 Found 3557 hits with Last Name = 'horne' and Initial = 'db'
Found 3557 hits with Last Name = 'horne' and Initial = 'db' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99011

(US8497265, 467)Show SMILES Cc1ccc(cn1)-c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 Show InChI InChI=1S/C28H30N6O2/c1-19-6-7-22(14-29-19)24-15-30-28(33-12-10-20(18-35)11-13-33)32-27(24)36-23-16-34(17-23)26-9-8-21-4-2-3-5-25(21)31-26/h2-9,14-15,20,23,35H,10-13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98900
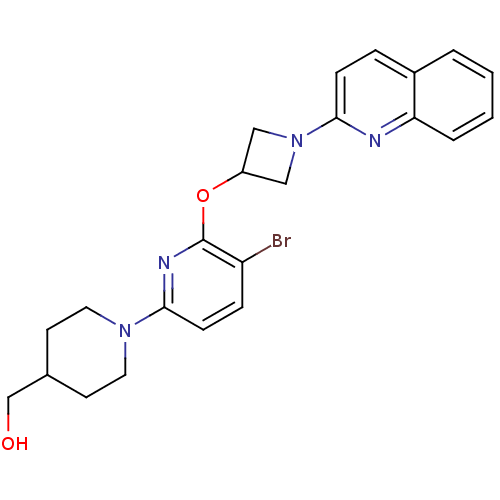
(US8497265, 226)Show SMILES OCC1CCN(CC1)c1ccc(Br)c(OC2CN(C2)c2ccc3ccccc3n2)n1 Show InChI InChI=1S/C23H25BrN4O2/c24-19-6-8-22(27-11-9-16(15-29)10-12-27)26-23(19)30-18-13-28(14-18)21-7-5-17-3-1-2-4-20(17)25-21/h1-8,16,18,29H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.229 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99009

(US8497265, 465)Show SMILES Cc1cc(ccn1)-c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 Show InChI InChI=1S/C28H30N6O2/c1-19-14-22(8-11-29-19)24-15-30-28(33-12-9-20(18-35)10-13-33)32-27(24)36-23-16-34(17-23)26-7-6-21-4-2-3-5-25(21)31-26/h2-8,11,14-15,20,23,35H,9-10,12-13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99013

(US8497265, 469)Show SMILES CC(C)(C)OC(=O)N1CCC(=CC1)c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 |c:10| Show InChI InChI=1S/C32H40N6O4/c1-32(2,3)42-31(40)37-16-12-23(13-17-37)26-18-33-30(36-14-10-22(21-39)11-15-36)35-29(26)41-25-19-38(20-25)28-9-8-24-6-4-5-7-27(24)34-28/h4-9,12,18,22,25,39H,10-11,13-17,19-21H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99048

(US8497265, 688)Show SMILES CC(=O)N1CCC(CC1)c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 Show InChI InChI=1S/C29H36N6O3/c1-20(37)33-14-10-22(11-15-33)25-16-30-29(34-12-8-21(19-36)9-13-34)32-28(25)38-24-17-35(18-24)27-7-6-23-4-2-3-5-26(23)31-27/h2-7,16,21-22,24,36H,8-15,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99010
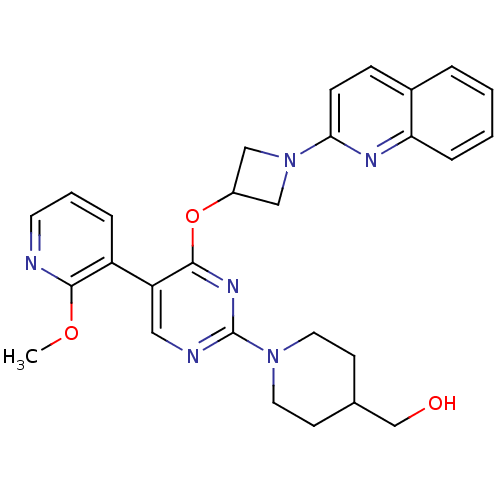
(US8497265, 466 | US8497265, 470)Show SMILES COc1ncccc1-c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 Show InChI InChI=1S/C28H30N6O3/c1-36-26-22(6-4-12-29-26)23-15-30-28(33-13-10-19(18-35)11-14-33)32-27(23)37-21-16-34(17-21)25-9-8-20-5-2-3-7-24(20)31-25/h2-9,12,15,19,21,35H,10-11,13-14,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98928
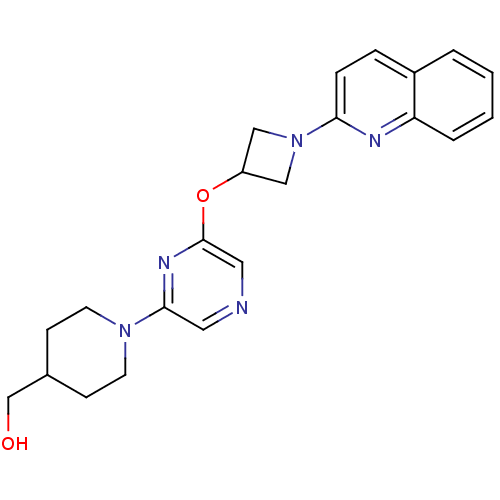
(US8497265, 254)Show SMILES OCC1CCN(CC1)c1cncc(OC2CN(C2)c2ccc3ccccc3n2)n1 Show InChI InChI=1S/C22H25N5O2/c28-15-16-7-9-26(10-8-16)21-11-23-12-22(25-21)29-18-13-27(14-18)20-6-5-17-3-1-2-4-19(17)24-20/h1-6,11-12,16,18,28H,7-10,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.639 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383847
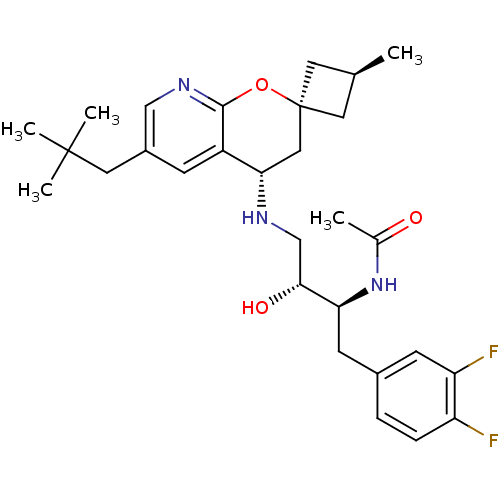
(CHEMBL2031143)Show SMILES C[C@H]1C[C@@]2(C1)C[C@H](NC[C@@H](O)[C@H](Cc1ccc(F)c(F)c1)NC(C)=O)c1cc(CC(C)(C)C)cnc1O2 |r,wU:6.7,wD:9.10,11.22,3.2,1.0,(46.38,-21.19,;45.29,-22.27,;43.75,-22.27,;43.75,-23.81,;45.29,-23.81,;42.41,-23.05,;41.07,-23.83,;39.73,-23.06,;38.4,-23.84,;37.06,-23.08,;37.06,-21.54,;35.73,-23.85,;35.74,-25.39,;34.41,-26.17,;34.42,-27.7,;33.1,-28.48,;31.75,-27.72,;30.42,-28.5,;31.75,-26.18,;30.41,-25.41,;33.07,-25.4,;34.39,-23.09,;34.39,-21.55,;33.05,-20.78,;35.72,-20.77,;41.08,-25.37,;39.75,-26.14,;39.75,-27.69,;38.41,-28.45,;38.41,-29.99,;37.08,-30.76,;39.74,-30.77,;38.4,-31.53,;41.08,-28.46,;42.42,-27.68,;42.42,-26.14,;43.75,-25.37,)| Show InChI InChI=1S/C29H39F2N3O3/c1-17-11-29(12-17)14-25(21-8-20(13-28(3,4)5)15-33-27(21)37-29)32-16-26(36)24(34-18(2)35)10-19-6-7-22(30)23(31)9-19/h6-9,15,17,24-26,32,36H,10-14,16H2,1-5H3,(H,34,35)/t17-,24-,25-,26+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383846
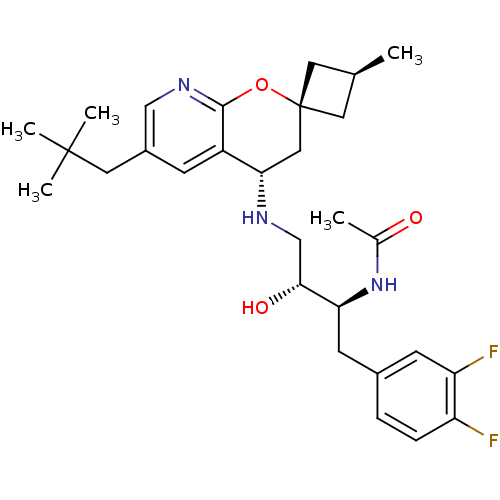
(CHEMBL2031142)Show SMILES C[C@H]1C[C@]2(C1)C[C@H](NC[C@@H](O)[C@H](Cc1ccc(F)c(F)c1)NC(C)=O)c1cc(CC(C)(C)C)cnc1O2 |r,wU:6.7,1.0,wD:9.10,11.22,3.3,(31.56,-19.68,;30.47,-20.77,;30.47,-22.31,;28.93,-22.31,;28.93,-20.77,;27.59,-21.54,;26.25,-22.32,;24.91,-21.56,;23.58,-22.33,;22.25,-21.57,;22.24,-20.03,;20.91,-22.34,;20.92,-23.88,;19.59,-24.66,;19.61,-26.19,;18.28,-26.97,;16.93,-26.21,;15.61,-26.99,;16.93,-24.67,;15.59,-23.9,;18.26,-23.89,;19.58,-21.58,;19.57,-20.04,;18.23,-19.28,;20.9,-19.26,;26.26,-23.86,;24.93,-24.63,;24.93,-26.18,;23.6,-26.95,;23.6,-28.49,;22.26,-29.25,;24.93,-29.26,;23.58,-30.02,;26.27,-26.95,;27.6,-26.18,;27.6,-24.63,;28.93,-23.86,)| Show InChI InChI=1S/C29H39F2N3O3/c1-17-11-29(12-17)14-25(21-8-20(13-28(3,4)5)15-33-27(21)37-29)32-16-26(36)24(34-18(2)35)10-19-6-7-22(30)23(31)9-19/h6-9,15,17,24-26,32,36H,10-14,16H2,1-5H3,(H,34,35)/t17-,24-,25-,26+,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99052

(US8497265, 736 | US8497265, 797)Show SMILES O=C(N1CC(C1)Oc1ncccc1C1=CCOCC1)c1nc2ccccc2[nH]1 |t:15| Show InChI InChI=1S/C21H20N4O3/c26-21(19-23-17-5-1-2-6-18(17)24-19)25-12-15(13-25)28-20-16(4-3-9-22-20)14-7-10-27-11-8-14/h1-7,9,15H,8,10-13H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.864 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99052

(US8497265, 736 | US8497265, 797)Show SMILES O=C(N1CC(C1)Oc1ncccc1C1=CCOCC1)c1nc2ccccc2[nH]1 |t:15| Show InChI InChI=1S/C21H20N4O3/c26-21(19-23-17-5-1-2-6-18(17)24-19)25-12-15(13-25)28-20-16(4-3-9-22-20)14-7-10-27-11-8-14/h1-7,9,15H,8,10-13H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.864 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383838
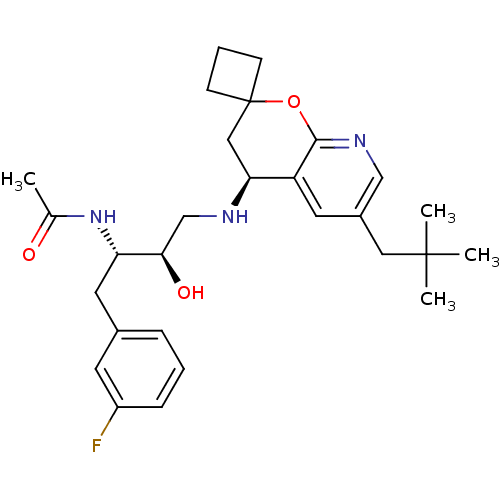
(CHEMBL2030996)Show SMILES CC(=O)N[C@@H](Cc1cccc(F)c1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38FN3O3/c1-18(33)32-23(13-19-7-5-8-21(29)11-19)25(34)17-30-24-15-28(9-6-10-28)35-26-22(24)12-20(16-31-26)14-27(2,3)4/h5,7-8,11-12,16,23-25,30,34H,6,9-10,13-15,17H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99012
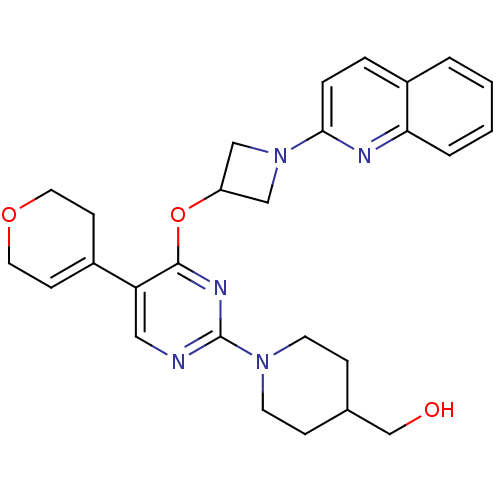
(US8497265, 468)Show SMILES OCC1CCN(CC1)c1ncc(C2=CCOCC2)c(OC2CN(C2)c2ccc3ccccc3n2)n1 |t:13| Show InChI InChI=1S/C27H31N5O3/c33-18-19-7-11-31(12-8-19)27-28-15-23(20-9-13-34-14-10-20)26(30-27)35-22-16-32(17-22)25-6-5-21-3-1-2-4-24(21)29-25/h1-6,9,15,19,22,33H,7-8,10-14,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98931

(US8497265, 343)Show SMILES Cc1cnc(OC2CN(C2)c2ccc3ccccc3n2)c(c1)N1CCC(CO)CC1 Show InChI InChI=1S/C24H28N4O2/c1-17-12-22(27-10-8-18(16-29)9-11-27)24(25-13-17)30-20-14-28(15-20)23-7-6-19-4-2-3-5-21(19)26-23/h2-7,12-13,18,20,29H,8-11,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98932

(US8497265, 344)Show SMILES OCC1CCN(CC1)c1cc(F)cnc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C23H25FN4O2/c24-18-11-21(27-9-7-16(15-29)8-10-27)23(25-12-18)30-19-13-28(14-19)22-6-5-17-3-1-2-4-20(17)26-22/h1-6,11-12,16,19,29H,7-10,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397712
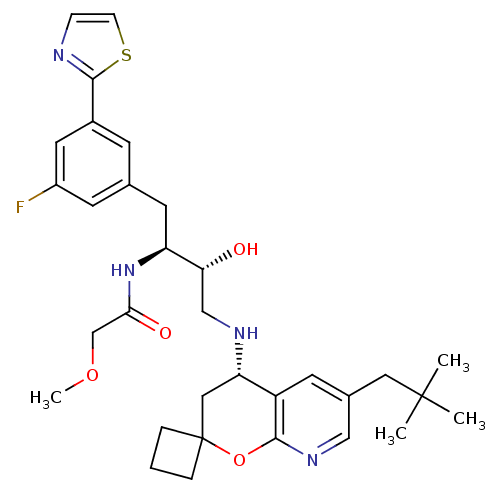
(CHEMBL2181902)Show SMILES COCC(=O)N[C@@H](Cc1cc(F)cc(c1)-c1nccs1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C32H41FN4O4S/c1-31(2,3)15-21-12-24-26(16-32(6-5-7-32)41-29(24)36-17-21)35-18-27(38)25(37-28(39)19-40-4)13-20-10-22(14-23(33)11-20)30-34-8-9-42-30/h8-12,14,17,25-27,35,38H,5-7,13,15-16,18-19H2,1-4H3,(H,37,39)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397708
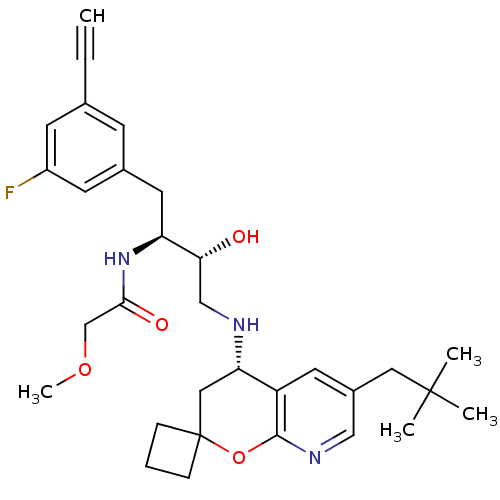
(CHEMBL2181906)Show SMILES COCC(=O)N[C@@H](Cc1cc(F)cc(c1)C#C)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C31H40FN3O4/c1-6-20-10-21(12-23(32)11-20)14-25(35-28(37)19-38-5)27(36)18-33-26-16-31(8-7-9-31)39-29-24(26)13-22(17-34-29)15-30(2,3)4/h1,10-13,17,25-27,33,36H,7-9,14-16,18-19H2,2-5H3,(H,35,37)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383851
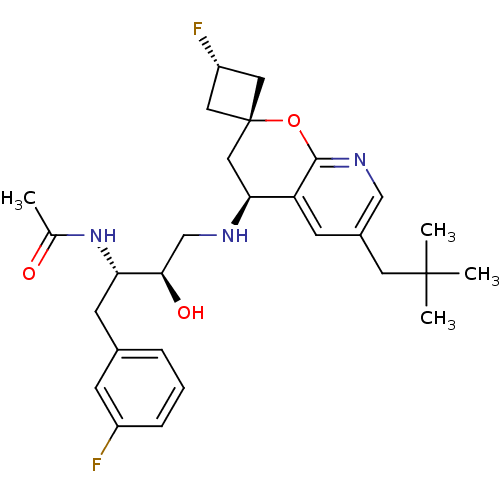
(CHEMBL2031145)Show SMILES CC(=O)N[C@@H](Cc1cccc(F)c1)[C@H](O)CN[C@H]1C[C@]2(C[C@H](F)C2)Oc2ncc(CC(C)(C)C)cc12 |r,wU:17.17,wD:13.14,4.3,19.20,21.22,(32.88,-32.05,;34.22,-32.82,;35.55,-32.04,;34.22,-34.36,;35.56,-35.12,;35.57,-36.66,;34.24,-37.44,;32.9,-36.67,;31.57,-37.45,;31.58,-38.99,;32.93,-39.75,;32.94,-41.29,;34.25,-38.97,;36.89,-34.35,;36.88,-32.81,;38.23,-35.11,;39.56,-34.33,;40.9,-35.1,;42.24,-34.32,;43.58,-35.08,;43.58,-33.54,;45.12,-33.54,;46.21,-32.46,;45.12,-35.08,;43.58,-36.64,;42.25,-37.41,;42.25,-38.95,;40.91,-39.73,;39.58,-38.96,;38.24,-39.72,;38.24,-41.26,;36.91,-42.03,;39.57,-42.04,;38.23,-42.8,;39.58,-37.41,;40.91,-36.64,)| Show InChI InChI=1S/C28H37F2N3O3/c1-17(34)33-23(10-18-6-5-7-20(29)8-18)25(35)16-31-24-14-28(12-21(30)13-28)36-26-22(24)9-19(15-32-26)11-27(2,3)4/h5-9,15,21,23-25,31,35H,10-14,16H2,1-4H3,(H,33,34)/t21-,23-,24-,25+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383837
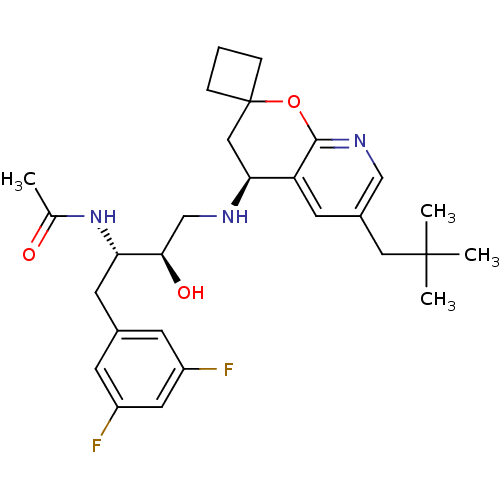
(CHEMBL2030998)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H37F2N3O3/c1-17(34)33-23(11-18-8-20(29)12-21(30)9-18)25(35)16-31-24-14-28(6-5-7-28)36-26-22(24)10-19(15-32-26)13-27(2,3)4/h8-10,12,15,23-25,31,35H,5-7,11,13-14,16H2,1-4H3,(H,33,34)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383855
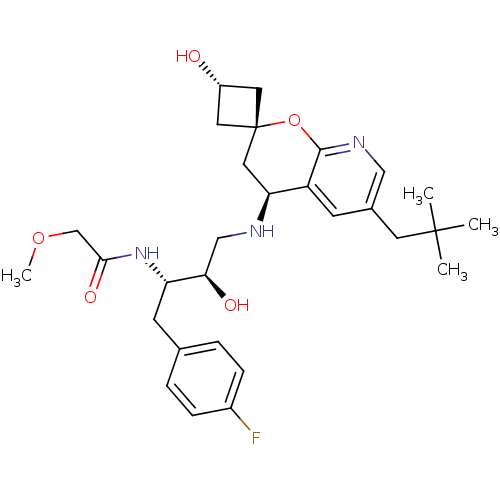
(CHEMBL2031149)Show SMILES COCC(=O)N[C@@H](Cc1ccc(F)cc1)[C@H](O)CN[C@H]1C[C@]2(C[C@H](O)C2)Oc2ncc(CC(C)(C)C)cc12 |r,wU:19.19,wD:15.16,6.5,21.22,23.24,(30.27,-40.49,;31.6,-41.26,;31.61,-42.8,;32.95,-43.56,;34.28,-42.78,;32.96,-45.1,;34.29,-45.86,;34.3,-47.4,;32.97,-48.18,;31.64,-47.41,;30.31,-48.19,;30.31,-49.73,;28.98,-50.51,;31.66,-50.49,;32.98,-49.71,;35.62,-45.09,;35.62,-43.55,;36.96,-45.85,;38.29,-45.08,;39.63,-45.84,;40.97,-45.06,;42.31,-45.83,;42.31,-44.29,;43.85,-44.29,;44.94,-43.2,;43.85,-45.83,;42.31,-47.38,;40.98,-48.15,;40.98,-49.7,;39.64,-50.47,;38.31,-49.7,;36.97,-50.47,;36.97,-52.01,;35.64,-52.77,;38.31,-52.78,;36.96,-53.54,;38.31,-48.15,;39.64,-47.38,)| Show InChI InChI=1S/C29H40FN3O5/c1-28(2,3)11-19-9-22-24(14-29(12-21(34)13-29)38-27(22)32-15-19)31-16-25(35)23(33-26(36)17-37-4)10-18-5-7-20(30)8-6-18/h5-9,15,21,23-25,31,34-35H,10-14,16-17H2,1-4H3,(H,33,36)/t21-,23-,24-,25+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383850
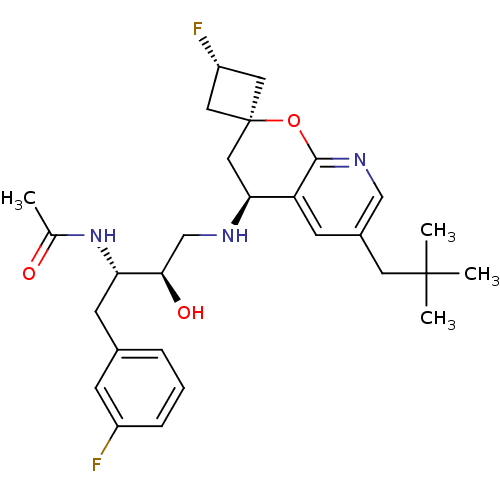
(CHEMBL2030994)Show SMILES CC(=O)N[C@@H](Cc1cccc(F)c1)[C@H](O)CN[C@H]1C[C@@]2(C[C@H](F)C2)Oc2ncc(CC(C)(C)C)cc12 |r,wU:17.17,21.22,wD:13.14,4.3,19.24,(17.15,-31.3,;18.49,-32.06,;19.82,-31.29,;18.5,-33.6,;19.83,-34.37,;19.84,-35.91,;18.51,-36.68,;17.18,-35.92,;15.85,-36.69,;15.85,-38.23,;17.2,-39,;17.21,-40.54,;18.52,-38.22,;21.16,-33.59,;21.16,-32.05,;22.5,-34.36,;23.83,-33.58,;25.17,-34.34,;26.51,-33.57,;27.85,-34.33,;29.39,-34.33,;29.39,-32.79,;30.48,-31.7,;27.85,-32.79,;27.85,-35.88,;26.52,-36.65,;26.52,-38.2,;25.18,-38.97,;23.85,-38.2,;22.52,-38.97,;22.51,-40.51,;21.18,-41.28,;23.85,-41.28,;22.5,-42.05,;23.85,-36.66,;25.18,-35.89,)| Show InChI InChI=1S/C28H37F2N3O3/c1-17(34)33-23(10-18-6-5-7-20(29)8-18)25(35)16-31-24-14-28(12-21(30)13-28)36-26-22(24)9-19(15-32-26)11-27(2,3)4/h5-9,15,21,23-25,31,35H,10-14,16H2,1-4H3,(H,33,34)/t21-,23-,24-,25+,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98891
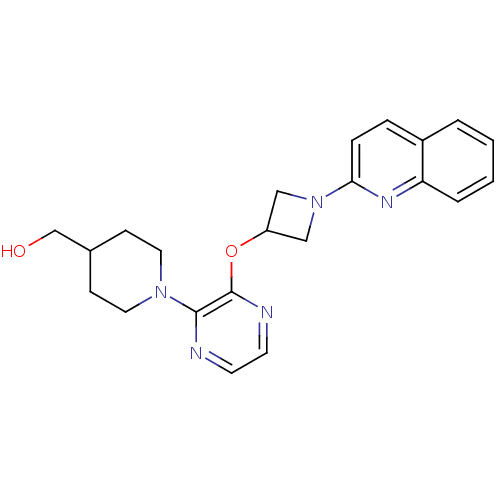
(US8497265, 217 | US8497265, 239)Show SMILES OCC1CCN(CC1)c1nccnc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C22H25N5O2/c28-15-16-7-11-26(12-8-16)21-22(24-10-9-23-21)29-18-13-27(14-18)20-6-5-17-3-1-2-4-19(17)25-20/h1-6,9-10,16,18,28H,7-8,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM98891

(US8497265, 217 | US8497265, 239)Show SMILES OCC1CCN(CC1)c1nccnc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C22H25N5O2/c28-15-16-7-11-26(12-8-16)21-22(24-10-9-23-21)29-18-13-27(14-18)20-6-5-17-3-1-2-4-19(17)25-20/h1-6,9-10,16,18,28H,7-8,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 using FAM-cAMP substrate by TR-FRET progressive binding assay |
Bioorg Med Chem 22: 6570-85 (2015)
Article DOI: 10.1016/j.bmc.2014.10.013
BindingDB Entry DOI: 10.7270/Q2RB766Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM99037
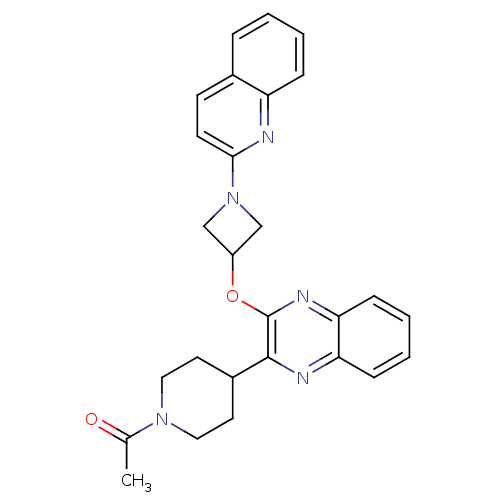
(US8497265, 677)Show SMILES CC(=O)N1CCC(CC1)c1nc2ccccc2nc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C27H27N5O2/c1-18(33)31-14-12-20(13-15-31)26-27(30-24-9-5-4-8-23(24)29-26)34-21-16-32(17-21)25-11-10-19-6-2-3-7-22(19)28-25/h2-11,20-21H,12-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 using FAM-cAMP substrate by TR-FRET progressive binding assay |
Bioorg Med Chem 22: 6570-85 (2015)
Article DOI: 10.1016/j.bmc.2014.10.013
BindingDB Entry DOI: 10.7270/Q2RB766Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397707

(CHEMBL2181907)Show SMILES COCC(=O)N[C@@H](Cc1cc(ccc1F)C#C)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C31H40FN3O4/c1-6-20-8-9-24(32)22(12-20)14-25(35-28(37)19-38-5)27(36)18-33-26-16-31(10-7-11-31)39-29-23(26)13-21(17-34-29)15-30(2,3)4/h1,8-9,12-13,17,25-27,33,36H,7,10-11,14-16,18-19H2,2-5H3,(H,35,37)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99037

(US8497265, 677)Show SMILES CC(=O)N1CCC(CC1)c1nc2ccccc2nc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C27H27N5O2/c1-18(33)31-14-12-20(13-15-31)26-27(30-24-9-5-4-8-23(24)29-26)34-21-16-32(17-21)25-11-10-19-6-2-3-7-22(19)28-25/h2-11,20-21H,12-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383854
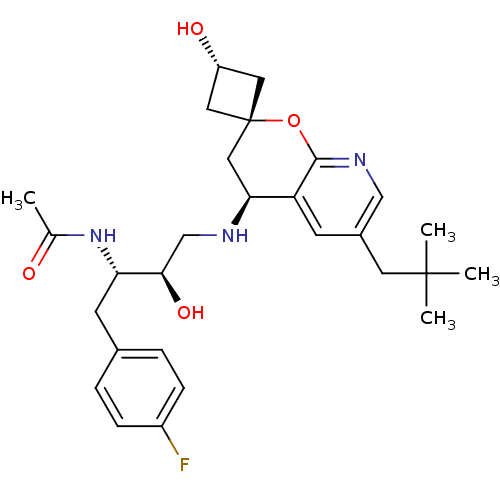
(CHEMBL2031148)Show SMILES CC(=O)N[C@@H](Cc1ccc(F)cc1)[C@H](O)CN[C@H]1C[C@]2(C[C@H](O)C2)Oc2ncc(CC(C)(C)C)cc12 |r,wU:17.17,wD:13.14,4.3,19.20,21.22,(16.97,-42.9,;18.31,-43.67,;19.64,-42.89,;18.32,-45.21,;19.65,-45.97,;19.66,-47.51,;18.33,-48.29,;17,-47.52,;15.67,-48.3,;15.67,-49.84,;14.35,-50.62,;17.02,-50.6,;18.35,-49.82,;20.99,-45.2,;20.98,-43.66,;22.32,-45.96,;23.65,-45.18,;24.99,-45.95,;26.33,-45.17,;27.67,-45.93,;27.67,-44.39,;29.21,-44.39,;30.3,-43.31,;29.21,-45.93,;27.67,-47.49,;26.34,-48.26,;26.34,-49.8,;25.01,-50.58,;23.67,-49.81,;22.34,-50.57,;22.34,-52.11,;21,-52.88,;23.67,-52.89,;22.32,-53.65,;23.67,-48.26,;25,-47.49,)| Show InChI InChI=1S/C28H38FN3O4/c1-17(33)32-23(10-18-5-7-20(29)8-6-18)25(35)16-30-24-14-28(12-21(34)13-28)36-26-22(24)9-19(15-31-26)11-27(2,3)4/h5-9,15,21,23-25,30,34-35H,10-14,16H2,1-4H3,(H,32,33)/t21-,23-,24-,25+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383839
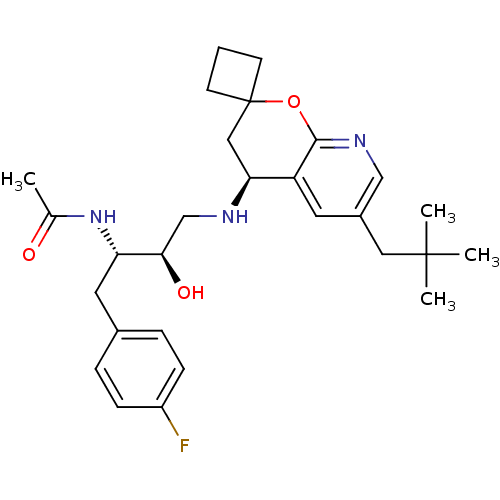
(CHEMBL2030997)Show SMILES CC(=O)N[C@@H](Cc1ccc(F)cc1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38FN3O3/c1-18(33)32-23(13-19-6-8-21(29)9-7-19)25(34)17-30-24-15-28(10-5-11-28)35-26-22(24)12-20(16-31-26)14-27(2,3)4/h6-9,12,16,23-25,30,34H,5,10-11,13-15,17H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99010

(US8497265, 466 | US8497265, 470)Show SMILES COc1ncccc1-c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 Show InChI InChI=1S/C28H30N6O3/c1-36-26-22(6-4-12-29-26)23-15-30-28(33-13-10-19(18-35)11-14-33)32-27(23)37-21-16-34(17-21)25-9-8-20-5-2-3-7-24(20)31-25/h2-9,12,15,19,21,35H,10-11,13-14,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397697
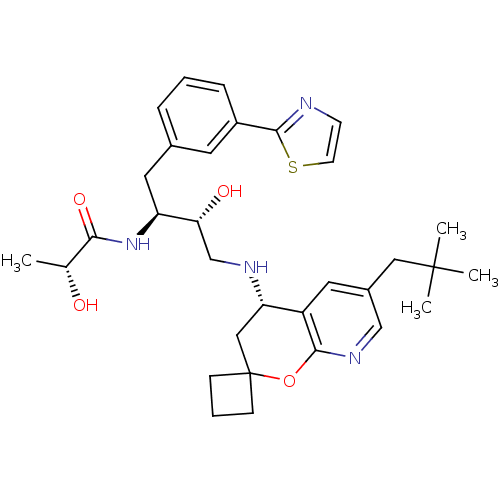
(CHEMBL2181917)Show SMILES C[C@@H](O)C(=O)N[C@@H](Cc1cccc(c1)-c1nccs1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C32H42N4O4S/c1-20(37)28(39)36-25(15-21-7-5-8-23(13-21)30-33-11-12-41-30)27(38)19-34-26-17-32(9-6-10-32)40-29-24(26)14-22(18-35-29)16-31(2,3)4/h5,7-8,11-14,18,20,25-27,34,37-38H,6,9-10,15-17,19H2,1-4H3,(H,36,39)/t20-,25+,26+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98990

(US8497265, 446)Show SMILES Nc1ncc2cc(ccc2n1)-c1nccnc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C24H19N7O/c25-24-28-12-17-11-16(5-7-20(17)30-24)22-23(27-10-9-26-22)32-18-13-31(14-18)21-8-6-15-3-1-2-4-19(15)29-21/h1-12,18H,13-14H2,(H2,25,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99008

(US8497265, 464)Show SMILES COc1cccc(c1)-c1cnc(nc1OC1CN(C1)c1ccc2ccccc2n1)N1CCC(CO)CC1 Show InChI InChI=1S/C29H31N5O3/c1-36-23-7-4-6-22(15-23)25-16-30-29(33-13-11-20(19-35)12-14-33)32-28(25)37-24-17-34(18-24)27-10-9-21-5-2-3-8-26(21)31-27/h2-10,15-16,20,24,35H,11-14,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397688

(CHEMBL2181896)Show SMILES COCC(=O)N[C@@H](Cc1cccc(c1)C#C)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C31H41N3O4/c1-6-21-9-7-10-22(13-21)15-25(34-28(36)20-37-5)27(35)19-32-26-17-31(11-8-12-31)38-29-24(26)14-23(18-33-29)16-30(2,3)4/h1,7,9-10,13-14,18,25-27,32,35H,8,11-12,15-17,19-20H2,2-5H3,(H,34,36)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397706

(CHEMBL2181908)Show SMILES CC(=O)N[C@@H](Cc1cccc(c1)-c1nccs1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C31H40N4O3S/c1-20(36)35-25(15-21-7-5-8-23(13-21)29-32-11-12-39-29)27(37)19-33-26-17-31(9-6-10-31)38-28-24(26)14-22(18-34-28)16-30(2,3)4/h5,7-8,11-14,18,25-27,33,37H,6,9-10,15-17,19H2,1-4H3,(H,35,36)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397709
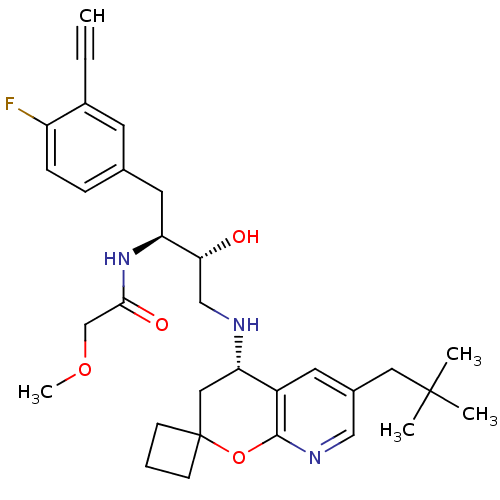
(CHEMBL2181905)Show SMILES COCC(=O)N[C@@H](Cc1ccc(F)c(c1)C#C)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C31H40FN3O4/c1-6-22-12-20(8-9-24(22)32)14-25(35-28(37)19-38-5)27(36)18-33-26-16-31(10-7-11-31)39-29-23(26)13-21(17-34-29)15-30(2,3)4/h1,8-9,12-13,17,25-27,33,36H,7,10-11,14-16,18-19H2,2-5H3,(H,35,37)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383845
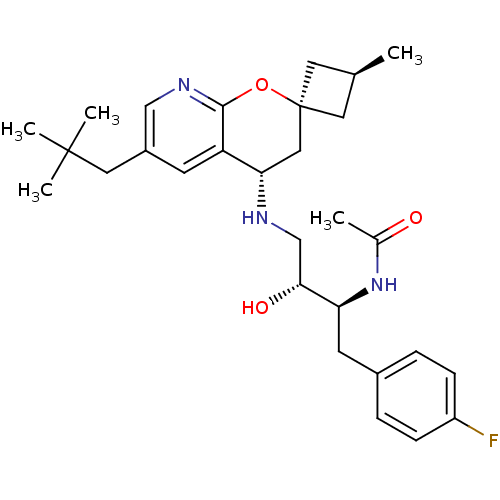
(CHEMBL2031141)Show SMILES C[C@H]1C[C@@]2(C1)C[C@H](NC[C@@H](O)[C@H](Cc1ccc(F)cc1)NC(C)=O)c1cc(CC(C)(C)C)cnc1O2 |r,wU:6.7,wD:9.10,11.21,3.2,1.0,(16.09,-18.99,;15,-20.08,;13.46,-20.08,;13.46,-21.62,;15,-21.62,;12.11,-20.86,;10.77,-21.64,;9.43,-20.87,;8.1,-21.65,;6.77,-20.88,;6.76,-19.34,;5.44,-21.66,;5.44,-23.2,;4.11,-23.97,;2.78,-23.21,;1.45,-23.98,;1.46,-25.52,;.13,-26.3,;2.8,-26.29,;4.13,-25.51,;4.1,-20.9,;4.09,-19.36,;2.76,-18.59,;5.42,-18.58,;10.78,-23.18,;9.46,-23.95,;9.45,-25.49,;8.12,-26.26,;8.12,-27.8,;6.78,-28.57,;9.45,-28.57,;8.1,-29.34,;10.79,-26.26,;12.12,-25.49,;12.12,-23.95,;13.45,-23.17,)| Show InChI InChI=1S/C29H40FN3O3/c1-18-12-29(13-18)15-25(23-10-21(14-28(3,4)5)16-32-27(23)36-29)31-17-26(35)24(33-19(2)34)11-20-6-8-22(30)9-7-20/h6-10,16,18,24-26,31,35H,11-15,17H2,1-5H3,(H,33,34)/t18-,24-,25-,26+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383838

(CHEMBL2030996)Show SMILES CC(=O)N[C@@H](Cc1cccc(F)c1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38FN3O3/c1-18(33)32-23(13-19-7-5-8-21(29)11-19)25(34)17-30-24-15-28(9-6-10-28)35-26-22(24)12-20(16-31-26)14-27(2,3)4/h5,7-8,11-12,16,23-25,30,34H,6,9-10,13-15,17H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383856
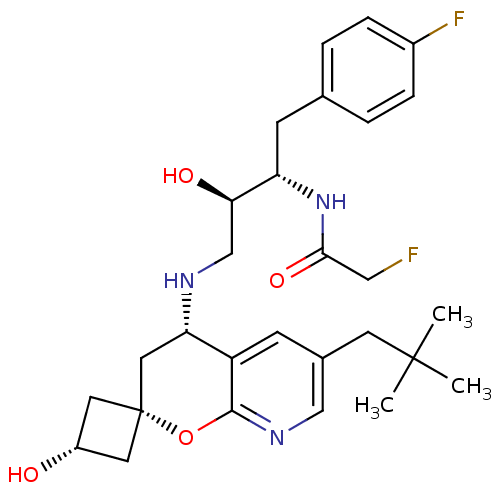
(CHEMBL2031150)Show SMILES CC(C)(C)Cc1cnc2O[C@@]3(C[C@H](O)C3)C[C@H](NC[C@@H](O)[C@H](Cc3ccc(F)cc3)NC(=O)CF)c2c1 |r,wU:16.17,wD:19.20,21.31,10.10,12.12,(-3.79,-4.61,;-2.45,-3.84,;-1.12,-4.61,;-2.47,-5.37,;-2.45,-2.3,;-1.12,-1.53,;.22,-2.3,;1.55,-1.53,;1.55,.02,;2.88,.79,;2.89,2.34,;2.89,3.88,;4.43,3.88,;5.52,4.97,;4.43,2.34,;1.54,3.1,;.2,2.33,;-1.14,3.09,;-2.47,2.32,;-3.8,3.08,;-3.81,4.62,;-5.13,2.3,;-5.13,.76,;-6.46,-.01,;-7.79,.76,;-9.12,-.02,;-9.11,-1.56,;-10.44,-2.34,;-7.77,-2.32,;-6.44,-1.55,;-6.47,3.07,;-6.48,4.61,;-5.15,5.38,;-7.81,5.37,;-7.82,6.91,;.21,.79,;-1.11,.02,)| Show InChI InChI=1S/C28H37F2N3O4/c1-27(2,3)10-18-8-21-23(13-28(11-20(34)12-28)37-26(21)32-15-18)31-16-24(35)22(33-25(36)14-29)9-17-4-6-19(30)7-5-17/h4-8,15,20,22-24,31,34-35H,9-14,16H2,1-3H3,(H,33,36)/t20-,22-,23-,24+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383846

(CHEMBL2031142)Show SMILES C[C@H]1C[C@]2(C1)C[C@H](NC[C@@H](O)[C@H](Cc1ccc(F)c(F)c1)NC(C)=O)c1cc(CC(C)(C)C)cnc1O2 |r,wU:6.7,1.0,wD:9.10,11.22,3.3,(31.56,-19.68,;30.47,-20.77,;30.47,-22.31,;28.93,-22.31,;28.93,-20.77,;27.59,-21.54,;26.25,-22.32,;24.91,-21.56,;23.58,-22.33,;22.25,-21.57,;22.24,-20.03,;20.91,-22.34,;20.92,-23.88,;19.59,-24.66,;19.61,-26.19,;18.28,-26.97,;16.93,-26.21,;15.61,-26.99,;16.93,-24.67,;15.59,-23.9,;18.26,-23.89,;19.58,-21.58,;19.57,-20.04,;18.23,-19.28,;20.9,-19.26,;26.26,-23.86,;24.93,-24.63,;24.93,-26.18,;23.6,-26.95,;23.6,-28.49,;22.26,-29.25,;24.93,-29.26,;23.58,-30.02,;26.27,-26.95,;27.6,-26.18,;27.6,-24.63,;28.93,-23.86,)| Show InChI InChI=1S/C29H39F2N3O3/c1-17-11-29(12-17)14-25(21-8-20(13-28(3,4)5)15-33-27(21)37-29)32-16-26(36)24(34-18(2)35)10-19-6-7-22(30)23(31)9-19/h6-9,15,17,24-26,32,36H,10-14,16H2,1-5H3,(H,34,35)/t17-,24-,25-,26+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397692

(CHEMBL2181892)Show SMILES COCC(=O)N[C@@H](Cc1cccc(c1)-c1nccs1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C32H42N4O4S/c1-31(2,3)16-22-14-24-26(17-32(9-6-10-32)40-29(24)35-18-22)34-19-27(37)25(36-28(38)20-39-4)15-21-7-5-8-23(13-21)30-33-11-12-41-30/h5,7-8,11-14,18,25-27,34,37H,6,9-10,15-17,19-20H2,1-4H3,(H,36,38)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99146

(US8497265, 895)Show SMILES CC(=O)N1CCC(=CC1)c1nccnc1OC1CN(C1)c1ccc2ccccc2n1 |c:6| Show InChI InChI=1S/C23H23N5O2/c1-16(29)27-12-8-18(9-13-27)22-23(25-11-10-24-22)30-19-14-28(15-19)21-7-6-17-4-2-3-5-20(17)26-21/h2-8,10-11,19H,9,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM99026

(US8497265, 659)Show SMILES FC1(F)CCN(CC1)c1nccnc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C21H21F2N5O/c22-21(23)7-11-27(12-8-21)19-20(25-10-9-24-19)29-16-13-28(14-16)18-6-5-15-3-1-2-4-17(15)26-18/h1-6,9-10,16H,7-8,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM98872

(US8497265, 198)Show SMILES OCC1CCN(CC1)c1nccnc1OC1CCN(CC1)c1ccc2ccccc2n1 Show InChI InChI=1S/C24H29N5O2/c30-17-18-7-13-29(14-8-18)23-24(26-12-11-25-23)31-20-9-15-28(16-10-20)22-6-5-19-3-1-2-4-21(19)27-22/h1-6,11-12,18,20,30H,7-10,13-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
Enzyme activity: An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale, Calif). |
US Patent US8497265 (2013)
BindingDB Entry DOI: 10.7270/Q2VD6X39 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM98933

(US8497265, 345)Show SMILES OCC1CCN(CC1)c1cnccc1OC1CN(C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C23H26N4O2/c28-16-17-8-11-26(12-9-17)21-13-24-10-7-22(21)29-19-14-27(15-19)23-6-5-18-3-1-2-4-20(18)25-23/h1-7,10,13,17,19,28H,8-9,11-12,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 using FAM-cAMP substrate by TR-FRET progressive binding assay |
Bioorg Med Chem 22: 6570-85 (2015)
Article DOI: 10.1016/j.bmc.2014.10.013
BindingDB Entry DOI: 10.7270/Q2RB766Q |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383854

(CHEMBL2031148)Show SMILES CC(=O)N[C@@H](Cc1ccc(F)cc1)[C@H](O)CN[C@H]1C[C@]2(C[C@H](O)C2)Oc2ncc(CC(C)(C)C)cc12 |r,wU:17.17,wD:13.14,4.3,19.20,21.22,(16.97,-42.9,;18.31,-43.67,;19.64,-42.89,;18.32,-45.21,;19.65,-45.97,;19.66,-47.51,;18.33,-48.29,;17,-47.52,;15.67,-48.3,;15.67,-49.84,;14.35,-50.62,;17.02,-50.6,;18.35,-49.82,;20.99,-45.2,;20.98,-43.66,;22.32,-45.96,;23.65,-45.18,;24.99,-45.95,;26.33,-45.17,;27.67,-45.93,;27.67,-44.39,;29.21,-44.39,;30.3,-43.31,;29.21,-45.93,;27.67,-47.49,;26.34,-48.26,;26.34,-49.8,;25.01,-50.58,;23.67,-49.81,;22.34,-50.57,;22.34,-52.11,;21,-52.88,;23.67,-52.89,;22.32,-53.65,;23.67,-48.26,;25,-47.49,)| Show InChI InChI=1S/C28H38FN3O4/c1-17(33)32-23(10-18-5-7-20(29)8-6-18)25(35)16-30-24-14-28(12-21(34)13-28)36-26-22(24)9-19(15-31-26)11-27(2,3)4/h5-9,15,21,23-25,30,34-35H,10-14,16H2,1-4H3,(H,32,33)/t21-,23-,24-,25+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human HEK293 cells stably expressing APP |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383839

(CHEMBL2030997)Show SMILES CC(=O)N[C@@H](Cc1ccc(F)cc1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38FN3O3/c1-18(33)32-23(13-19-6-8-21(29)9-7-19)25(34)17-30-24-15-28(10-5-11-28)35-26-22(24)12-20(16-31-26)14-27(2,3)4/h6-9,12,16,23-25,30,34H,5,10-11,13-15,17H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50383857
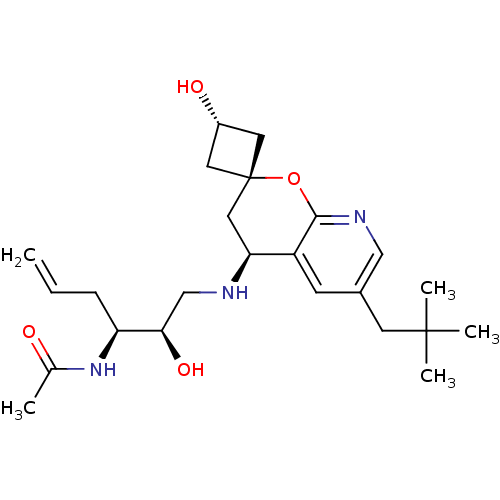
(CHEMBL2031151)Show SMILES CC(=O)N[C@@H](CC=C)[C@H](O)CN[C@H]1C[C@]2(C[C@H](O)C2)Oc2ncc(CC(C)(C)C)cc12 |r,wU:12.11,wD:8.8,4.3,14.14,16.16,(9.62,4.78,;10.96,4.02,;12.29,4.79,;10.97,2.48,;12.3,1.71,;12.31,.17,;10.98,-.6,;9.65,.16,;13.64,2.49,;13.63,4.03,;14.97,1.72,;16.3,2.5,;17.64,1.74,;18.98,2.51,;20.32,1.75,;20.32,3.29,;21.86,3.29,;22.95,4.38,;21.86,1.75,;20.32,.2,;18.99,-.57,;18.99,-2.12,;17.66,-2.89,;16.32,-2.12,;14.99,-2.89,;14.99,-4.43,;13.65,-5.2,;16.32,-5.2,;14.97,-5.97,;16.32,-.58,;17.65,.2,)| Show InChI InChI=1S/C24H37N3O4/c1-6-7-19(27-15(2)28)21(30)14-25-20-12-24(10-17(29)11-24)31-22-18(20)8-16(13-26-22)9-23(3,4)5/h6,8,13,17,19-21,25,29-30H,1,7,9-12,14H2,2-5H3,(H,27,28)/t17-,19-,20-,21+,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50463853
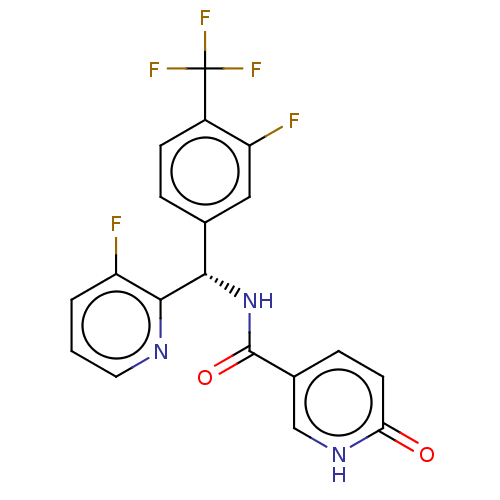
(CHEMBL4237618)Show SMILES Fc1cccnc1[C@@H](NC(=O)c1ccc(=O)[nH]c1)c1ccc(c(F)c1)C(F)(F)F |r| Show InChI InChI=1S/C19H12F5N3O2/c20-13-2-1-7-25-17(13)16(27-18(29)11-4-6-15(28)26-9-11)10-3-5-12(14(21)8-10)19(22,23)24/h1-9,16H,(H,26,28)(H,27,29)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in CHO cells assessed as inhibition of icilin-induced intracellular calcium levels preincubated for 2.5 ... |
J Med Chem 61: 8186-8201 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00518
BindingDB Entry DOI: 10.7270/Q2TH8QCW |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383841

(CHEMBL2031000)Show SMILES C[C@H]1CC[C@]11C[C@H](NC[C@@H](O)[C@H](Cc2ccc(F)cc2)NC(C)=O)c2cc(CC(C)(C)C)cnc2O1 |r| Show InChI InChI=1S/C29H40FN3O3/c1-18-10-11-29(18)15-25(23-12-21(14-28(3,4)5)16-32-27(23)36-29)31-17-26(35)24(33-19(2)34)13-20-6-8-22(30)9-7-20/h6-9,12,16,18,24-26,31,35H,10-11,13-15,17H2,1-5H3,(H,33,34)/t18-,24-,25-,26+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50397691
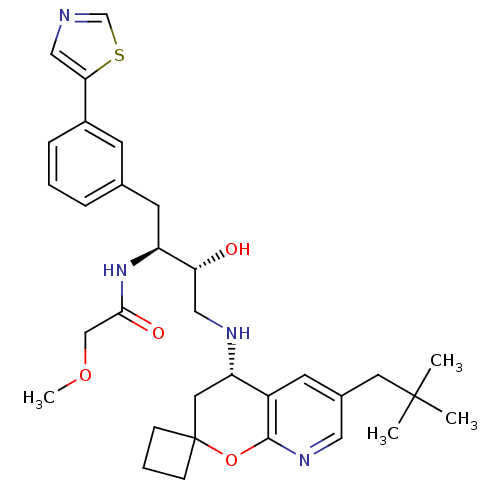
(CHEMBL2181893)Show SMILES COCC(=O)N[C@@H](Cc1cccc(c1)-c1cncs1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C32H42N4O4S/c1-31(2,3)14-22-12-24-26(15-32(9-6-10-32)40-30(24)35-16-22)34-17-27(37)25(36-29(38)19-39-4)13-21-7-5-8-23(11-21)28-18-33-20-41-28/h5,7-8,11-12,16,18,20,25-27,34,37H,6,9-10,13-15,17,19H2,1-4H3,(H,36,38)/t25-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... |
J Med Chem 55: 9025-44 (2012)
Article DOI: 10.1021/jm300118s
BindingDB Entry DOI: 10.7270/Q298885R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data