 Found 209 hits with Last Name = 'houghton' and Initial = 'j'
Found 209 hits with Last Name = 'houghton' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15186
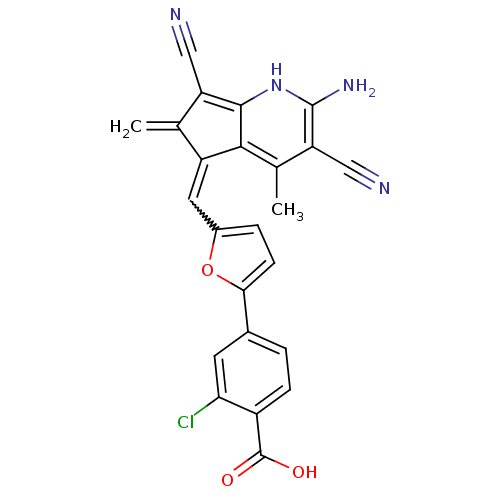
(4-(5-{[(5E)-2-amino-3,7-dicyano-4,6-dimethyl-5H-cy...)Show SMILES Cc1c(C#N)c(N)[nH]c2c(C#N)c(=C)c(=Cc3ccc(o3)-c3ccc(C(O)=O)c(Cl)c3)c12 |w:15.15| Show InChI InChI=1S/C24H15ClN4O3/c1-11-16(21-12(2)18(10-27)23(28)29-22(21)17(11)9-26)8-14-4-6-20(32-14)13-3-5-15(24(30)31)19(25)7-13/h3-8,29H,1,28H2,2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | -34.0 | 2.60E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
The Burnham Institute
| Assay Description
The Z-LYTE assay (Invitrogen Corporation) employs a FRET-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated ... |
J Med Chem 48: 2278-81 (2005)
Article DOI: 10.1021/jm048962u
BindingDB Entry DOI: 10.7270/Q2KD1W4Z |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15187
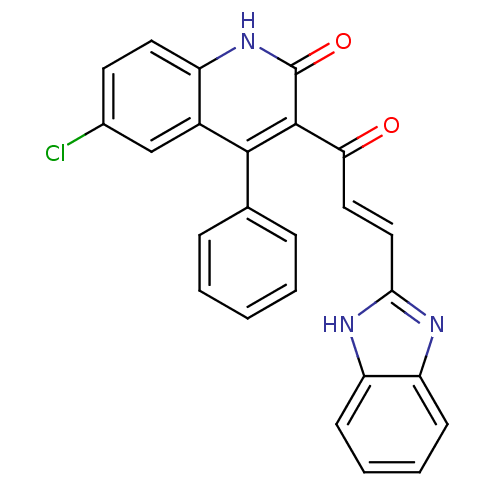
(3-[(2E)-3-(1H-1,3-benzodiazol-2-yl)prop-2-enoyl]-6...)Show SMILES Clc1ccc2[nH]c(=O)c(C(=O)\C=C\c3nc4ccccc4[nH]3)c(-c3ccccc3)c2c1 Show InChI InChI=1S/C25H16ClN3O2/c26-16-10-11-18-17(14-16)23(15-6-2-1-3-7-15)24(25(31)29-18)21(30)12-13-22-27-19-8-4-5-9-20(19)28-22/h1-14H,(H,27,28)(H,29,31)/b13-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.90E+3 | -30.9 | 4.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
The Burnham Institute
| Assay Description
The Z-LYTE assay (Invitrogen Corporation) employs a FRET-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated ... |
J Med Chem 48: 2278-81 (2005)
Article DOI: 10.1021/jm048962u
BindingDB Entry DOI: 10.7270/Q2KD1W4Z |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50228778

(4,4''-Dihydroxyoctafluoroazobenzene | CHEMBL79036)Show SMILES Oc1c(F)c(F)c(\N=N\c2c(F)c(F)c(O)c(F)c2F)c(F)c1F Show InChI InChI=1S/C12H2F8N2O2/c13-1-5(17)11(23)6(18)2(14)9(1)21-22-10-3(15)7(19)12(24)8(20)4(10)16/h23-24H/b22-21+ | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding constant of Testosterone-5 alpha-reductase activity at pH 7.4 |
J Med Chem 33: 2452-5 (1990)
BindingDB Entry DOI: 10.7270/Q2ZS2VGM |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50015844
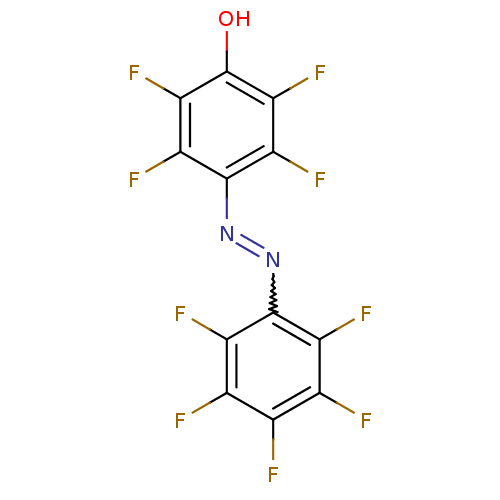
(2,3,5,6-Tetrafluoro-4-pentafluorophenylazo-phenol ...)Show SMILES Oc1c(F)c(F)c(N=Nc2c(F)c(F)c(F)c(F)c2F)c(F)c1F |w:8.8| Show InChI InChI=1S/C12HF9N2O/c13-1-2(14)4(16)10(5(17)3(1)15)22-23-11-6(18)8(20)12(24)9(21)7(11)19/h24H | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 17-alpha-hydroxylase/17,20 lyase from rat testes microsomal preparation |
J Med Chem 33: 2452-5 (1990)
BindingDB Entry DOI: 10.7270/Q2ZS2VGM |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15188
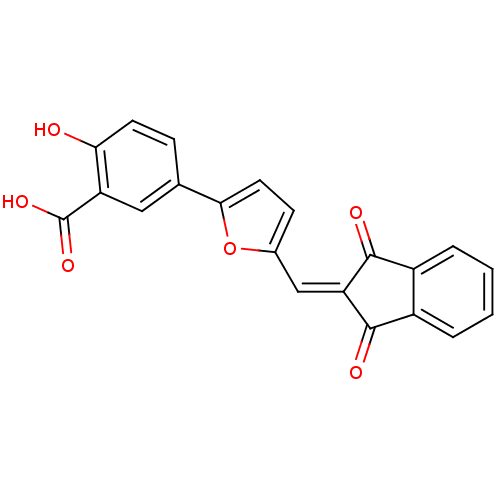
(5-{5-[(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)me...)Show SMILES [#8]-[#6](=O)-c1cc(ccc1-[#8])-c1ccc(\[#6]=[#6]-2/[#6](=O)-c3ccccc3-[#6]-2=O)o1 Show InChI InChI=1S/C21H12O6/c22-17-7-5-11(9-15(17)21(25)26)18-8-6-12(27-18)10-16-19(23)13-3-1-2-4-14(13)20(16)24/h1-10,22H,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.08E+4 | -26.7 | 2.51E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
The Burnham Institute
| Assay Description
The Z-LYTE assay (Invitrogen Corporation) employs a FRET-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated ... |
J Med Chem 48: 2278-81 (2005)
Article DOI: 10.1021/jm048962u
BindingDB Entry DOI: 10.7270/Q2KD1W4Z |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052668
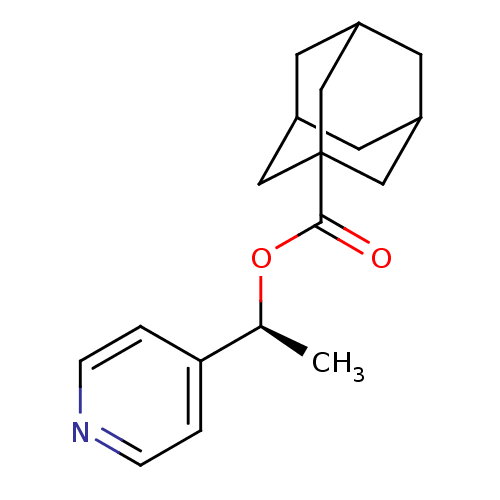
(Adamantane-1-carboxylic acid (S)-1-pyridin-4-yl-et...)Show SMILES C[C@H](OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:12:7:14:13.11.10,12:11:14:6.7.8,THB:10:11:6:14.9.8,10:9:6:13.11.12| Show InChI InChI=1S/C18H23NO2/c1-12(16-2-4-19-5-3-16)21-17(20)18-9-13-6-14(10-18)8-15(7-13)11-18/h2-5,12-15H,6-11H2,1H3/t12-,13?,14?,15?,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052669
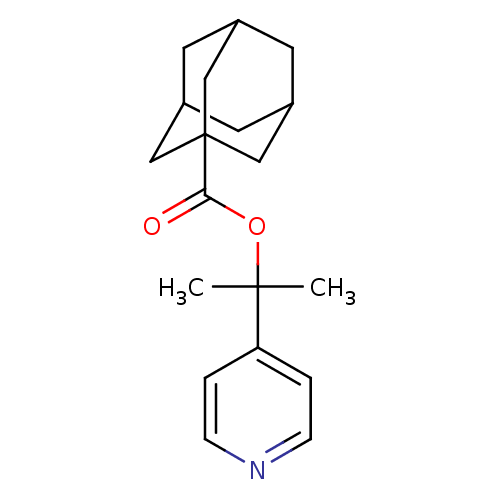
(Adamantane-1-carboxylic acid 1-methyl-1-pyridin-4-...)Show SMILES CC(C)(OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:9:10:14:7.8.13,13:8:15:14.12.11,13:12:15:7.8.9,THB:9:8:14:15.10.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-3-5-20-6-4-16)22-17(21)19-10-13-7-14(11-19)9-15(8-13)12-19/h3-6,13-15H,7-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052668

(Adamantane-1-carboxylic acid (S)-1-pyridin-4-yl-et...)Show SMILES C[C@H](OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:12:7:14:13.11.10,12:11:14:6.7.8,THB:10:11:6:14.9.8,10:9:6:13.11.12| Show InChI InChI=1S/C18H23NO2/c1-12(16-2-4-19-5-3-16)21-17(20)18-9-13-6-14(10-18)8-15(7-13)11-18/h2-5,12-15H,6-11H2,1H3/t12-,13?,14?,15?,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029238
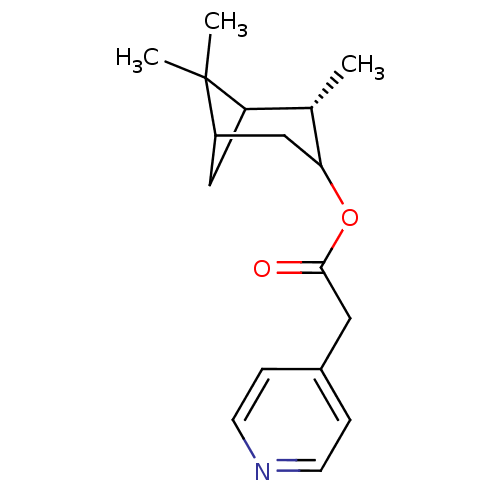
(CHEMBL132072 | Pyridin-4-yl-acetic acid (S)-2,6,6-...)Show SMILES C[C@H]1C2CC(CC1OC(=O)Cc1ccncc1)C2(C)C |TLB:7:6:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-9-13(17(14,2)3)10-15(11)20-16(19)8-12-4-6-18-7-5-12/h4-7,11,13-15H,8-10H2,1-3H3/t11-,13?,14?,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029237
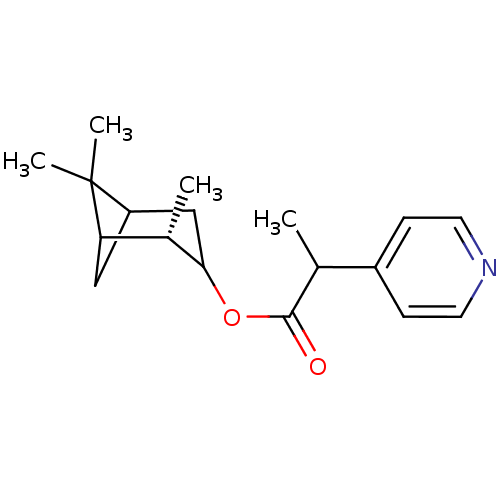
(2-Pyridin-4-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-5-7-19-8-6-13)17(20)21-16-10-14-9-15(12(16)2)18(14,3)4/h5-8,11-12,14-16H,9-10H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029237

(2-Pyridin-4-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-5-7-19-8-6-13)17(20)21-16-10-14-9-15(12(16)2)18(14,3)4/h5-8,11-12,14-16H,9-10H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052669

(Adamantane-1-carboxylic acid 1-methyl-1-pyridin-4-...)Show SMILES CC(C)(OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:9:10:14:7.8.13,13:8:15:14.12.11,13:12:15:7.8.9,THB:9:8:14:15.10.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-3-5-20-6-4-16)22-17(21)19-10-13-7-14(11-19)9-15(8-13)12-19/h3-6,13-15H,7-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029228
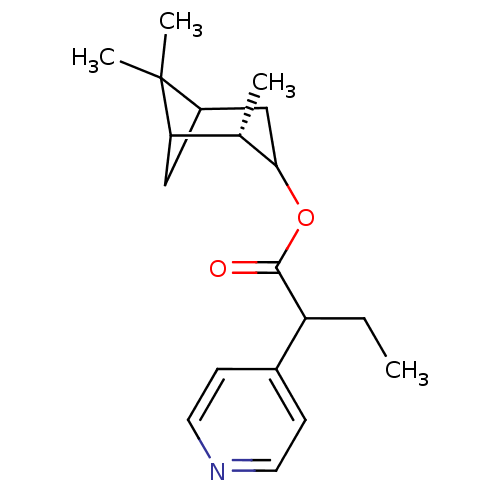
(2-Pyridin-4-yl-butyric acid (S)-2,6,6-trimethyl-bi...)Show SMILES CCC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:5:6:13:9| Show InChI InChI=1S/C19H27NO2/c1-5-15(13-6-8-20-9-7-13)18(21)22-17-11-14-10-16(12(17)2)19(14,3)4/h6-9,12,14-17H,5,10-11H2,1-4H3/t12-,14?,15?,16?,17?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029228

(2-Pyridin-4-yl-butyric acid (S)-2,6,6-trimethyl-bi...)Show SMILES CCC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:5:6:13:9| Show InChI InChI=1S/C19H27NO2/c1-5-15(13-6-8-20-9-7-13)18(21)22-17-11-14-10-16(12(17)2)19(14,3)4/h6-9,12,14-17H,5,10-11H2,1-4H3/t12-,14?,15?,16?,17?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029240
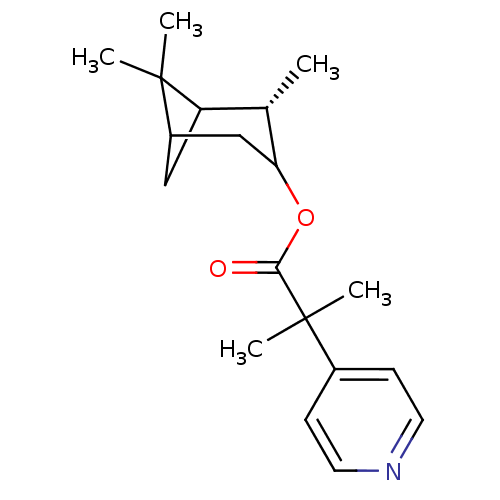
(2-Methyl-2-pyridin-4-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1ccncc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-10-14(18(15,2)3)11-16(12)22-17(21)19(4,5)13-6-8-20-9-7-13/h6-9,12,14-16H,10-11H2,1-5H3/t12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029229
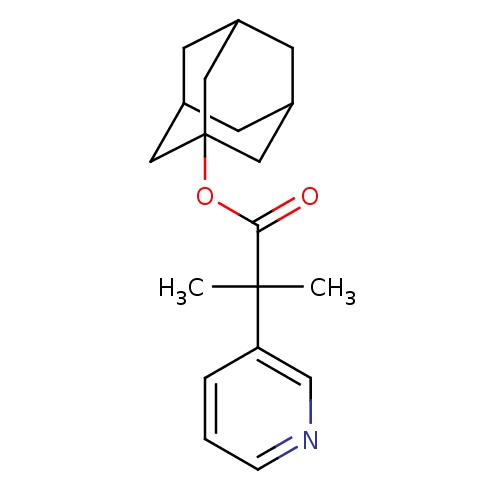
(2-Methyl-2-pyridin-3-yl-propionic acid adamantan-1...)Show SMILES CC(C)(C(=O)OC12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:9:10:14:8.7.13,13:8:15:12.14.11,13:12:15:8.7.9,THB:9:8:14:10.15.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-4-3-5-20-12-16)17(21)22-19-9-13-6-14(10-19)8-15(7-13)11-19/h3-5,12-15H,6-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029229

(2-Methyl-2-pyridin-3-yl-propionic acid adamantan-1...)Show SMILES CC(C)(C(=O)OC12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:9:10:14:8.7.13,13:8:15:12.14.11,13:12:15:8.7.9,THB:9:8:14:10.15.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-4-3-5-20-12-16)17(21)22-19-9-13-6-14(10-19)8-15(7-13)11-19/h3-5,12-15H,6-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029230
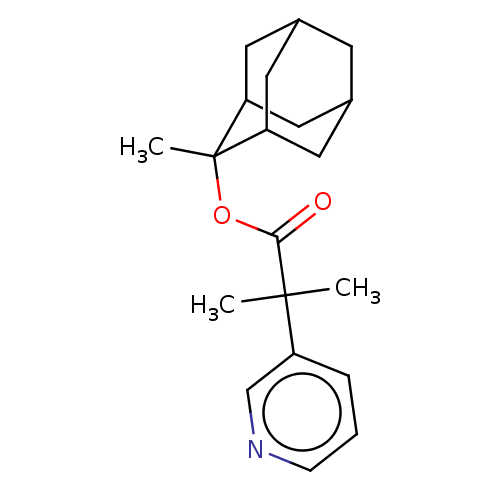
(2-Methyl-2-pyridin-3-yl-propionic acid 2-methyl-ad...)Show SMILES CC(C)(C(=O)OC1(C)C2CC3CC(C2)CC1C3)c1cccnc1 |TLB:7:6:10.9.16:12.13.14,5:6:13:10.9.11,16:15:13:10.9.11,THB:7:6:13:10.9.11,5:6:10.9.16:12.13.14,16:10:13:6.15.14,11:10:6:12.13.14,11:12:6:10.9.16,(4.95,-9.51,;4.66,-11.03,;4.37,-12.54,;6.18,-11.31,;6.68,-12.77,;7.18,-10.15,;8.69,-10.43,;8.91,-11.96,;10.07,-9.74,;10.07,-8.34,;10.91,-6.87,;12.28,-7.56,;12.28,-8.96,;11.44,-10.43,;10.91,-9.66,;9.53,-8.96,;9.53,-7.56,;3.15,-10.74,;2.64,-9.28,;1.13,-9,;.12,-10.16,;.63,-11.62,;2.14,-11.9,)| Show InChI InChI=1S/C20H27NO2/c1-19(2,15-5-4-6-21-12-15)18(22)23-20(3)16-8-13-7-14(10-16)11-17(20)9-13/h4-6,12-14,16-17H,7-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029241
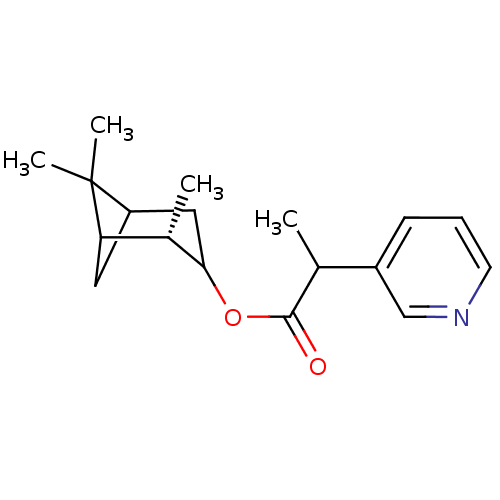
(2-Pyridin-3-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1cccnc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-6-5-7-19-10-13)17(20)21-16-9-14-8-15(12(16)2)18(14,3)4/h5-7,10-12,14-16H,8-9H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029238

(CHEMBL132072 | Pyridin-4-yl-acetic acid (S)-2,6,6-...)Show SMILES C[C@H]1C2CC(CC1OC(=O)Cc1ccncc1)C2(C)C |TLB:7:6:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-9-13(17(14,2)3)10-15(11)20-16(19)8-12-4-6-18-7-5-12/h4-7,11,13-15H,8-10H2,1-3H3/t11-,13?,14?,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular Steroid 17-alpha-hydroxylase/17,20 lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029242

(2-Methyl-2-pyridin-3-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1cccnc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-9-14(18(15,2)3)10-16(12)22-17(21)19(4,5)13-7-6-8-20-11-13/h6-8,11-12,14-16H,9-10H2,1-5H3/t12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029242

(2-Methyl-2-pyridin-3-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1cccnc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-9-14(18(15,2)3)10-16(12)22-17(21)19(4,5)13-7-6-8-20-11-13/h6-8,11-12,14-16H,9-10H2,1-5H3/t12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052671
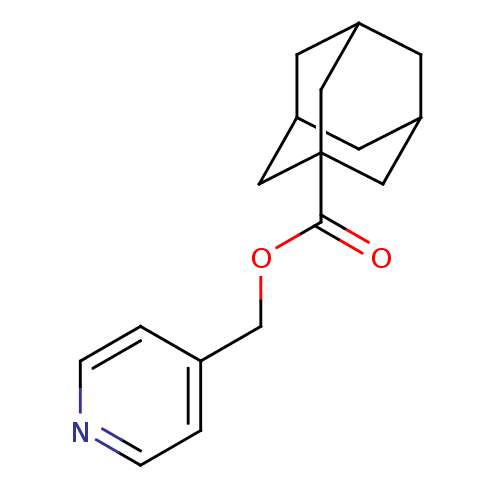
(Adamantane-1-carboxylic acid pyridin-4-ylmethyl es...)Show SMILES O=C(OCc1ccncc1)C12CC3CC(CC(C3)C1)C2 |TLB:13:14:18:11.12.17,THB:13:12:18:19.14.15,15:16:11:19.14.13,15:14:11:18.16.17| Show InChI InChI=1S/C17H21NO2/c19-16(20-11-12-1-3-18-4-2-12)17-8-13-5-14(9-17)7-15(6-13)10-17/h1-4,13-15H,5-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029240

(2-Methyl-2-pyridin-4-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1ccncc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-10-14(18(15,2)3)11-16(12)22-17(21)19(4,5)13-6-8-20-9-7-13/h6-9,12,14-16H,10-11H2,1-5H3/t12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular Steroid 17-alpha-hydroxylase/17,20 lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029226
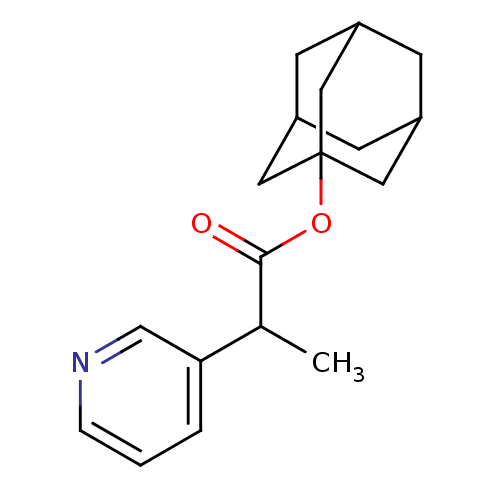
(2-Pyridin-3-yl-propionic acid adamantan-1-yl ester...)Show SMILES CC(C(=O)OC12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:8:9:13:7.6.12,12:7:14:11.13.10,12:11:14:7.6.8,THB:8:7:13:9.14.10| Show InChI InChI=1S/C18H23NO2/c1-12(16-3-2-4-19-11-16)17(20)21-18-8-13-5-14(9-18)7-15(6-13)10-18/h2-4,11-15H,5-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029236
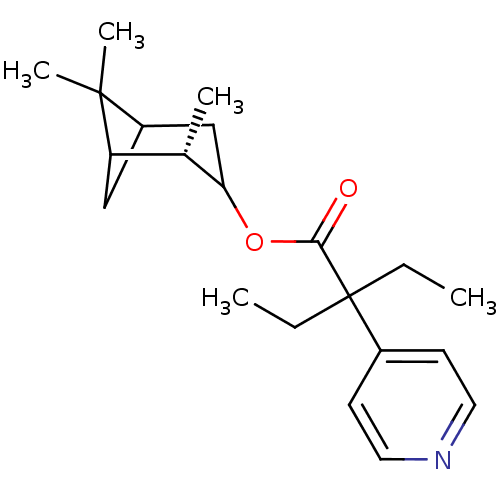
(2-Ethyl-2-pyridin-4-yl-butyric acid (S)-2,6,6-trim...)Show SMILES CCC(CC)(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:7:8:15:11| Show InChI InChI=1S/C21H31NO2/c1-6-21(7-2,15-8-10-22-11-9-15)19(23)24-18-13-16-12-17(14(18)3)20(16,4)5/h8-11,14,16-18H,6-7,12-13H2,1-5H3/t14-,16?,17?,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029226

(2-Pyridin-3-yl-propionic acid adamantan-1-yl ester...)Show SMILES CC(C(=O)OC12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:8:9:13:7.6.12,12:7:14:11.13.10,12:11:14:7.6.8,THB:8:7:13:9.14.10| Show InChI InChI=1S/C18H23NO2/c1-12(16-3-2-4-19-11-16)17(20)21-18-8-13-5-14(9-18)7-15(6-13)10-18/h2-4,11-15H,5-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029227
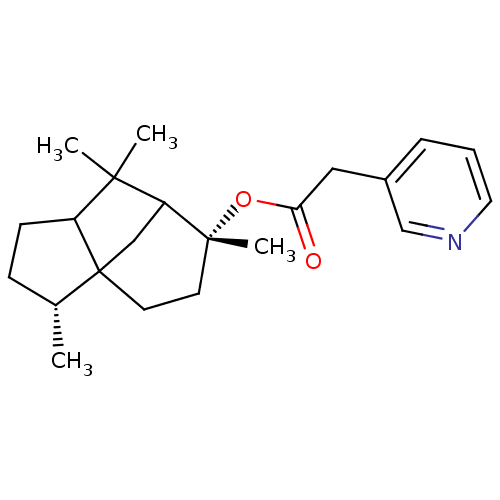
(CHEMBL135095 | Pyridin-3-yl-acetic acid (3R,6R)-3,...)Show SMILES C[C@@H]1CCC2C(C)(C)C3CC12CC[C@@]3(C)OC(=O)Cc1cccnc1 |TLB:15:13:5.4:9,3:4:9:13.11.12,THB:14:13:5.4:9| Show InChI InChI=1S/C22H31NO2/c1-15-7-8-17-20(2,3)18-13-22(15,17)10-9-21(18,4)25-19(24)12-16-6-5-11-23-14-16/h5-6,11,14-15,17-18H,7-10,12-13H2,1-4H3/t15-,17?,18?,21-,22?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029227

(CHEMBL135095 | Pyridin-3-yl-acetic acid (3R,6R)-3,...)Show SMILES C[C@@H]1CCC2C(C)(C)C3CC12CC[C@@]3(C)OC(=O)Cc1cccnc1 |TLB:15:13:5.4:9,3:4:9:13.11.12,THB:14:13:5.4:9| Show InChI InChI=1S/C22H31NO2/c1-15-7-8-17-20(2,3)18-13-22(15,17)10-9-21(18,4)25-19(24)12-16-6-5-11-23-14-16/h5-6,11,14-15,17-18H,7-10,12-13H2,1-4H3/t15-,17?,18?,21-,22?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052671

(Adamantane-1-carboxylic acid pyridin-4-ylmethyl es...)Show SMILES O=C(OCc1ccncc1)C12CC3CC(CC(C3)C1)C2 |TLB:13:14:18:11.12.17,THB:13:12:18:19.14.15,15:16:11:19.14.13,15:14:11:18.16.17| Show InChI InChI=1S/C17H21NO2/c19-16(20-11-12-1-3-18-4-2-12)17-8-13-5-14(9-17)7-15(6-13)10-17/h1-4,13-15H,5-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50029242

(2-Methyl-2-pyridin-3-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1cccnc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-9-14(18(15,2)3)10-16(12)22-17(21)19(4,5)13-7-6-8-20-11-13/h6-8,11-12,14-16H,9-10H2,1-5H3/t12-,14?,15?,16?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular Steroid 17-alpha-hydroxylase/17,20 lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50029242

(2-Methyl-2-pyridin-3-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1cccnc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-9-14(18(15,2)3)10-16(12)22-17(21)19(4,5)13-7-6-8-20-11-13/h6-8,11-12,14-16H,9-10H2,1-5H3/t12-,14?,15?,16?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular C17,20-Lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029232
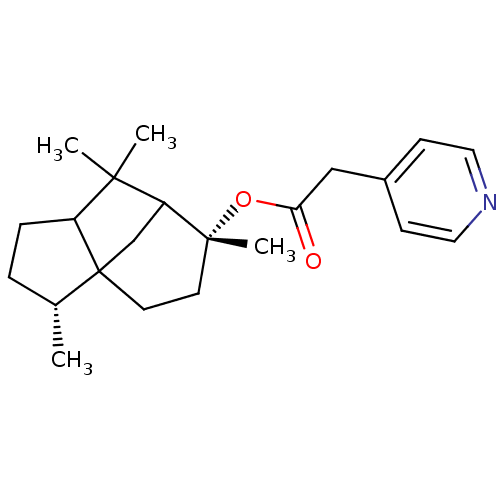
(CHEMBL134442 | Pyridin-4-yl-acetic acid (3R,6R)-3,...)Show SMILES C[C@@H]1CCC2C(C)(C)C3CC12CC[C@@]3(C)OC(=O)Cc1ccncc1 |TLB:15:13:5.4:9,3:4:9:13.11.12,THB:14:13:5.4:9| Show InChI InChI=1S/C22H31NO2/c1-15-5-6-17-20(2,3)18-14-22(15,17)10-9-21(18,4)25-19(24)13-16-7-11-23-12-8-16/h7-8,11-12,15,17-18H,5-6,9-10,13-14H2,1-4H3/t15-,17?,18?,21-,22?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular Steroid 17-alpha-hydroxylase/17,20 lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular Steroid 17-alpha-hydroxylase/17,20 lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052672
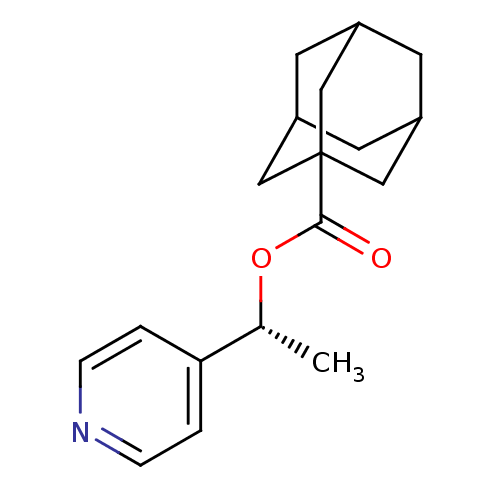
(Adamantane-1-carboxylic acid (R)-1-pyridin-4-yl-et...)Show SMILES C[C@@H](OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:12:7:14:13.11.10,12:11:14:6.7.8,THB:10:11:6:14.9.8,10:9:6:13.11.12| Show InChI InChI=1S/C18H23NO2/c1-12(16-2-4-19-5-3-16)21-17(20)18-9-13-6-14(10-18)8-15(7-13)11-18/h2-5,12-15H,6-11H2,1H3/t12-,13?,14?,15?,18?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50029230

(2-Methyl-2-pyridin-3-yl-propionic acid 2-methyl-ad...)Show SMILES CC(C)(C(=O)OC1(C)C2CC3CC(C2)CC1C3)c1cccnc1 |TLB:7:6:10.9.16:12.13.14,5:6:13:10.9.11,16:15:13:10.9.11,THB:7:6:13:10.9.11,5:6:10.9.16:12.13.14,16:10:13:6.15.14,11:10:6:12.13.14,11:12:6:10.9.16,(4.95,-9.51,;4.66,-11.03,;4.37,-12.54,;6.18,-11.31,;6.68,-12.77,;7.18,-10.15,;8.69,-10.43,;8.91,-11.96,;10.07,-9.74,;10.07,-8.34,;10.91,-6.87,;12.28,-7.56,;12.28,-8.96,;11.44,-10.43,;10.91,-9.66,;9.53,-8.96,;9.53,-7.56,;3.15,-10.74,;2.64,-9.28,;1.13,-9,;.12,-10.16,;.63,-11.62,;2.14,-11.9,)| Show InChI InChI=1S/C20H27NO2/c1-19(2,15-5-4-6-21-12-15)18(22)23-20(3)16-8-13-7-14(10-16)11-17(20)9-13/h4-6,12-14,16-17H,7-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human placental Cytochrome P450 19A1 |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029230

(2-Methyl-2-pyridin-3-yl-propionic acid 2-methyl-ad...)Show SMILES CC(C)(C(=O)OC1(C)C2CC3CC(C2)CC1C3)c1cccnc1 |TLB:7:6:10.9.16:12.13.14,5:6:13:10.9.11,16:15:13:10.9.11,THB:7:6:13:10.9.11,5:6:10.9.16:12.13.14,16:10:13:6.15.14,11:10:6:12.13.14,11:12:6:10.9.16,(4.95,-9.51,;4.66,-11.03,;4.37,-12.54,;6.18,-11.31,;6.68,-12.77,;7.18,-10.15,;8.69,-10.43,;8.91,-11.96,;10.07,-9.74,;10.07,-8.34,;10.91,-6.87,;12.28,-7.56,;12.28,-8.96,;11.44,-10.43,;10.91,-9.66,;9.53,-8.96,;9.53,-7.56,;3.15,-10.74,;2.64,-9.28,;1.13,-9,;.12,-10.16,;.63,-11.62,;2.14,-11.9,)| Show InChI InChI=1S/C20H27NO2/c1-19(2,15-5-4-6-21-12-15)18(22)23-20(3)16-8-13-7-14(10-16)11-17(20)9-13/h4-6,12-14,16-17H,7-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029241

(2-Pyridin-3-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1cccnc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-6-5-7-19-10-13)17(20)21-16-9-14-8-15(12(16)2)18(14,3)4/h5-7,10-12,14-16H,8-9H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular Steroid 17-alpha-hydroxylase/17,20 lyase |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029239

(CHEMBL131984 | Pyridin-3-yl-acetic acid (S)-2,6,6-...)Show SMILES C[C@H]1C2CC(CC1OC(=O)Cc1cccnc1)C2(C)C |TLB:7:6:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-8-13(17(14,2)3)9-15(11)20-16(19)7-12-5-4-6-18-10-12/h4-6,10-11,13-15H,7-9H2,1-3H3/t11-,13?,14?,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014462
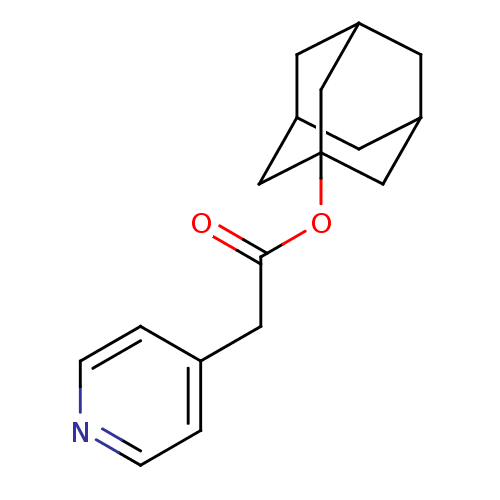
(CHEMBL104047 | Pyridin-4-yl-acetic acid adamantan-...)Show SMILES O=C(Cc1ccncc1)OC12CC3CC(CC(C3)C1)C2 |THB:18:10:13:16.17.15,18:16:10.11.19:13,15:16:11:14.19.13,15:14:11:16.18.17| Show InChI InChI=1S/C17H21NO2/c19-16(8-12-1-3-18-4-2-12)20-17-9-13-5-14(10-17)7-15(6-13)11-17/h1-4,13-15H,5-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A1. |
J Med Chem 33: 3050-5 (1990)
BindingDB Entry DOI: 10.7270/Q23N22CM |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052666
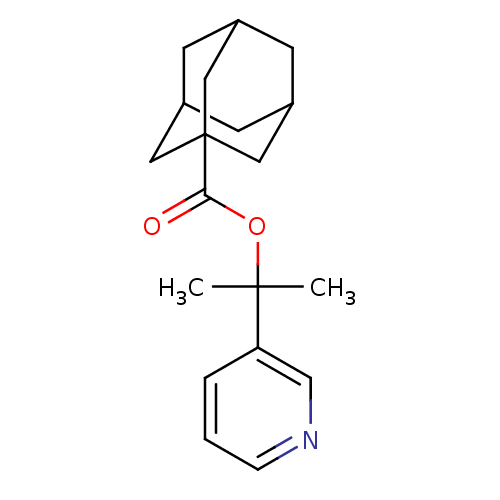
(Adamantane-1-carboxylic acid 1-methyl-1-pyridin-3-...)Show SMILES CC(C)(OC(=O)C12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:9:10:14:7.8.13,13:8:15:14.12.11,13:12:15:7.8.9,THB:9:8:14:15.10.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-4-3-5-20-12-16)22-17(21)19-9-13-6-14(10-19)8-15(7-13)11-19/h3-5,12-15H,6-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014449

((1R,2R,3R,5S)-(-)-Pyridin-4-yl-acetic acid 2,6,6-t...)Show SMILES CC1C2CC(CC1OC(=O)Cc1ccncc1)C2(C)C |TLB:7:6:17:3,THB:0:1:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-9-13(17(14,2)3)10-15(11)20-16(19)8-12-4-6-18-7-5-12/h4-7,11,13-15H,8-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A1. |
J Med Chem 33: 3050-5 (1990)
BindingDB Entry DOI: 10.7270/Q23N22CM |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014449

((1R,2R,3R,5S)-(-)-Pyridin-4-yl-acetic acid 2,6,6-t...)Show SMILES CC1C2CC(CC1OC(=O)Cc1ccncc1)C2(C)C |TLB:7:6:17:3,THB:0:1:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-9-13(17(14,2)3)10-15(11)20-16(19)8-12-4-6-18-7-5-12/h4-7,11,13-15H,8-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of rat testicular Cytochrome P450 steroid 17alpha-hydroxylase/17,20 lyase was determined |
J Med Chem 33: 3050-5 (1990)
BindingDB Entry DOI: 10.7270/Q23N22CM |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014456
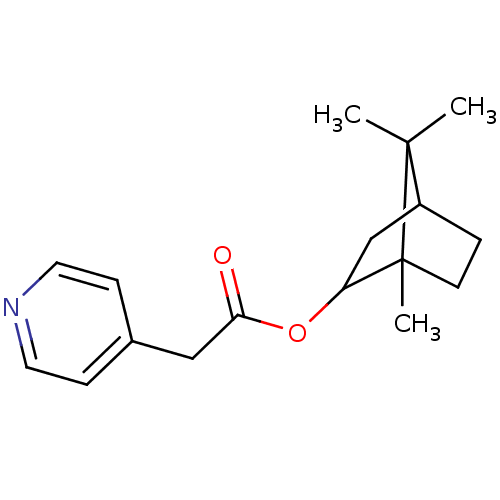
((+)-Pyridin-4-yl-acetic acid 1,7,7-trimethyl-bicyc...)Show InChI InChI=1S/C17H23NO2/c1-16(2)13-4-7-17(16,3)14(11-13)20-15(19)10-12-5-8-18-9-6-12/h5-6,8-9,13-14H,4,7,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of rat testicular Cytochrome P450 steroid 17alpha-hydroxylase/17,20 lyase was determined |
J Med Chem 33: 3050-5 (1990)
BindingDB Entry DOI: 10.7270/Q23N22CM |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014456

((+)-Pyridin-4-yl-acetic acid 1,7,7-trimethyl-bicyc...)Show InChI InChI=1S/C17H23NO2/c1-16(2)13-4-7-17(16,3)14(11-13)20-15(19)10-12-5-8-18-9-6-12/h5-6,8-9,13-14H,4,7,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A1. |
J Med Chem 33: 3050-5 (1990)
BindingDB Entry DOI: 10.7270/Q23N22CM |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014457
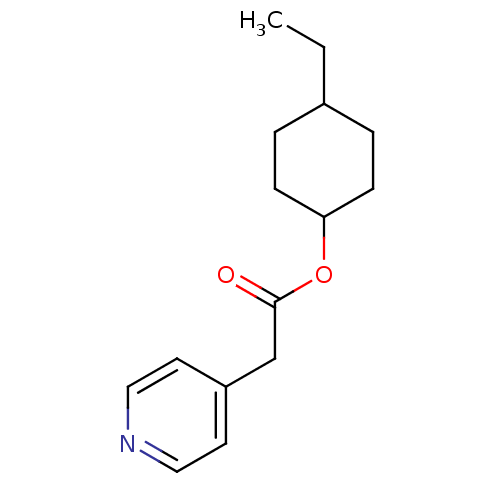
(CHEMBL101743 | Pyridin-4-yl-acetic acid 4-ethyl-cy...)Show SMILES CCC1CCC(CC1)OC(=O)Cc1ccncc1 |(-2.68,-8.66,;-1.35,-7.89,;-.02,-8.68,;-.02,-10.22,;1.31,-11,;2.64,-10.23,;2.64,-8.69,;1.33,-7.92,;3.97,-11,;5.3,-10.23,;5.3,-8.69,;6.63,-11,;7.98,-10.23,;9.31,-11,;10.65,-10.23,;10.65,-8.69,;9.31,-7.92,;7.98,-8.69,)| Show InChI InChI=1S/C15H21NO2/c1-2-12-3-5-14(6-4-12)18-15(17)11-13-7-9-16-10-8-13/h7-10,12,14H,2-6,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A1. |
J Med Chem 33: 3050-5 (1990)
BindingDB Entry DOI: 10.7270/Q23N22CM |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50029238

(CHEMBL132072 | Pyridin-4-yl-acetic acid (S)-2,6,6-...)Show SMILES C[C@H]1C2CC(CC1OC(=O)Cc1ccncc1)C2(C)C |TLB:7:6:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-9-13(17(14,2)3)10-15(11)20-16(19)8-12-4-6-18-7-5-12/h4-7,11,13-15H,8-10H2,1-3H3/t11-,13?,14?,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human placental Cytochrome P450 19A1 |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data