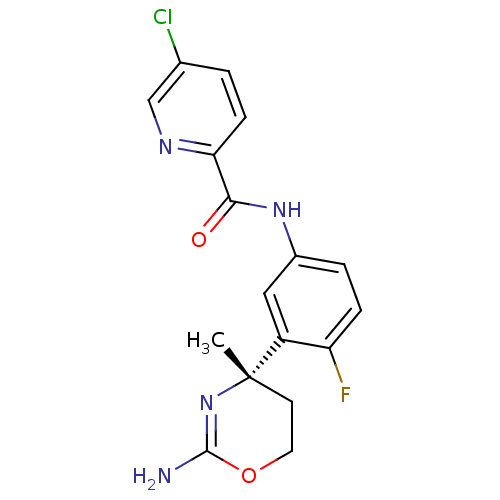 (CHEMBL2347209 | US8999980, I-8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50432620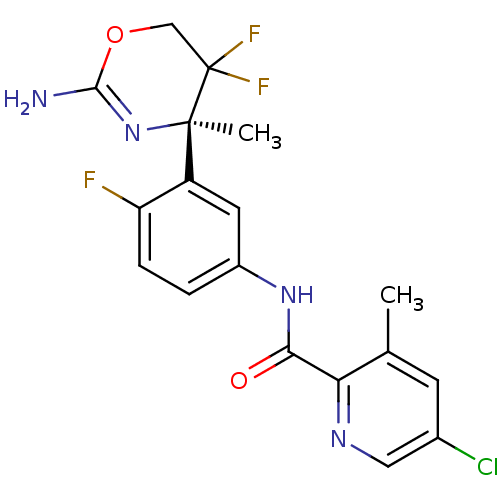 (CHEMBL2347192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE2 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50432631 (CHEMBL2347212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE2 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432602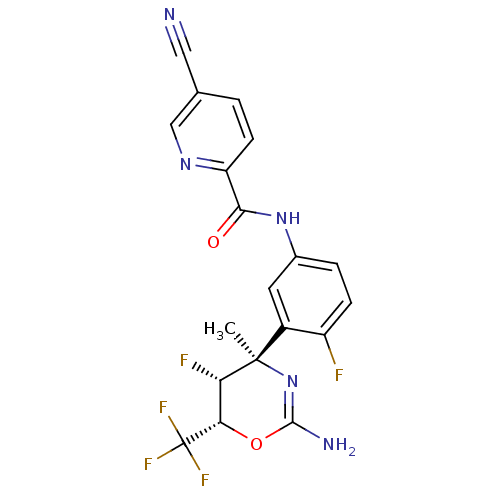 (CHEMBL2347188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541787 (CHEMBL4634463) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50432610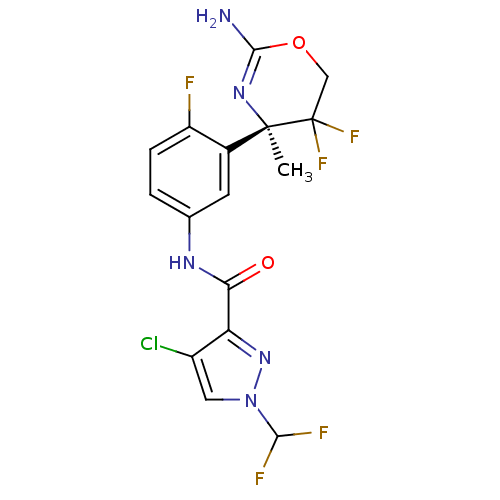 (CHEMBL2347202) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE2 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432601 (CHEMBL2347208 | US9296734, 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50432619 (CHEMBL2347193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE2 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541770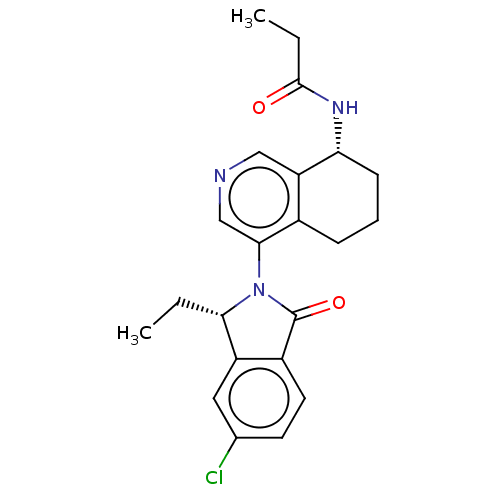 (CHEMBL4640951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432602 (CHEMBL2347188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432619 (CHEMBL2347193) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432615 (CHEMBL2347197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432603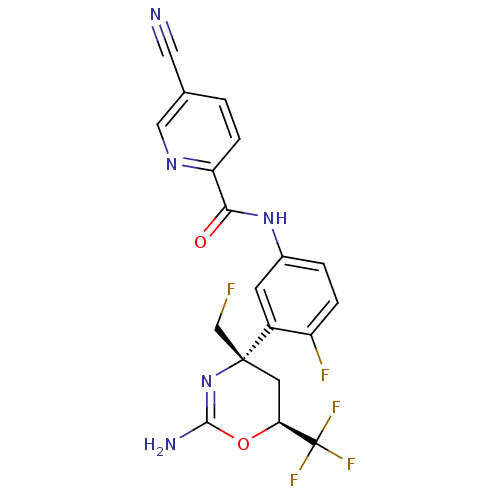 (CHEMBL2347187 | US9296734, 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432610 (CHEMBL2347202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432629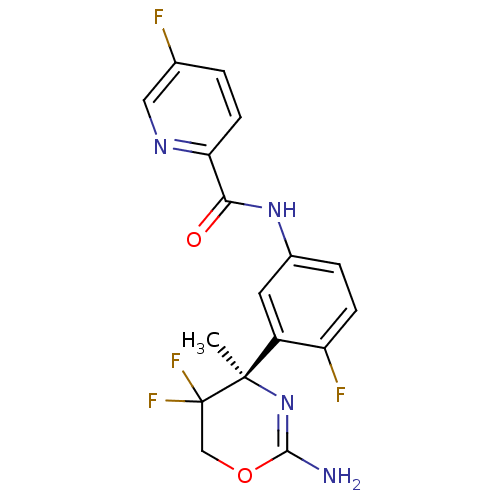 (CHEMBL2347214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432609 (CHEMBL2347203 | US8999980, I-6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM279736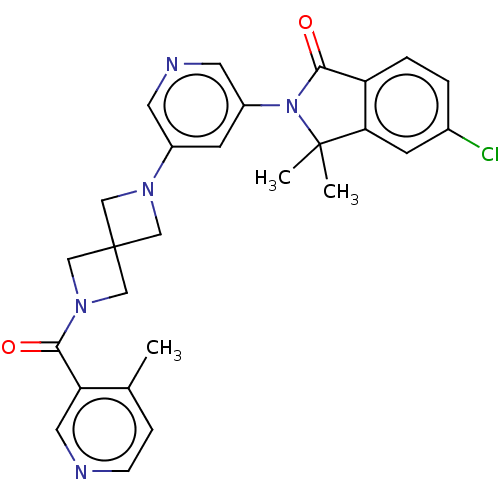 (US10035804, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432631 (CHEMBL2347212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50432598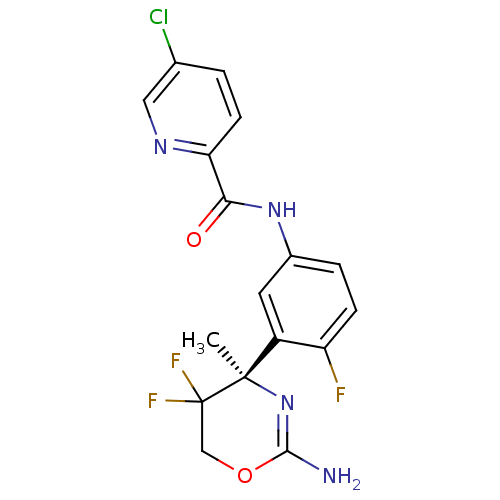 (CHEMBL2347210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE2 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541794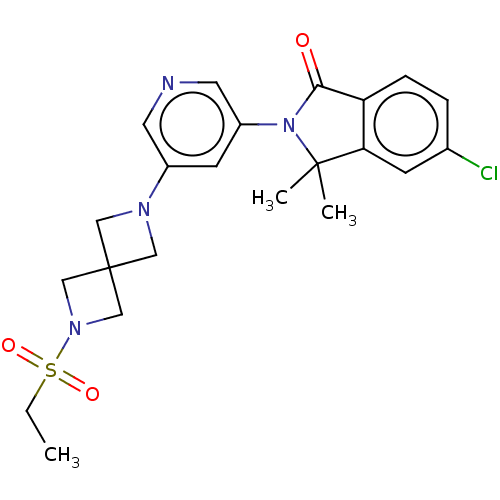 (CHEMBL4640361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432632 (CHEMBL2347211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432620 (CHEMBL2347192) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432620 (CHEMBL2347192) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 219 total ) | Next | Last >> |