 Found 117 hits with Last Name = 'hovdal' and Initial = 'd'
Found 117 hits with Last Name = 'hovdal' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017
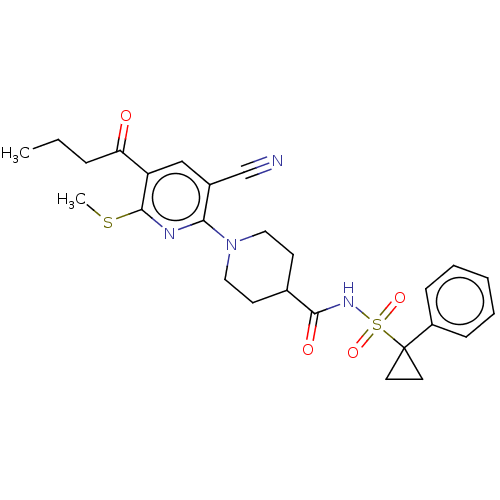
(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced platelet aggregation after 5 to 90 mins by spectro... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595667
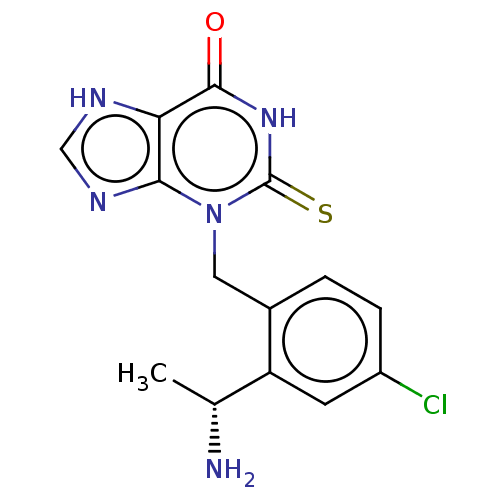
(CHEMBL5181350)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2nc[nH]c2c(=O)[nH]c1=S |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 mins |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595661
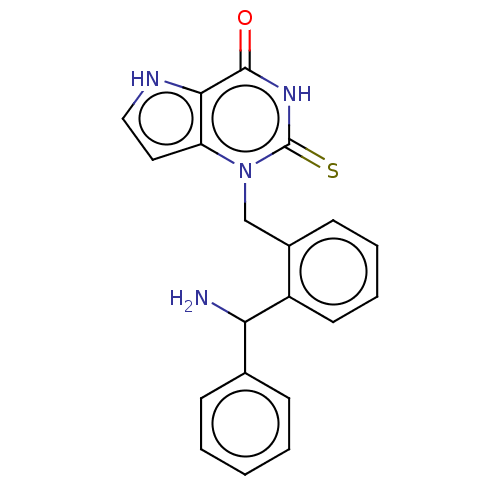
(CHEMBL5197968)Show SMILES NC(c1ccccc1)c1ccccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595661

(CHEMBL5197968)Show SMILES NC(c1ccccc1)c1ccccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277
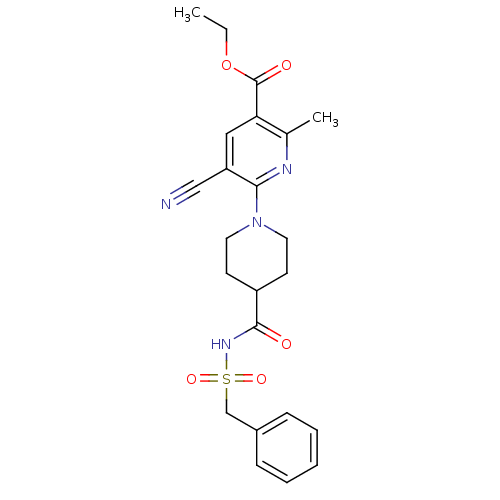
(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 mins |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277

(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced platelet aggregation after 5 to 90 mins by spectro... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595666

(CHEMBL5200126)Show SMILES CN[C@H](C)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277

(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 seconds |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595660

(CHEMBL5185576)Show SMILES O=c1[nH]c(=S)n(Cc2ccccc2CN2CCC2)c2cc[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312244
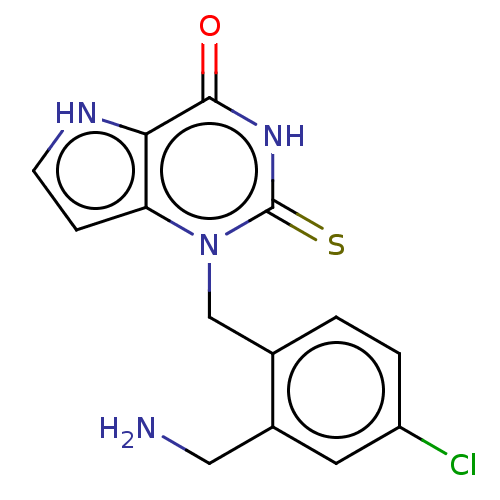
(1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,...)Show SMILES NCc1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C14H13ClN4OS/c15-10-2-1-8(9(5-10)6-16)7-19-11-3-4-17-12(11)13(20)18-14(19)21/h1-5,17H,6-7,16H2,(H,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312244

(1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,...)Show SMILES NCc1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C14H13ClN4OS/c15-10-2-1-8(9(5-10)6-16)7-19-11-3-4-17-12(11)13(20)18-14(19)21/h1-5,17H,6-7,16H2,(H,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312245

(1-{4-Chloro-2-[(methylamino)methyl]benzyl}-2-thiox...)Show SMILES CNCc1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;HN;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-17-7-10-6-11(16)3-2-9(10)8-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,17-18H,7-8H2,1H3,(H,19,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019019
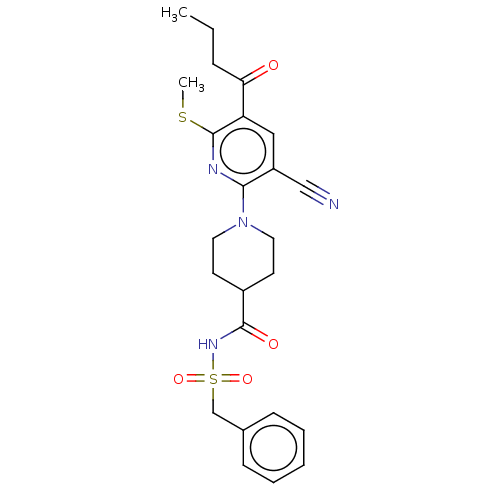
(CHEMBL3288127)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C24H28N4O4S2/c1-3-7-21(29)20-14-19(15-25)22(26-24(20)33-2)28-12-10-18(11-13-28)23(30)27-34(31,32)16-17-8-5-4-6-9-17/h4-6,8-9,14,18H,3,7,10-13,16H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277

(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 30 seconds |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 seconds |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277

(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312173
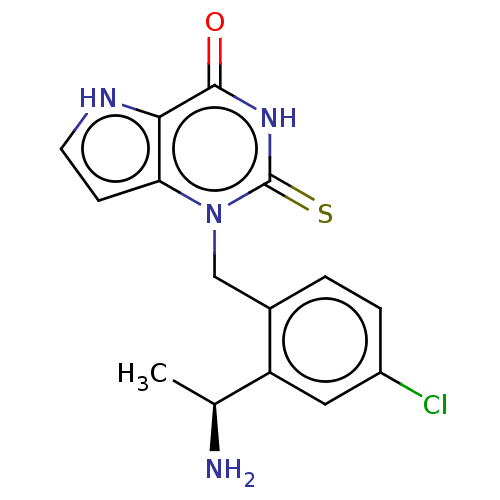
(1-{2-[(1S)-1-Aminoethyl]-4-chlorobenzyl}-2-thioxo-...)Show SMILES C[C@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595665
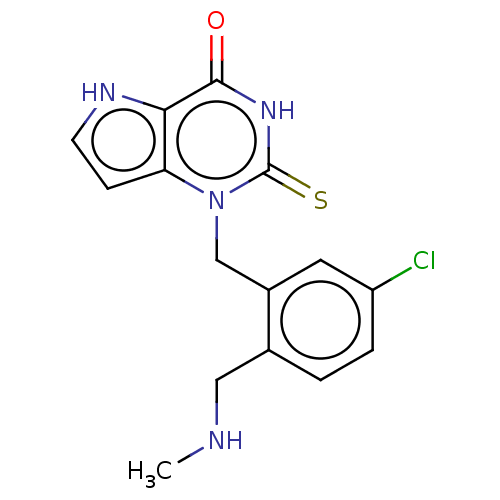
(CHEMBL5186129) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312261

(1-{2-[(Propan-2-ylamino)methyl]benzyl}-2-thioxo-1,...)Show SMILES CC(C)NCc1ccccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;;;HN;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C17H20N4OS/c1-11(2)19-9-12-5-3-4-6-13(12)10-21-14-7-8-18-15(14)16(22)20-17(21)23/h3-8,11,18-19H,9-10H2,1-2H3,(H,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM92467
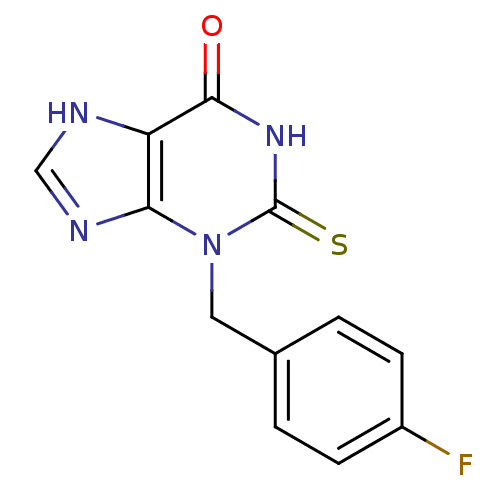
(2-Thioxanthine, TX2)Show InChI InChI=1S/C12H9FN4OS/c13-8-3-1-7(2-4-8)5-17-10-9(14-6-15-10)11(18)16-12(17)19/h1-4,6H,5H2,(H,14,15)(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM92467

(2-Thioxanthine, TX2)Show InChI InChI=1S/C12H9FN4OS/c13-8-3-1-7(2-4-8)5-17-10-9(14-6-15-10)11(18)16-12(17)19/h1-4,6H,5H2,(H,14,15)(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595662
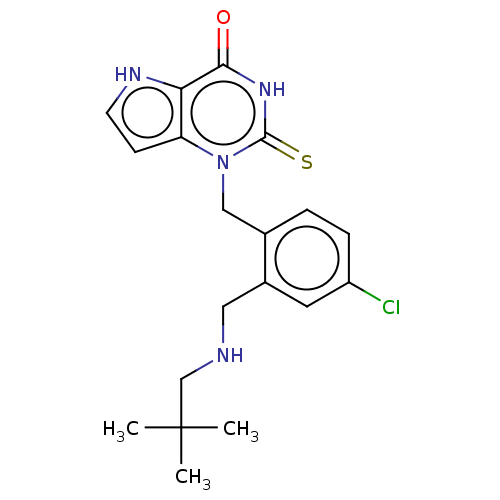
(CHEMBL5197383)Show SMILES CC(C)(C)CNCc1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595664

(CHEMBL5207346) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595659

(CHEMBL5181827) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 30 seconds |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019020
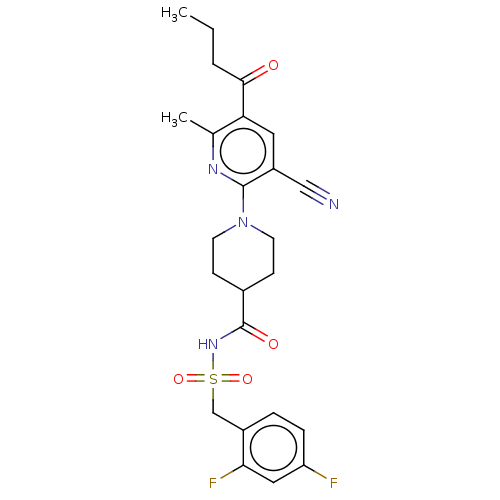
(CHEMBL3288126)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccc(F)cc1F Show InChI InChI=1S/C24H26F2N4O4S/c1-3-4-22(31)20-11-18(13-27)23(28-15(20)2)30-9-7-16(8-10-30)24(32)29-35(33,34)14-17-5-6-19(25)12-21(17)26/h5-6,11-12,16H,3-4,7-10,14H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277

(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 10 seconds |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019021
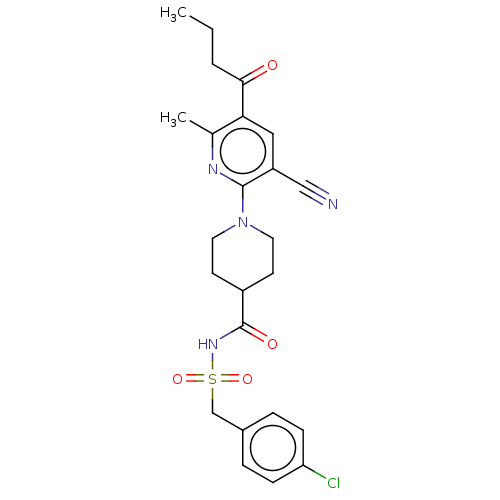
(CHEMBL3288129)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C24H27ClN4O4S/c1-3-4-22(30)21-13-19(14-26)23(27-16(21)2)29-11-9-18(10-12-29)24(31)28-34(32,33)15-17-5-7-20(25)8-6-17/h5-8,13,18H,3-4,9-12,15H2,1-2H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019023
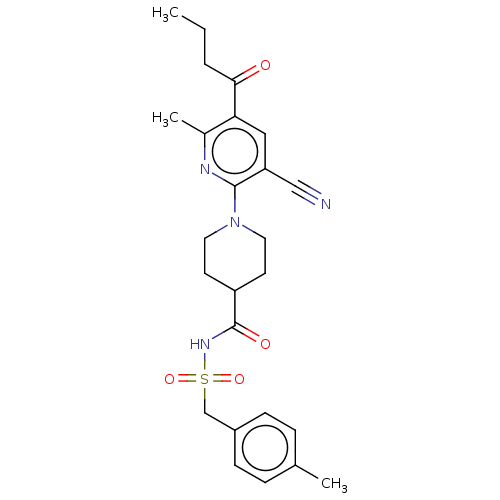
(CHEMBL3288128)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccc(C)cc1 Show InChI InChI=1S/C25H30N4O4S/c1-4-5-23(30)22-14-21(15-26)24(27-18(22)3)29-12-10-20(11-13-29)25(31)28-34(32,33)16-19-8-6-17(2)7-9-19/h6-9,14,20H,4-5,10-13,16H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019024
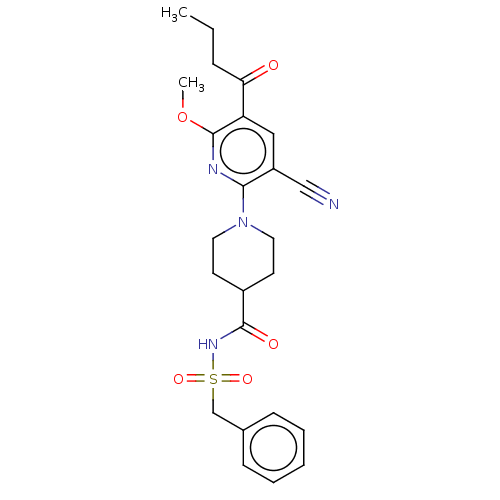
(CHEMBL3288132)Show SMILES CCCC(=O)c1cc(C#N)c(nc1OC)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C24H28N4O5S/c1-3-7-21(29)20-14-19(15-25)22(26-24(20)33-2)28-12-10-18(11-13-28)23(30)27-34(31,32)16-17-8-5-4-6-9-17/h4-6,8-9,14,18H,3,7,10-13,16H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019025

(CHEMBL3288131)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C(F)F)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C24H26F2N4O4S/c1-2-6-20(31)19-13-18(14-27)23(28-21(19)22(25)26)30-11-9-17(10-12-30)24(32)29-35(33,34)15-16-7-4-3-5-8-16/h3-5,7-8,13,17,22H,2,6,9-12,15H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019026

(CHEMBL3288130)Show SMILES CCCC(=O)c1cc(C#N)c(nc1CF)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C24H27FN4O4S/c1-2-6-22(30)20-13-19(15-26)23(27-21(20)14-25)29-11-9-18(10-12-29)24(31)28-34(32,33)16-17-7-4-3-5-8-17/h3-5,7-8,13,18H,2,6,9-12,14,16H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019027
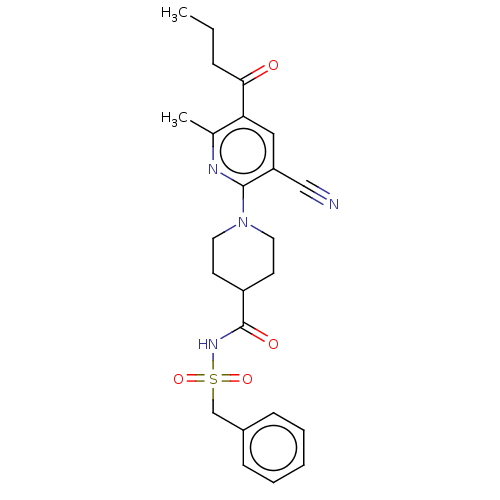
(CHEMBL3288134)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C24H28N4O4S/c1-3-7-22(29)21-14-20(15-25)23(26-17(21)2)28-12-10-19(11-13-28)24(30)27-33(31,32)16-18-8-5-4-6-9-18/h4-6,8-9,14,19H,3,7,10-13,16H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 10 seconds |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019028
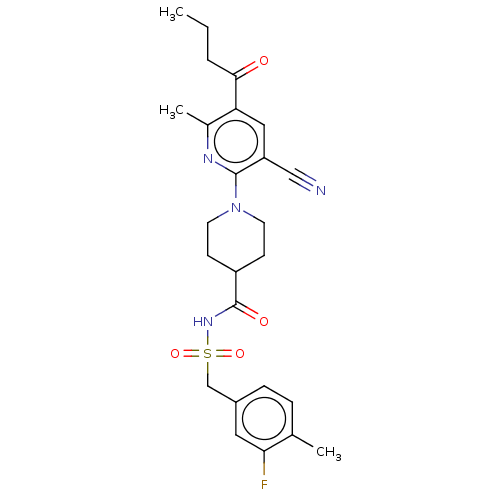
(CHEMBL3288125)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccc(C)c(F)c1 Show InChI InChI=1S/C25H29FN4O4S/c1-4-5-23(31)21-13-20(14-27)24(28-17(21)3)30-10-8-19(9-11-30)25(32)29-35(33,34)15-18-7-6-16(2)22(26)12-18/h6-7,12-13,19H,4-5,8-11,15H2,1-3H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595663

(CHEMBL5175091)Show SMILES CC(=O)NCc1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019029
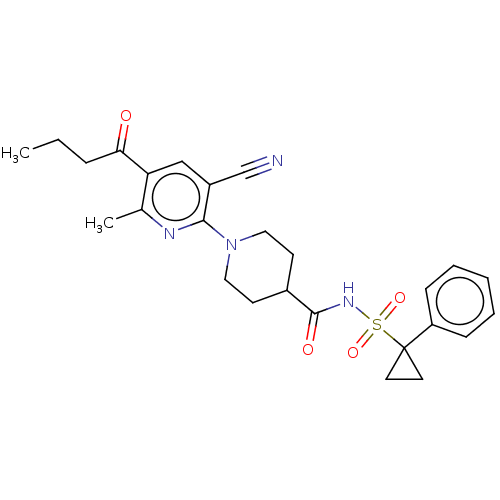
(CHEMBL3288122)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S/c1-3-7-23(31)22-16-20(17-27)24(28-18(22)2)30-14-10-19(11-15-30)25(32)29-35(33,34)26(12-13-26)21-8-5-4-6-9-21/h4-6,8-9,16,19H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019030
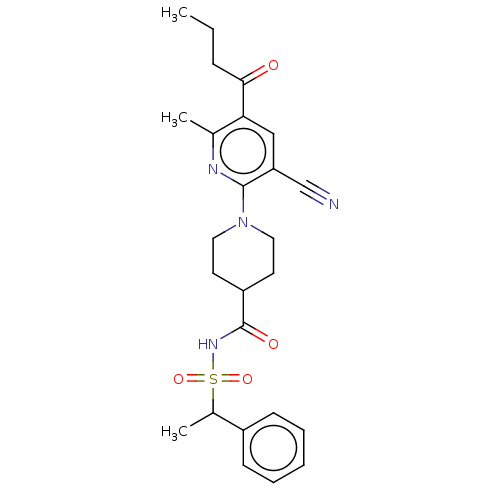
(CHEMBL3288121)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)C(C)c1ccccc1 Show InChI InChI=1S/C25H30N4O4S/c1-4-8-23(30)22-15-21(16-26)24(27-17(22)2)29-13-11-20(12-14-29)25(31)28-34(32,33)18(3)19-9-6-5-7-10-19/h5-7,9-10,15,18,20H,4,8,11-14H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019032
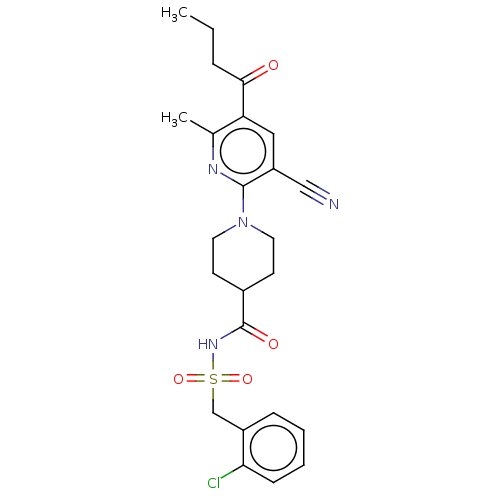
(CHEMBL3288124)Show SMILES CCCC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1Cl Show InChI InChI=1S/C24H27ClN4O4S/c1-3-6-22(30)20-13-19(14-26)23(27-16(20)2)29-11-9-17(10-12-29)24(31)28-34(32,33)15-18-7-4-5-8-21(18)25/h4-5,7-8,13,17H,3,6,9-12,15H2,1-2H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019018
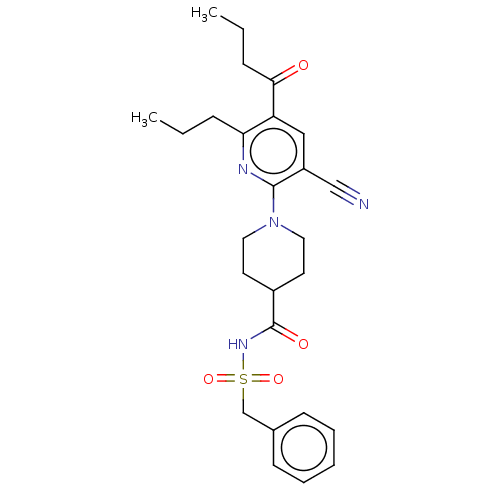
(CHEMBL3288133)Show SMILES CCCC(=O)c1cc(C#N)c(nc1CCC)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H32N4O4S/c1-3-8-23-22(24(31)9-4-2)16-21(17-27)25(28-23)30-14-12-20(13-15-30)26(32)29-35(33,34)18-19-10-6-5-7-11-19/h5-7,10-11,16,20H,3-4,8-9,12-15,18H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of ADP-induced [35S]GTPgammaS binding after 45 mins by scintillation co... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM92469

(2-Thioxanthine, TX4)Show SMILES O=c1[nH]c(=S)n(C[C@H]2CCCO2)c2nc[nH]c12 |r| Show InChI InChI=1S/C10H12N4O2S/c15-9-7-8(12-5-11-7)14(10(17)13-9)4-6-2-1-3-16-6/h5-6H,1-4H2,(H,11,12)(H,13,15,17)/t6-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM92469

(2-Thioxanthine, TX4)Show SMILES O=c1[nH]c(=S)n(C[C@H]2CCCO2)c2nc[nH]c12 |r| Show InChI InChI=1S/C10H12N4O2S/c15-9-7-8(12-5-11-7)14(10(17)13-9)4-6-2-1-3-16-6/h5-6H,1-4H2,(H,11,12)(H,13,15,17)/t6-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid peroxidase
(Homo sapiens (Human)) | BDBM92467

(2-Thioxanthine, TX2)Show InChI InChI=1S/C12H9FN4OS/c13-8-3-1-7(2-4-8)5-17-10-9(14-6-15-10)11(18)16-12(17)19/h1-4,6H,5H2,(H,14,15)(H,16,18,19) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity P2Y12 receptor in human blood assessed as inhibition of ADP-induced platelet aggregation measured as residual platelet count afte... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Thyroid peroxidase
(Homo sapiens (Human)) | BDBM50595667

(CHEMBL5181350)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2nc[nH]c2c(=O)[nH]c1=S |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data