 Found 505 hits with Last Name = 'hong' and Initial = 'sp'
Found 505 hits with Last Name = 'hong' and Initial = 'sp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353455

(CHEMBL1830049)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2N(CCc2c1)C1CNC1 Show InChI InChI=1S/C23H23N3O2/c27-23-13-21(28-16-17-4-2-1-3-5-17)9-11-26(23)19-6-7-22-18(12-19)8-10-25(22)20-14-24-15-20/h1-7,9,11-13,20,24H,8,10,14-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353456
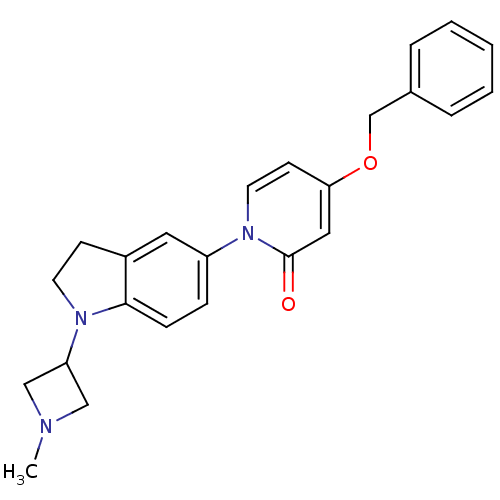
(CHEMBL1830050)Show SMILES CN1CC(C1)N1CCc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C24H25N3O2/c1-25-15-21(16-25)26-11-9-19-13-20(7-8-23(19)26)27-12-10-22(14-24(27)28)29-17-18-5-3-2-4-6-18/h2-8,10,12-14,21H,9,11,15-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353453
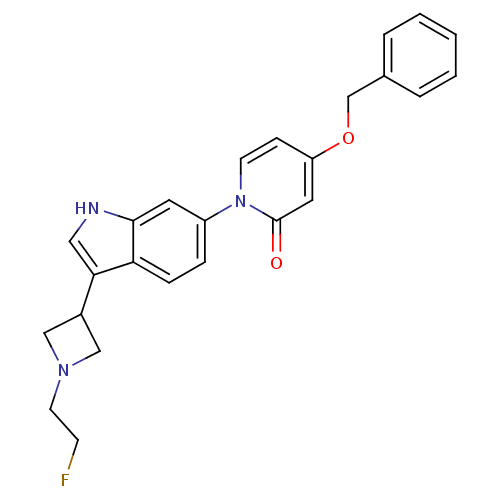
(CHEMBL1830047)Show SMILES FCCN1CC(C1)c1c[nH]c2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C25H24FN3O2/c26-9-11-28-15-19(16-28)23-14-27-24-12-20(6-7-22(23)24)29-10-8-21(13-25(29)30)31-17-18-4-2-1-3-5-18/h1-8,10,12-14,19,27H,9,11,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353458

(CHEMBL1830052)Show SMILES CN1CC(C1)N1CCc2cc(ccc12)-n1ccc(cc1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H22ClN3O/c1-25-14-21(15-25)26-10-9-18-12-20(6-7-22(18)26)27-11-8-17(13-23(27)28)16-2-4-19(24)5-3-16/h2-8,11-13,21H,9-10,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353457
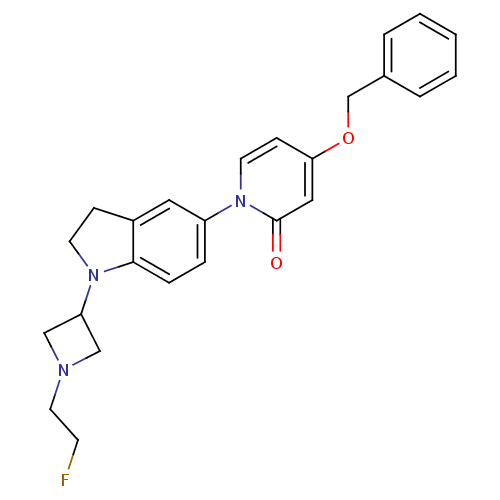
(CHEMBL1830051)Show SMILES FCCN1CC(C1)N1CCc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C25H26FN3O2/c26-10-13-27-16-22(17-27)28-11-8-20-14-21(6-7-24(20)28)29-12-9-23(15-25(29)30)31-18-19-4-2-1-3-5-19/h1-7,9,12,14-15,22H,8,10-11,13,16-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353452

(CHEMBL1830046)Show SMILES COCCN1CC(C1)c1c[nH]c2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C26H27N3O3/c1-31-12-11-28-16-20(17-28)24-15-27-25-13-21(7-8-23(24)25)29-10-9-22(14-26(29)30)32-18-19-5-3-2-4-6-19/h2-10,13-15,20,27H,11-12,16-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353459
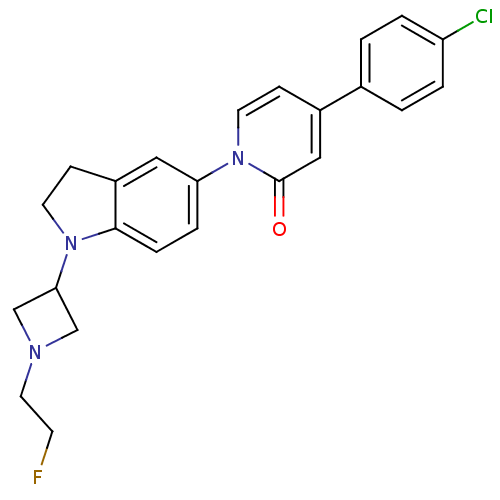
(CHEMBL1830053)Show SMILES FCCN1CC(C1)N1CCc2cc(ccc12)-n1ccc(cc1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H23ClFN3O/c25-20-3-1-17(2-4-20)18-7-11-29(24(30)14-18)21-5-6-23-19(13-21)8-10-28(23)22-15-27(16-22)12-9-26/h1-7,11,13-14,22H,8-10,12,15-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353449
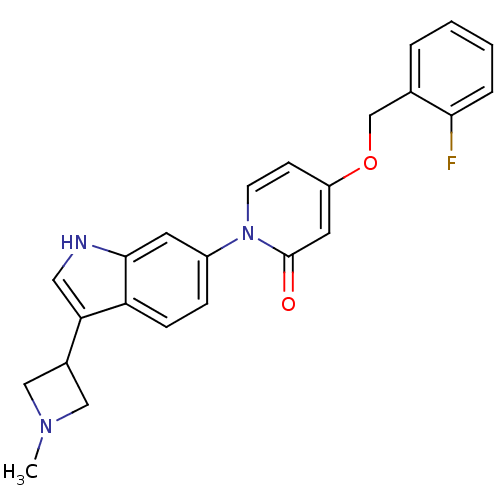
(CHEMBL1830043)Show SMILES CN1CC(C1)c1c[nH]c2cc(ccc12)-n1ccc(OCc2ccccc2F)cc1=O Show InChI InChI=1S/C24H22FN3O2/c1-27-13-17(14-27)21-12-26-23-10-18(6-7-20(21)23)28-9-8-19(11-24(28)29)30-15-16-4-2-3-5-22(16)25/h2-12,17,26H,13-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353450

(CHEMBL1830044)Show SMILES CN1CC(C1)c1c[nH]c2cc(ccc12)-n1ccc(cc1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H20ClN3O/c1-26-13-17(14-26)21-12-25-22-11-19(6-7-20(21)22)27-9-8-16(10-23(27)28)15-2-4-18(24)5-3-15/h2-12,17,25H,13-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353454
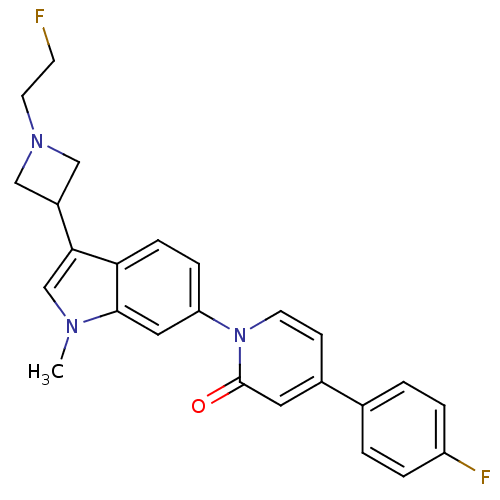
(CHEMBL1830048)Show SMILES Cn1cc(C2CN(CCF)C2)c2ccc(cc12)-n1ccc(cc1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C25H23F2N3O/c1-28-16-23(19-14-29(15-19)11-9-26)22-7-6-21(13-24(22)28)30-10-8-18(12-25(30)31)17-2-4-20(27)5-3-17/h2-8,10,12-13,16,19H,9,11,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353451
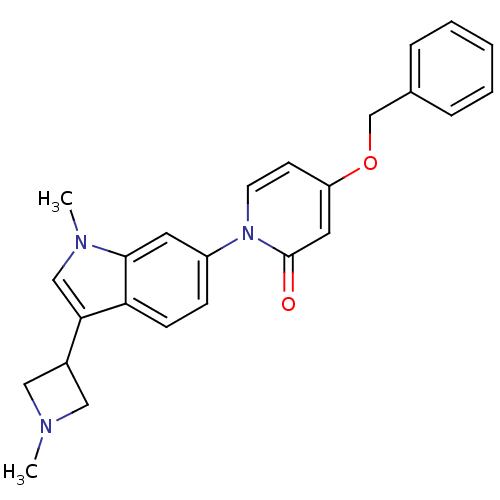
(CHEMBL1830045)Show SMILES CN1CC(C1)c1cn(C)c2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C25H25N3O2/c1-26-14-19(15-26)23-16-27(2)24-12-20(8-9-22(23)24)28-11-10-21(13-25(28)29)30-17-18-6-4-3-5-7-18/h3-13,16,19H,14-15,17H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50353448
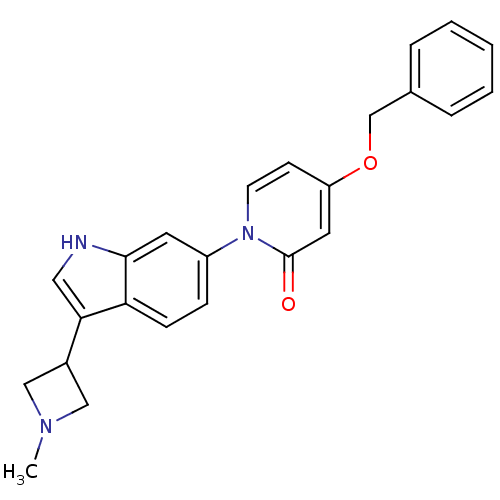
(CHEMBL1830042)Show SMILES CN1CC(C1)c1c[nH]c2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C24H23N3O2/c1-26-14-18(15-26)22-13-25-23-11-19(7-8-21(22)23)27-10-9-20(12-24(27)28)29-16-17-5-3-2-4-6-17/h2-13,18,25H,14-16H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-S36057 from rat MCH1 receptor |
Bioorg Med Chem Lett 21: 5310-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.020
BindingDB Entry DOI: 10.7270/Q2DJ5G0J |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50390028
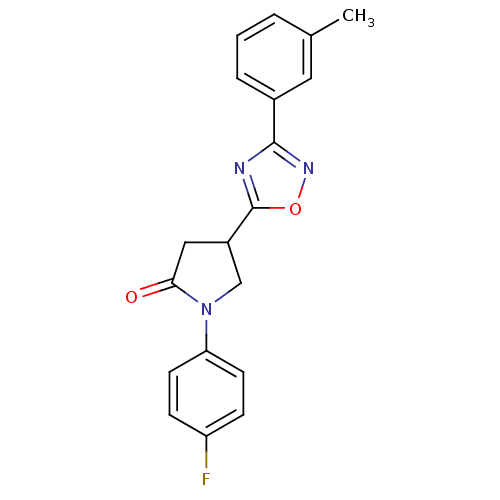
(CHEMBL2069398)Show SMILES Cc1cccc(c1)-c1noc(n1)C1CN(C(=O)C1)c1ccc(F)cc1 Show InChI InChI=1S/C19H16FN3O2/c1-12-3-2-4-13(9-12)18-21-19(25-22-18)14-10-17(24)23(11-14)16-7-5-15(20)6-8-16/h2-9,14H,10-11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAM from mGluR5 |
Bioorg Med Chem Lett 22: 5658-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.094
BindingDB Entry DOI: 10.7270/Q2348MF7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50390029

(CHEMBL2069397)Show SMILES Fc1ccc(cc1)N1CC(CC1=O)c1nc(no1)-c1cccc(Cl)c1 Show InChI InChI=1S/C18H13ClFN3O2/c19-13-3-1-2-11(8-13)17-21-18(25-22-17)12-9-16(24)23(10-12)15-6-4-14(20)5-7-15/h1-8,12H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAM from mGluR5 |
Bioorg Med Chem Lett 22: 5658-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.094
BindingDB Entry DOI: 10.7270/Q2348MF7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50390029

(CHEMBL2069397)Show SMILES Fc1ccc(cc1)N1CC(CC1=O)c1nc(no1)-c1cccc(Cl)c1 Show InChI InChI=1S/C18H13ClFN3O2/c19-13-3-1-2-11(8-13)17-21-18(25-22-17)12-9-16(24)23(10-12)15-6-4-14(20)5-7-15/h1-8,12H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAM from mGluR5 |
Bioorg Med Chem Lett 22: 5658-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.094
BindingDB Entry DOI: 10.7270/Q2348MF7 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112149
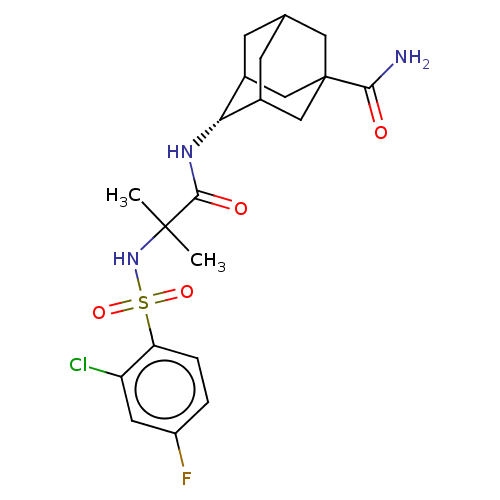
(CHEMBL3609877)Show SMILES CC(C)(NS(=O)(=O)c1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-14.11,.25,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27ClFN3O4S/c1-20(2,26-31(29,30)16-4-3-14(23)7-15(16)22)19(28)25-17-12-5-11-6-13(17)10-21(8-11,9-12)18(24)27/h3-4,7,11-13,17,26H,5-6,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112154
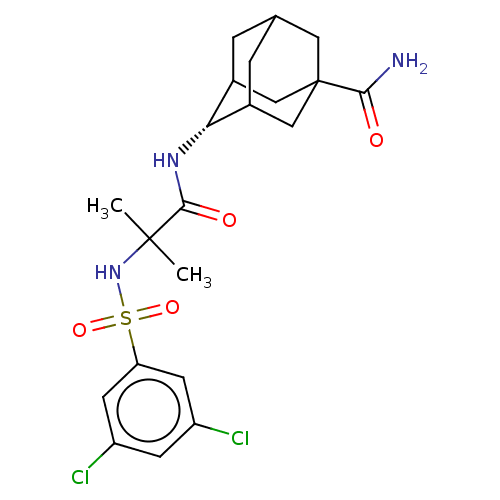
(CHEMBL3608403)Show SMILES CC(C)(NS(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27Cl2N3O4S/c1-20(2,26-31(29,30)16-6-14(22)5-15(23)7-16)19(28)25-17-12-3-11-4-13(17)10-21(8-11,9-12)18(24)27/h5-7,11-13,17,26H,3-4,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112151
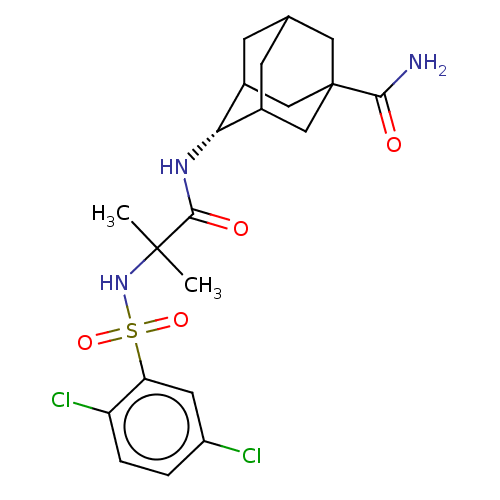
(CHEMBL3608400)Show SMILES CC(C)(NS(=O)(=O)c1cc(Cl)ccc1Cl)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27Cl2N3O4S/c1-20(2,26-31(29,30)16-7-14(22)3-4-15(16)23)19(28)25-17-12-5-11-6-13(17)10-21(8-11,9-12)18(24)27/h3-4,7,11-13,17,26H,5-6,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112152
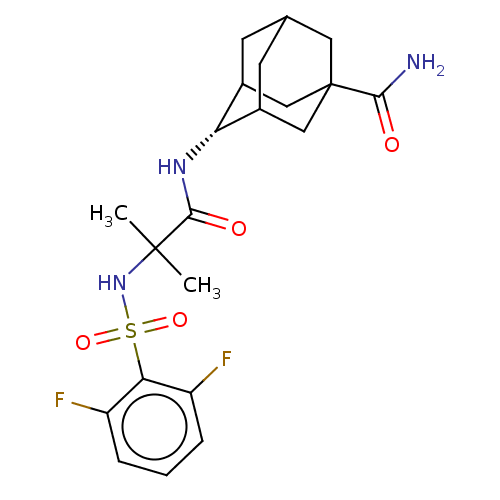
(CHEMBL3608401 | US9464044, 84)Show SMILES CC(C)(NS(=O)(=O)c1c(F)cccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-11.31,-2.31,;-11.14,-3.53,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27F2N3O4S/c1-20(2,26-31(29,30)17-14(22)4-3-5-15(17)23)19(28)25-16-12-6-11-7-13(16)10-21(8-11,9-12)18(24)27/h3-5,11-13,16,26H,6-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,16-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112133
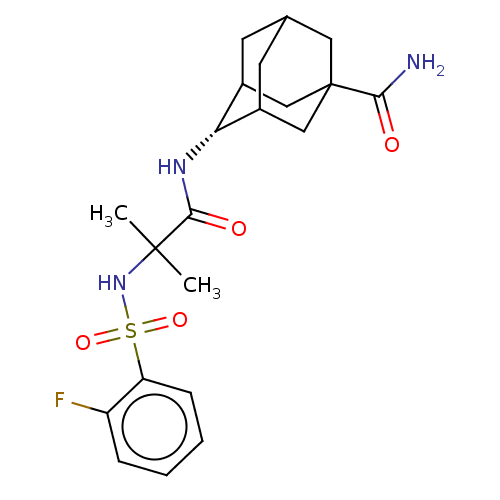
(CHEMBL3609861 | US9464044, 71)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:16:17:21:23.25.24,26:24:21:19.18.17,THB:27:24:21:19.18.17,27:24:21.20.19:17,25:20:17:23.24.26,25:24:21.20.19:17,26:18:21:23.25.24,(-5.85,-2.77,;-6.03,-1.55,;-7,-2.31,;-7.46,-.97,;-8.67,-1.93,;-8.49,-3.15,;-7.53,-2.38,;-10.1,-1.35,;-11.31,-2.31,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.17,;3.58,2.29,;3.79,.17,)| Show InChI InChI=1S/C21H28FN3O4S/c1-20(2,25-30(28,29)16-6-4-3-5-15(16)22)19(27)24-17-13-7-12-8-14(17)11-21(9-12,10-13)18(23)26/h3-6,12-14,17,25H,7-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112143
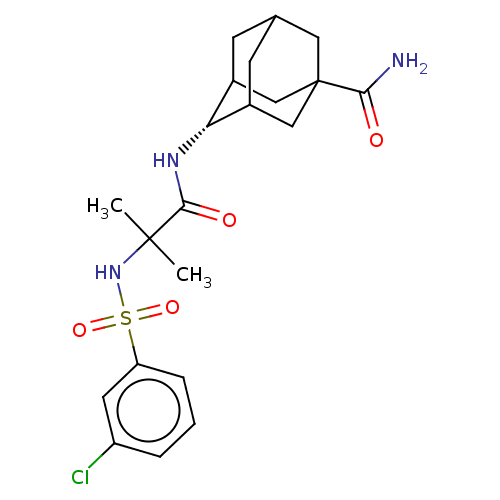
(CHEMBL3609871 | US9464044, 73)Show SMILES CC(C)(NS(=O)(=O)c1cccc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:21:22:26:25.19.20,21:20:26:17.22.23,17:18:25:22.21.23,THB:16:17:26:25.19.20,17:22:25:26.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H28ClN3O4S/c1-20(2,25-30(28,29)16-5-3-4-15(22)8-16)19(27)24-17-13-6-12-7-14(17)11-21(9-12,10-13)18(23)26/h3-5,8,12-14,17,25H,6-7,9-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112127
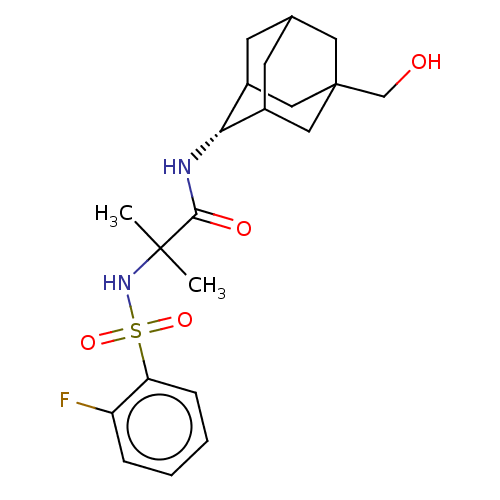
(CHEMBL3608407 | US9464044, 76)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(CO)(C3)C2 |r,wD:17.17,TLB:21:22:28:27.19.20,21:20:28:17.22.23,17:18:27:22.21.23,THB:16:17:28:27.19.20,17:22:27:28.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;3.07,1.15,;3.59,2.27,;1.56,2.59,;.56,-.22,)| Show InChI InChI=1S/C21H29FN2O4S/c1-20(2,24-29(27,28)17-6-4-3-5-16(17)22)19(26)23-18-14-7-13-8-15(18)11-21(9-13,10-14)12-25/h3-6,13-15,18,24-25H,7-12H2,1-2H3,(H,23,26)/t13?,14?,15?,18-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112145
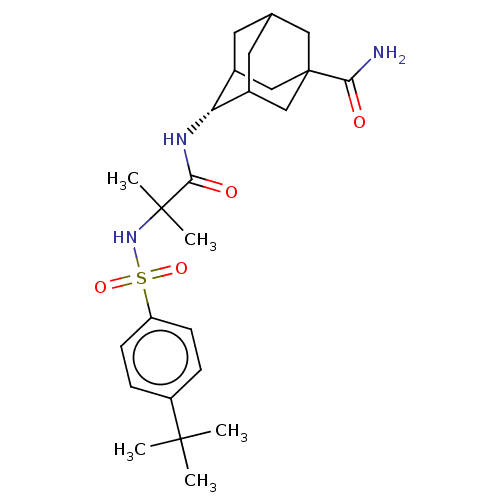
(CHEMBL3609873)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)NC(C)(C)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:20.20,TLB:24:25:29:28.22.23,24:23:29:20.25.26,20:21:28:25.24.26,THB:19:20:29:28.22.23,20:25:28:29.22.21,(-14.57,1.58,;-14.39,.36,;-15.36,-.4,;-15.54,.82,;-12.96,-.21,;-12.74,-1.74,;-11.31,-2.31,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-8.67,-1.93,;-7.53,-2.38,;-8.5,-3.15,;-7.46,-.97,;-6.03,-1.55,;-7,-2.31,;-5.85,-2.77,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C25H37N3O4S/c1-23(2,3)18-6-8-19(9-7-18)33(31,32)28-24(4,5)22(30)27-20-16-10-15-11-17(20)14-25(12-15,13-16)21(26)29/h6-9,15-17,20,28H,10-14H2,1-5H3,(H2,26,29)(H,27,30)/t15?,16?,17?,20-,25? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112142
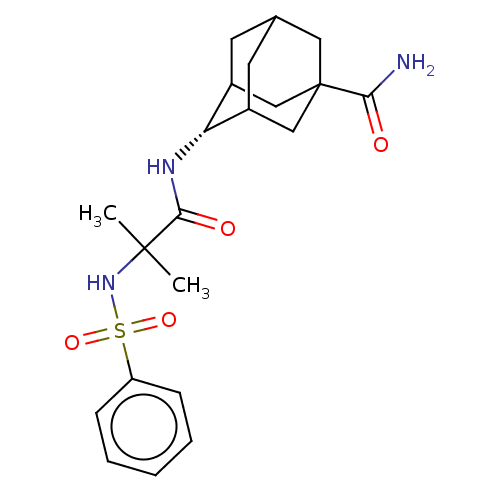
(CHEMBL3609870 | US9464044, 81)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:16.16,TLB:20:21:25:24.18.19,20:19:25:16.21.22,16:17:24:21.20.22,THB:15:16:25:24.18.19,16:21:24:25.18.17,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-11.31,-2.31,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H29N3O4S/c1-20(2,24-29(27,28)16-6-4-3-5-7-16)19(26)23-17-14-8-13-9-15(17)12-21(10-13,11-14)18(22)25/h3-7,13-15,17,24H,8-12H2,1-2H3,(H2,22,25)(H,23,26)/t13?,14?,15?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473585
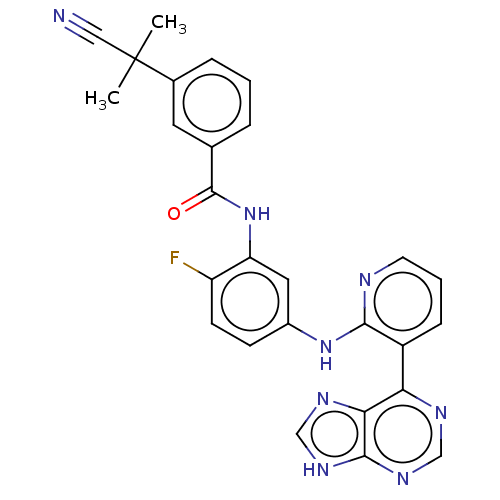
(US10844062, Example 21)Show SMILES CC(C)(C#N)c1cccc(c1)C(=O)Nc1cc(Nc2ncccc2-c2ncnc3[nH]cnc23)ccc1F Show InChI InChI=1S/C27H21FN8O/c1-27(2,13-29)17-6-3-5-16(11-17)26(37)36-21-12-18(8-9-20(21)28)35-24-19(7-4-10-30-24)22-23-25(33-14-31-22)34-15-32-23/h3-12,14-15H,1-2H3,(H,30,35)(H,36,37)(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112141
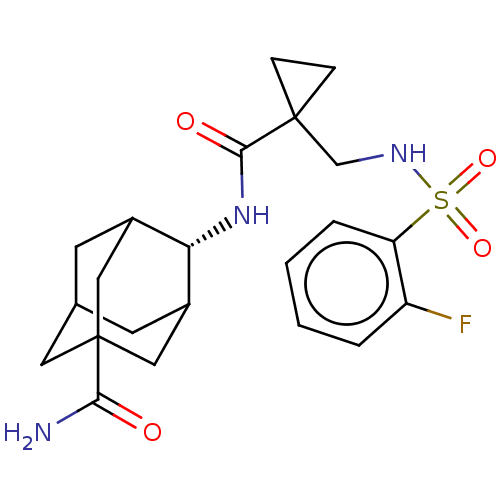
(CHEMBL3609869 | US9464044, 3)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)C1(CNS(=O)(=O)c4ccccc4F)CC1)C(C3)C2 |r,wD:9.10,TLB:10:9:29:3.4.30,8:3:29:9.7.6,THB:1:3:29:9.7.6,9:28:4:8.7.6,9:7:4:29.30.28,8:7:29:3.4.30,(3.79,.16,;3.07,1.16,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-4.99,.63,;-6.03,-1.55,;-5.81,-3.07,;-7.02,-4.02,;-6.8,-5.55,;-5.66,-6.01,;-5.83,-4.79,;-8.01,-6.5,;-9.44,-5.93,;-10.65,-6.88,;-10.43,-8.41,;-9,-8.98,;-7.79,-8.03,;-6.65,-8.48,;-6.85,-.39,;-7.43,-1.82,;-1.2,1.02,;-1.2,2.69,;.34,.44,)| Show InChI InChI=1S/C22H28FN3O4S/c23-16-3-1-2-4-17(16)31(29,30)25-12-21(5-6-21)20(28)26-18-14-7-13-8-15(18)11-22(9-13,10-14)19(24)27/h1-4,13-15,18,25H,5-12H2,(H2,24,27)(H,26,28)/t13?,14?,15?,18-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112154

(CHEMBL3608403)Show SMILES CC(C)(NS(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27Cl2N3O4S/c1-20(2,26-31(29,30)16-6-14(22)5-15(23)7-16)19(28)25-17-12-3-11-4-13(17)10-21(8-11,9-12)18(24)27/h5-7,11-13,17,26H,3-4,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112140
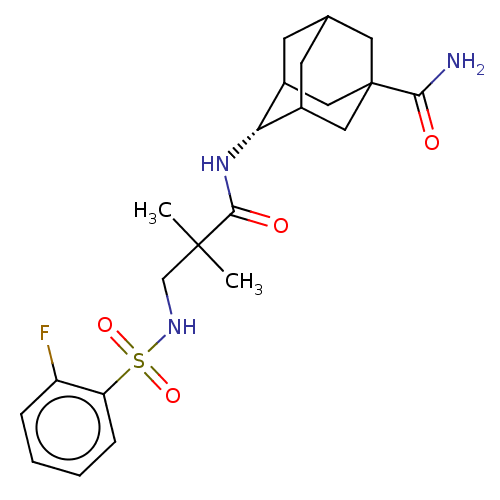
(CHEMBL3609868 | US9464044, 68)Show SMILES CC(C)(CNS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:17:18:22:25.26.24,18:19:26:22.24.23,27:25:22:18.19.20,THB:28:25:22:18.19.20,18:23:26:27.19.20,27:19:22:25.26.24,(-5.85,-2.77,;-6.03,-1.55,;-7,-2.31,;-7.46,-.97,;-8.67,-1.93,;-10.1,-1.35,;-10.28,-.14,;-9.13,-.59,;-11.31,-2.31,;-12.74,-1.73,;-13.95,-2.69,;-13.73,-4.21,;-12.3,-4.78,;-11.09,-3.83,;-9.95,-4.29,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.16,;3.79,.16,;3.58,2.29,)| Show InChI InChI=1S/C22H30FN3O4S/c1-21(2,12-25-31(29,30)17-6-4-3-5-16(17)23)20(28)26-18-14-7-13-8-15(18)11-22(9-13,10-14)19(24)27/h3-6,13-15,18,25H,7-12H2,1-2H3,(H2,24,27)(H,26,28)/t13?,14?,15?,18-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473613
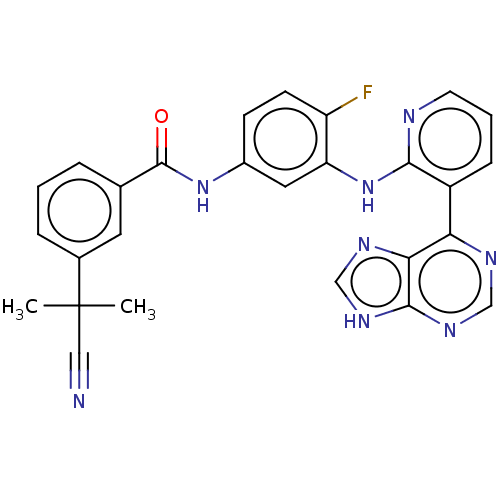
(US10844062, Example 49)Show SMILES CC(C)(C#N)c1cccc(c1)C(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1 Show InChI InChI=1S/C27H21FN8O/c1-27(2,13-29)17-6-3-5-16(11-17)26(37)35-18-8-9-20(28)21(12-18)36-24-19(7-4-10-30-24)22-23-25(33-14-31-22)34-15-32-23/h3-12,14-15H,1-2H3,(H,30,36)(H,35,37)(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112149

(CHEMBL3609877)Show SMILES CC(C)(NS(=O)(=O)c1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-14.11,.25,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27ClFN3O4S/c1-20(2,26-31(29,30)16-4-3-14(23)7-15(16)22)19(28)25-17-12-5-11-6-13(17)10-21(8-11,9-12)18(24)27/h3-4,7,11-13,17,26H,5-6,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
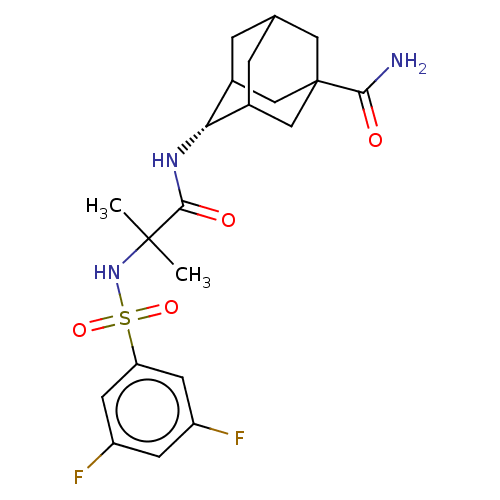
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112153
(CHEMBL3608402 | US9464044, 83)Show SMILES CC(C)(NS(=O)(=O)c1cc(F)cc(F)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27F2N3O4S/c1-20(2,26-31(29,30)16-6-14(22)5-15(23)7-16)19(28)25-17-12-3-11-4-13(17)10-21(8-11,9-12)18(24)27/h5-7,11-13,17,26H,3-4,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50112133

(CHEMBL3609861 | US9464044, 71)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:16:17:21:23.25.24,26:24:21:19.18.17,THB:27:24:21:19.18.17,27:24:21.20.19:17,25:20:17:23.24.26,25:24:21.20.19:17,26:18:21:23.25.24,(-5.85,-2.77,;-6.03,-1.55,;-7,-2.31,;-7.46,-.97,;-8.67,-1.93,;-8.49,-3.15,;-7.53,-2.38,;-10.1,-1.35,;-11.31,-2.31,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.17,;3.58,2.29,;3.79,.17,)| Show InChI InChI=1S/C21H28FN3O4S/c1-20(2,25-30(28,29)16-6-4-3-5-15(16)22)19(27)24-17-13-7-12-8-14(17)11-21(9-12,10-13)18(23)26/h3-6,12-14,17,25H,7-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD2 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473190
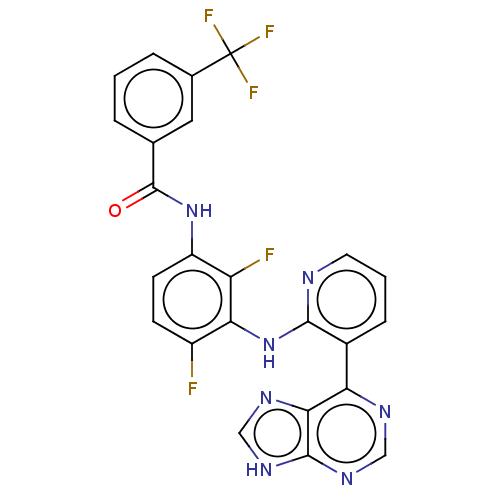
(US10844062, Example 1)Show SMILES Fc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C24H14F5N7O/c25-15-6-7-16(35-23(37)12-3-1-4-13(9-12)24(27,28)29)17(26)19(15)36-21-14(5-2-8-30-21)18-20-22(33-10-31-18)34-11-32-20/h1-11H,(H,30,36)(H,35,37)(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473594
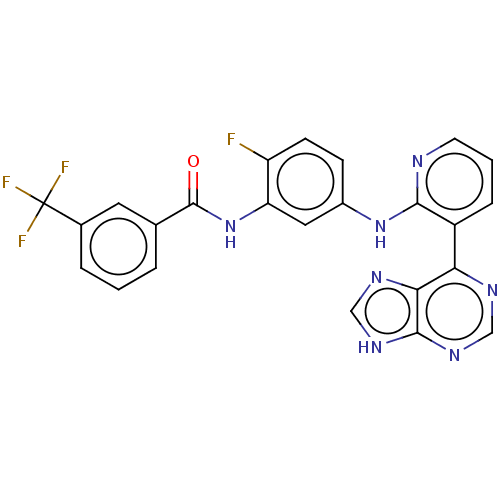
(US10844062, Example 30)Show SMILES Fc1ccc(Nc2ncccc2-c2ncnc3[nH]cnc23)cc1NC(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H15F4N7O/c25-17-7-6-15(10-18(17)35-23(36)13-3-1-4-14(9-13)24(26,27)28)34-21-16(5-2-8-29-21)19-20-22(32-11-30-19)33-12-31-20/h1-12H,(H,29,34)(H,35,36)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473640
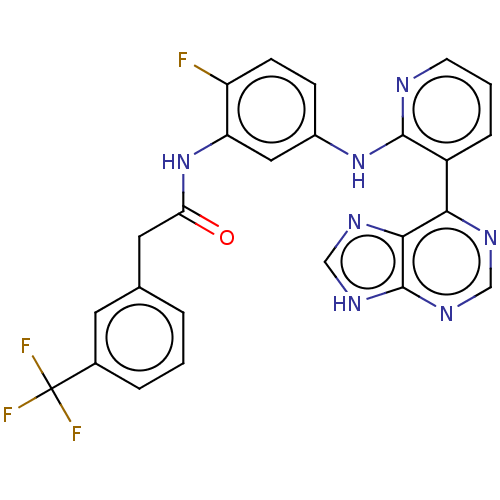
(US10844062, Example 76)Show SMILES Fc1ccc(Nc2ncccc2-c2ncnc3[nH]cnc23)cc1NC(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C25H17F4N7O/c26-18-7-6-16(11-19(18)36-20(37)10-14-3-1-4-15(9-14)25(27,28)29)35-23-17(5-2-8-30-23)21-22-24(33-12-31-21)34-13-32-22/h1-9,11-13H,10H2,(H,30,35)(H,36,37)(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112143

(CHEMBL3609871 | US9464044, 73)Show SMILES CC(C)(NS(=O)(=O)c1cccc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:21:22:26:25.19.20,21:20:26:17.22.23,17:18:25:22.21.23,THB:16:17:26:25.19.20,17:22:25:26.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H28ClN3O4S/c1-20(2,25-30(28,29)16-5-3-4-15(22)8-16)19(27)24-17-13-6-12-7-14(17)11-21(9-12,10-13)18(23)26/h3-5,8,12-14,17,25H,6-7,9-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112152

(CHEMBL3608401 | US9464044, 84)Show SMILES CC(C)(NS(=O)(=O)c1c(F)cccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-11.31,-2.31,;-11.14,-3.53,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27F2N3O4S/c1-20(2,26-31(29,30)17-14(22)4-3-5-15(17)23)19(28)25-16-12-6-11-7-13(16)10-21(8-11,9-12)18(24)27/h3-5,11-13,16,26H,6-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,16-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473593
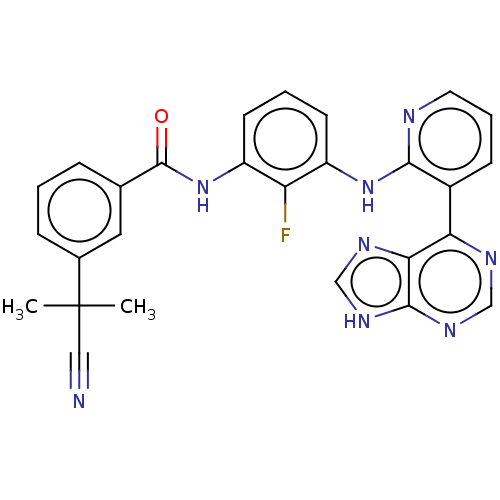
(US10844062, Example 29)Show SMILES CC(C)(C#N)c1cccc(c1)C(=O)Nc1cccc(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C27H21FN8O/c1-27(2,13-29)17-7-3-6-16(12-17)26(37)36-20-10-4-9-19(21(20)28)35-24-18(8-5-11-30-24)22-23-25(33-14-31-22)34-15-32-23/h3-12,14-15H,1-2H3,(H,30,35)(H,36,37)(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473573
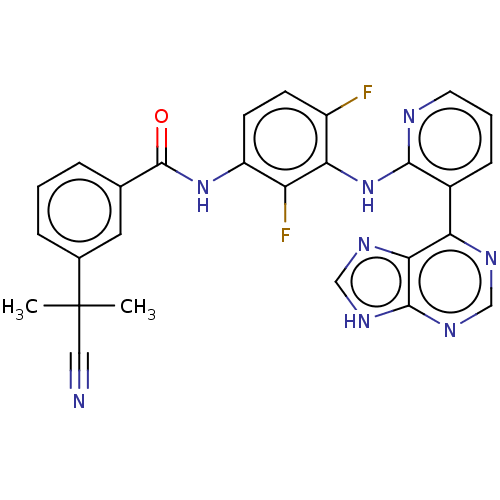
(US10844062, Example 9)Show SMILES CC(C)(C#N)c1cccc(c1)C(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C27H20F2N8O/c1-27(2,12-30)16-6-3-5-15(11-16)26(38)36-19-9-8-18(28)22(20(19)29)37-24-17(7-4-10-31-24)21-23-25(34-13-32-21)35-14-33-23/h3-11,13-14H,1-2H3,(H,31,37)(H,36,38)(H,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473600
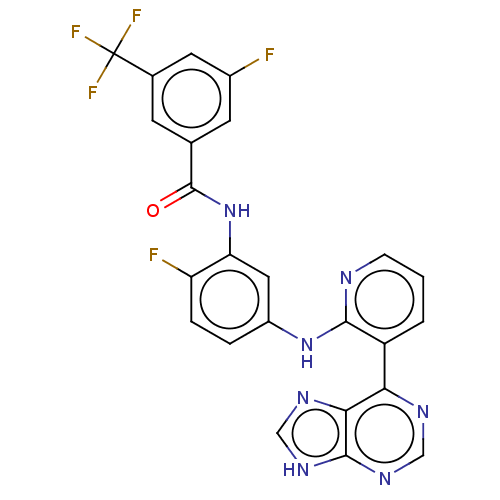
(US10844062, Example 36)Show SMILES Fc1cc(cc(c1)C(F)(F)F)C(=O)Nc1cc(Nc2ncccc2-c2ncnc3[nH]cnc23)ccc1F Show InChI InChI=1S/C24H14F5N7O/c25-14-7-12(6-13(8-14)24(27,28)29)23(37)36-18-9-15(3-4-17(18)26)35-21-16(2-1-5-30-21)19-20-22(33-10-31-19)34-11-32-20/h1-11H,(H,30,35)(H,36,37)(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112148
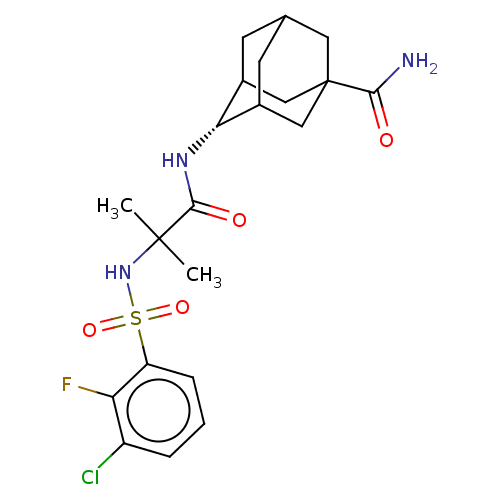
(CHEMBL3609876)Show SMILES CC(C)(NS(=O)(=O)c1cccc(Cl)c1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27ClFN3O4S/c1-20(2,26-31(29,30)15-5-3-4-14(22)16(15)23)19(28)25-17-12-6-11-7-13(17)10-21(8-11,9-12)18(24)27/h3-5,11-13,17,26H,6-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112141

(CHEMBL3609869 | US9464044, 3)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)C1(CNS(=O)(=O)c4ccccc4F)CC1)C(C3)C2 |r,wD:9.10,TLB:10:9:29:3.4.30,8:3:29:9.7.6,THB:1:3:29:9.7.6,9:28:4:8.7.6,9:7:4:29.30.28,8:7:29:3.4.30,(3.79,.16,;3.07,1.16,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-4.99,.63,;-6.03,-1.55,;-5.81,-3.07,;-7.02,-4.02,;-6.8,-5.55,;-5.66,-6.01,;-5.83,-4.79,;-8.01,-6.5,;-9.44,-5.93,;-10.65,-6.88,;-10.43,-8.41,;-9,-8.98,;-7.79,-8.03,;-6.65,-8.48,;-6.85,-.39,;-7.43,-1.82,;-1.2,1.02,;-1.2,2.69,;.34,.44,)| Show InChI InChI=1S/C22H28FN3O4S/c23-16-3-1-2-4-17(16)31(29,30)25-12-21(5-6-21)20(28)26-18-14-7-13-8-15(18)11-22(9-13,10-14)19(24)27/h1-4,13-15,18,25H,5-12H2,(H2,24,27)(H,26,28)/t13?,14?,15?,18-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473587
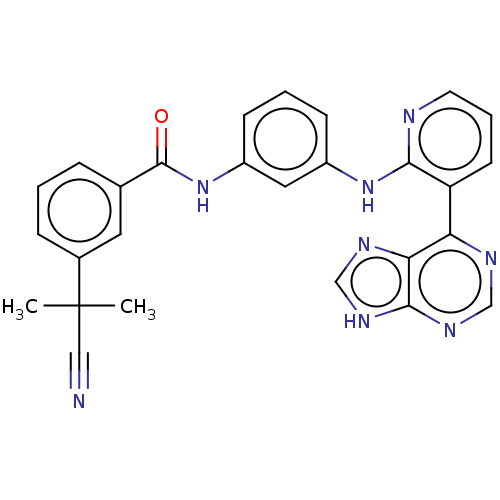
(US10844062, Example 23)Show SMILES CC(C)(C#N)c1cccc(c1)C(=O)Nc1cccc(Nc2ncccc2-c2ncnc3[nH]cnc23)c1 Show InChI InChI=1S/C27H22N8O/c1-27(2,14-28)18-7-3-6-17(12-18)26(36)35-20-9-4-8-19(13-20)34-24-21(10-5-11-29-24)22-23-25(32-15-30-22)33-16-31-23/h3-13,15-16H,1-2H3,(H,29,34)(H,35,36)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112150
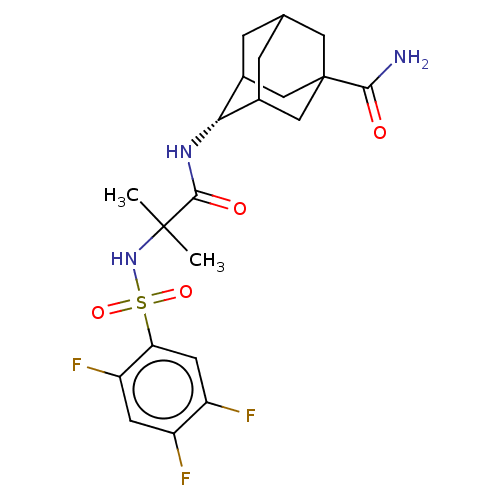
(CHEMBL3608399)Show SMILES CC(C)(NS(=O)(=O)c1cc(F)c(F)cc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:19.19,TLB:23:24:28:27.21.22,23:22:28:19.24.25,19:20:27:24.23.25,THB:18:19:28:27.21.22,19:24:27:28.21.20,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-14.11,.25,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H26F3N3O4S/c1-20(2,27-32(30,31)16-6-14(23)13(22)5-15(16)24)19(29)26-17-11-3-10-4-12(17)9-21(7-10,8-11)18(25)28/h5-6,10-12,17,27H,3-4,7-9H2,1-2H3,(H2,25,28)(H,26,29)/t10?,11?,12?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112131
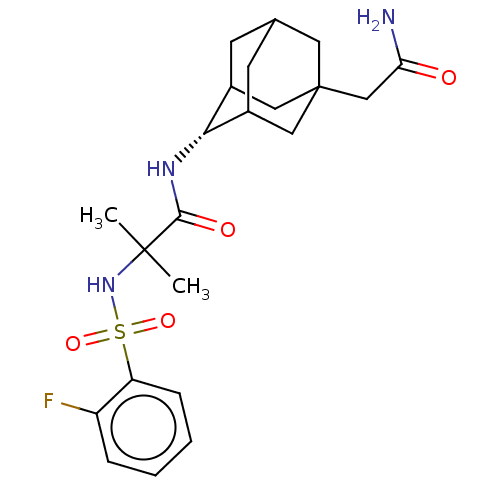
(CHEMBL3608411 | US9464044, 80)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(CC(N)=O)(C3)C2 |r,wD:17.17,TLB:17:18:29:22.21.23,21:22:30:29.19.20,21:20:30:17.22.23,THB:16:17:30:29.19.20,17:22:29:30.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;3.07,1.15,;3.72,2.55,;3.01,3.56,;4.95,2.66,;1.56,2.59,;.56,-.22,)| Show InChI InChI=1S/C22H30FN3O4S/c1-21(2,26-31(29,30)17-6-4-3-5-16(17)23)20(28)25-19-14-7-13-8-15(19)11-22(9-13,10-14)12-18(24)27/h3-6,13-15,19,26H,7-12H2,1-2H3,(H2,24,27)(H,25,28)/t13?,14?,15?,19-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112153

(CHEMBL3608402 | US9464044, 83)Show SMILES CC(C)(NS(=O)(=O)c1cc(F)cc(F)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27F2N3O4S/c1-20(2,26-31(29,30)16-6-14(22)5-15(23)7-16)19(28)25-17-12-3-11-4-13(17)10-21(8-11,9-12)18(24)27/h5-7,11-13,17,26H,3-4,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473629
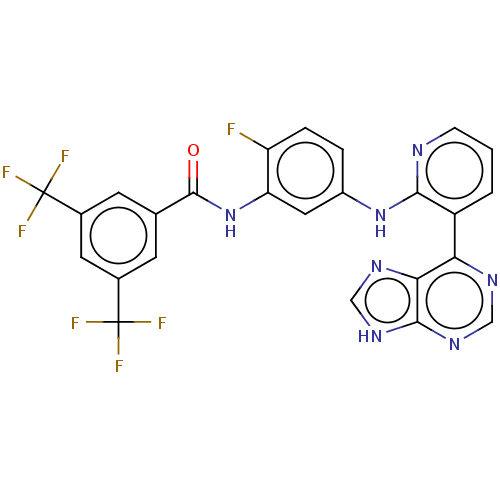
(US10844062, Example 65)Show SMILES Fc1ccc(Nc2ncccc2-c2ncnc3[nH]cnc23)cc1NC(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C25H14F7N7O/c26-17-4-3-15(38-21-16(2-1-5-33-21)19-20-22(36-10-34-19)37-11-35-20)9-18(17)39-23(40)12-6-13(24(27,28)29)8-14(7-12)25(30,31)32/h1-11H,(H,33,38)(H,39,40)(H,34,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50112127

(CHEMBL3608407 | US9464044, 76)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(CO)(C3)C2 |r,wD:17.17,TLB:21:22:28:27.19.20,21:20:28:17.22.23,17:18:27:22.21.23,THB:16:17:28:27.19.20,17:22:27:28.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;3.07,1.15,;3.59,2.27,;1.56,2.59,;.56,-.22,)| Show InChI InChI=1S/C21H29FN2O4S/c1-20(2,24-29(27,28)17-6-4-3-5-16(17)22)19(26)23-18-14-7-13-8-15(18)11-21(9-13,10-14)12-25/h3-6,13-15,18,24-25H,7-12H2,1-2H3,(H,23,26)/t13?,14?,15?,18-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM473648
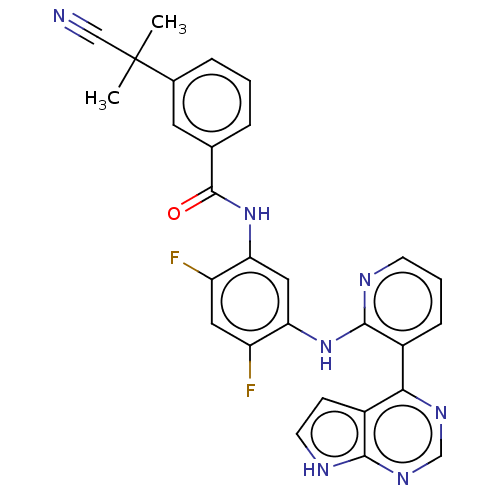
(US10844062, Example 84)Show SMILES CC(C)(C#N)c1cccc(c1)C(=O)Nc1cc(Nc2ncccc2-c2ncnc3[nH]ccc23)c(F)cc1F Show InChI InChI=1S/C28H21F2N7O/c1-28(2,14-31)17-6-3-5-16(11-17)27(38)37-23-13-22(20(29)12-21(23)30)36-26-18(7-4-9-32-26)24-19-8-10-33-25(19)35-15-34-24/h3-13,15H,1-2H3,(H,32,36)(H,37,38)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon University Industry Academic Cooperation Foundation; Samjin Pharmaceutical Co., Ltd.; Bamichem Co., Ltd.
US Patent
| Assay Description
The inhibitory activities of the compounds of the present invention against VEGFR-2 tyrosine kinase were analyzed using ADP-Glo™ kinase assay kit com... |
US Patent US10844062 (2020)
BindingDB Entry DOI: 10.7270/Q2NG4TRN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112136
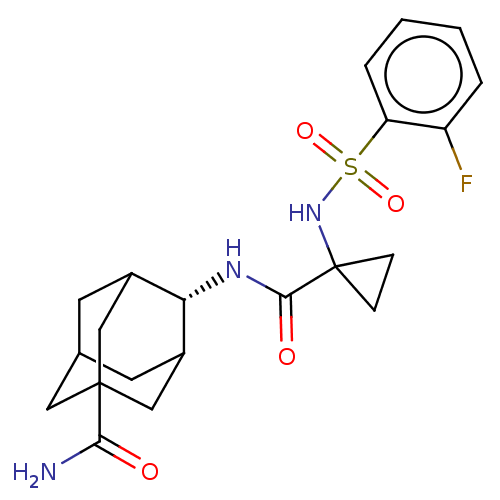
(CHEMBL3609864 | US9464044, 39)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)C1(CC1)NS(=O)(=O)c1ccccc1F)C(C3)C2 |r,wD:9.10,TLB:28:27:8:4.6.5,THB:28:5:8:9.27.29,10:9:8:4.6.5,9:27:4:8.6.7,9:7:4:27.28.29,(3.78,.15,;3.07,1.15,;3.59,2.27,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-4.99,.63,;-6.03,-1.55,;-5.1,-2.62,;-6.62,-2.84,;-7.46,-.97,;-7.68,.55,;-6.54,.09,;-6.71,1.31,;-9.11,1.12,;-9.33,2.65,;-10.76,3.22,;-11.97,2.27,;-11.75,.74,;-10.32,.17,;-10.15,-1.05,;-1.2,1.02,;-1.2,2.69,;.34,.44,)| Show InChI InChI=1S/C21H26FN3O4S/c22-15-3-1-2-4-16(15)30(28,29)25-21(5-6-21)19(27)24-17-13-7-12-8-14(17)11-20(9-12,10-13)18(23)26/h1-4,12-14,17,25H,5-11H2,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,20? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data