 Found 317 hits with Last Name = 'hurst' and Initial = 'dn'
Found 317 hits with Last Name = 'hurst' and Initial = 'dn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160917
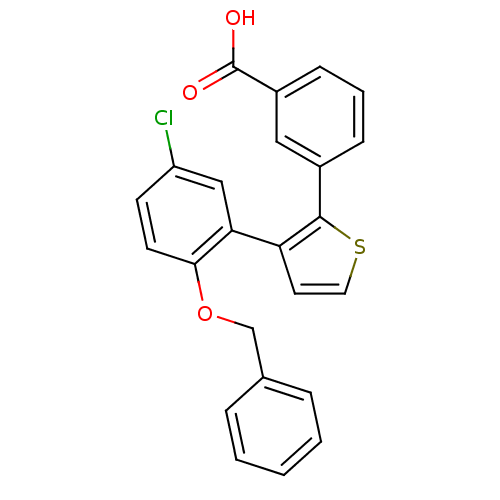
(3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...)Show SMILES OC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClO3S/c25-19-9-10-22(28-15-16-5-2-1-3-6-16)21(14-19)20-11-12-29-23(20)17-7-4-8-18(13-17)24(26)27/h1-14H,15H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR |
Bioorg Med Chem Lett 16: 2666-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.014
BindingDB Entry DOI: 10.7270/Q2J102RM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50376788
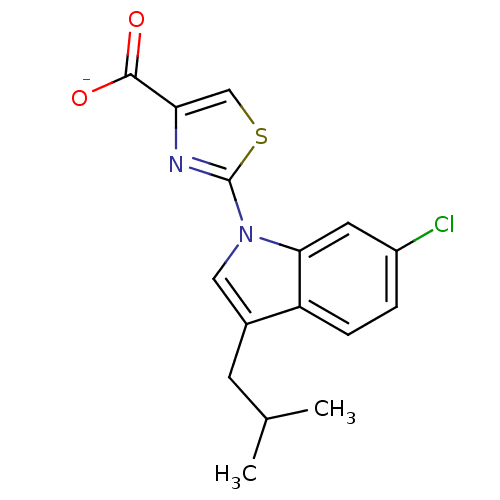
(CHEMBL257997)Show SMILES CC(C)Cc1cn(-c2nc(cs2)C([O-])=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C16H15ClN2O2S/c1-9(2)5-10-7-19(14-6-11(17)3-4-12(10)14)16-18-13(8-22-16)15(20)21/h3-4,6-9H,5H2,1-2H3,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP1 receptor expressed in CHOK1 cells assessed as inhibition of PGE2-induced intracellular calcium mobilization by ... |
Bioorg Med Chem Lett 18: 2684-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.018
BindingDB Entry DOI: 10.7270/Q2PC338K |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259611
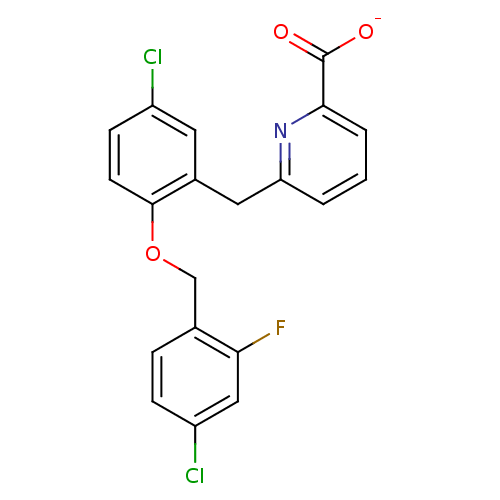
(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of TP receptor |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259613

(CHEMBL467720 | sodium 6-(5-chloro-2-(2,4-dichlorob...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl3NO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50183182
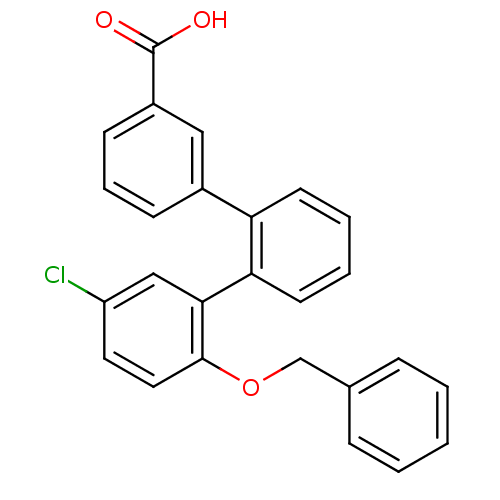
(2''-benzyloxy-5''-chloro-[1,1';2',1'']terphenyl-3-...)Show SMILES OC(=O)c1cccc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H19ClO3/c27-21-13-14-25(30-17-18-7-2-1-3-8-18)24(16-21)23-12-5-4-11-22(23)19-9-6-10-20(15-19)26(28)29/h1-16H,17H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259612
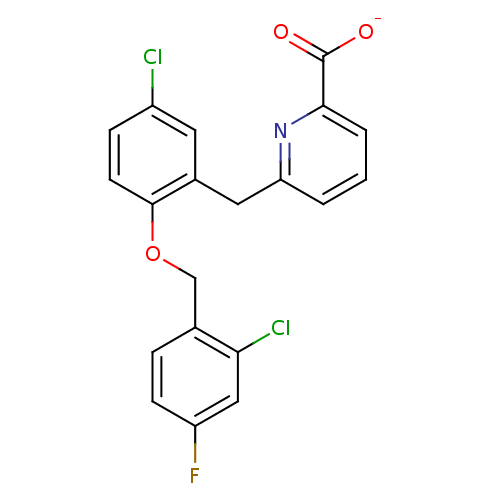
(CHEMBL513491 | sodium 6-(5-chloro-2-(2-chloro-4-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(F)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-5-7-19(27-11-12-4-6-15(23)10-17(12)22)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TP receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP3 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Activity at EP3 receptor by FLIPR method |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414022

(CHEMBL2110364 | GSK-180100B)Show SMILES Cc1cc(nn1Cc1cc(Br)ccc1OCc1ccc(Cl)cc1Cl)C([O-])=O Show InChI InChI=1S/C19H15BrCl2N2O3/c1-11-6-17(19(25)26)23-24(11)9-13-7-14(20)3-5-18(13)27-10-12-2-4-15(21)8-16(12)22/h2-8H,9-10H2,1H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419410

(CHEMBL1915252)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H15ClN2O3/c1-12-7-17(20(24)25)22-23(12)11-15-9-16(21)8-14-10-18(26-19(14)15)13-5-3-2-4-6-13/h2-10H,11H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413672

(CHEMBL516324)Show SMILES CCCC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C30H26ClNO4/c1-2-8-29(33)32-24-16-21(15-22(17-24)30(34)35)25-11-6-7-12-26(25)27-18-23(31)13-14-28(27)36-19-20-9-4-3-5-10-20/h3-7,9-18H,2,8,19H2,1H3,(H,32,33)(H,34,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50197898

(6-(2-(2-(2,4-difluorobenzyloxy)-5-chlorophenyl)cyc...)Show SMILES OC(=O)c1cccc(n1)C1=C(CCC1)c1cc(Cl)ccc1OCc1ccc(F)cc1F |t:10| Show InChI InChI=1S/C24H18ClF2NO3/c25-15-8-10-23(31-13-14-7-9-16(26)12-20(14)27)19(11-15)17-3-1-4-18(17)21-5-2-6-22(28-21)24(29)30/h2,5-12H,1,3-4,13H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50197898

(6-(2-(2-(2,4-difluorobenzyloxy)-5-chlorophenyl)cyc...)Show SMILES OC(=O)c1cccc(n1)C1=C(CCC1)c1cc(Cl)ccc1OCc1ccc(F)cc1F |t:10| Show InChI InChI=1S/C24H18ClF2NO3/c25-15-8-10-23(31-13-14-7-9-16(26)12-20(14)27)19(11-15)17-3-1-4-18(17)21-5-2-6-22(28-21)24(29)30/h2,5-12H,1,3-4,13H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50423557
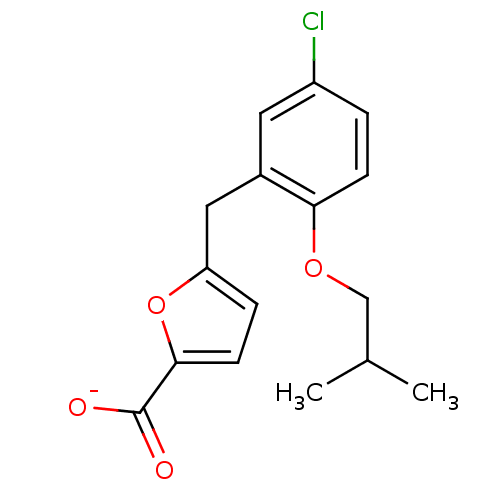
(CHEMBL403330)Show InChI InChI=1S/C16H17ClO4/c1-10(2)9-20-14-5-3-12(17)7-11(14)8-13-4-6-15(21-13)16(18)19/h3-7,10H,8-9H2,1-2H3,(H,18,19)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413667
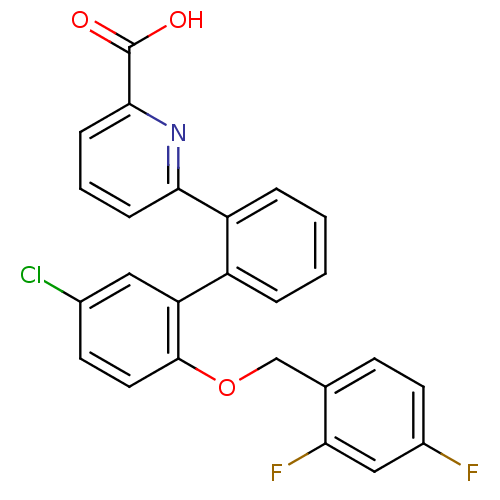
(CHEMBL514574)Show SMILES OC(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccc(F)cc1F Show InChI InChI=1S/C25H16ClF2NO3/c26-16-9-11-24(32-14-15-8-10-17(27)13-21(15)28)20(12-16)18-4-1-2-5-19(18)22-6-3-7-23(29-22)25(30)31/h1-13H,14H2,(H,30,31) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413670
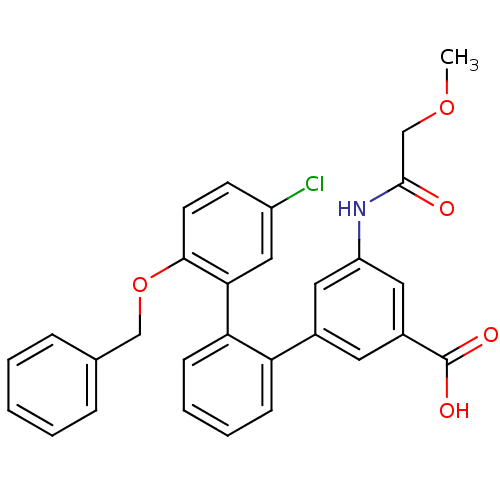
(CHEMBL458452)Show SMILES COCC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C29H24ClNO5/c1-35-18-28(32)31-23-14-20(13-21(15-23)29(33)34)24-9-5-6-10-25(24)26-16-22(30)11-12-27(26)36-17-19-7-3-2-4-8-19/h2-16H,17-18H2,1H3,(H,31,32)(H,33,34) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419415
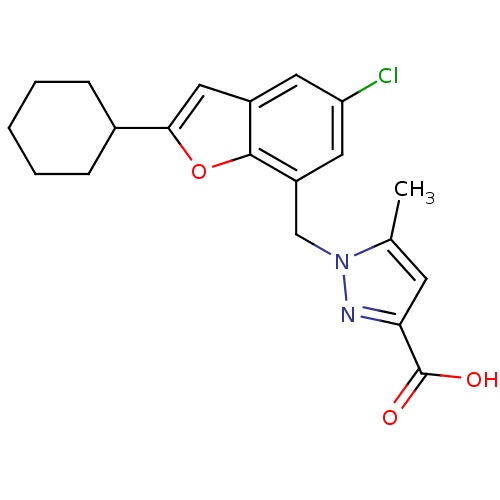
(CHEMBL1915015)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)C1CCCCC1)C(O)=O Show InChI InChI=1S/C20H21ClN2O3/c1-12-7-17(20(24)25)22-23(12)11-15-9-16(21)8-14-10-18(26-19(14)15)13-5-3-2-4-6-13/h7-10,13H,2-6,11H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096734

((S)-4-[(S)-3-Carboxy-2-(3-carboxy-propionylamino)-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)NCc1ccc(O)cc1 Show InChI InChI=1S/C47H62F3N7O15/c1-24(2)19-30(41(68)56-33(22-47(48,49)50)38(66)44(71)51-23-26-11-13-28(58)14-12-26)55-45(72)39(46(4,5)6)57-43(70)31(20-27-10-8-7-9-25(27)3)54-40(67)29(15-17-35(60)61)53-42(69)32(21-37(64)65)52-34(59)16-18-36(62)63/h7-14,24,29-33,39,58H,15-23H2,1-6H3,(H,51,71)(H,52,59)(H,53,69)(H,54,67)(H,55,72)(H,56,68)(H,57,70)(H,60,61)(H,62,63)(H,64,65)/t29-,30-,31-,32-,33+,39+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096726
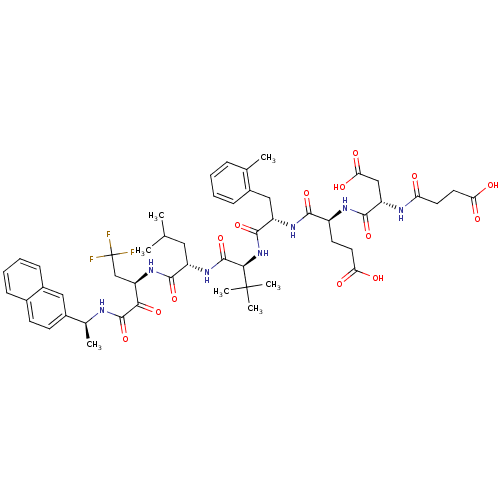
(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@@H](C)c1ccc2ccccc2c1 Show InChI InChI=1S/C52H66F3N7O14/c1-27(2)22-35(46(72)61-38(26-52(53,54)55)43(70)49(75)56-29(4)32-17-16-30-13-10-11-15-33(30)23-32)60-50(76)44(51(5,6)7)62-48(74)36(24-31-14-9-8-12-28(31)3)59-45(71)34(18-20-40(64)65)58-47(73)37(25-42(68)69)57-39(63)19-21-41(66)67/h8-17,23,27,29,34-38,44H,18-22,24-26H2,1-7H3,(H,56,75)(H,57,63)(H,58,73)(H,59,71)(H,60,76)(H,61,72)(H,62,74)(H,64,65)(H,66,67)(H,68,69)/t29-,34-,35-,36-,37-,38+,44+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096729

((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Benzylaminooxaly...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C47H62F3N7O14/c1-25(2)20-30(41(67)56-33(23-47(48,49)50)38(65)44(70)51-24-27-13-8-7-9-14-27)55-45(71)39(46(4,5)6)57-43(69)31(21-28-15-11-10-12-26(28)3)54-40(66)29(16-18-35(59)60)53-42(68)32(22-37(63)64)52-34(58)17-19-36(61)62/h7-15,25,29-33,39H,16-24H2,1-6H3,(H,51,70)(H,52,58)(H,53,68)(H,54,66)(H,55,71)(H,56,67)(H,57,69)(H,59,60)(H,61,62)(H,63,64)/t29-,30-,31-,32-,33+,39+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096730

(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C48H64F3N7O14/c1-25(2)21-31(42(68)57-34(24-48(49,50)51)39(66)45(71)52-27(4)28-14-9-8-10-15-28)56-46(72)40(47(5,6)7)58-44(70)32(22-29-16-12-11-13-26(29)3)55-41(67)30(17-19-36(60)61)54-43(69)33(23-38(64)65)53-35(59)18-20-37(62)63/h8-16,25,27,30-34,40H,17-24H2,1-7H3,(H,52,71)(H,53,59)(H,54,69)(H,55,67)(H,56,72)(H,57,68)(H,58,70)(H,60,61)(H,62,63)(H,64,65)/t27-,30-,31-,32-,33-,34+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50423570
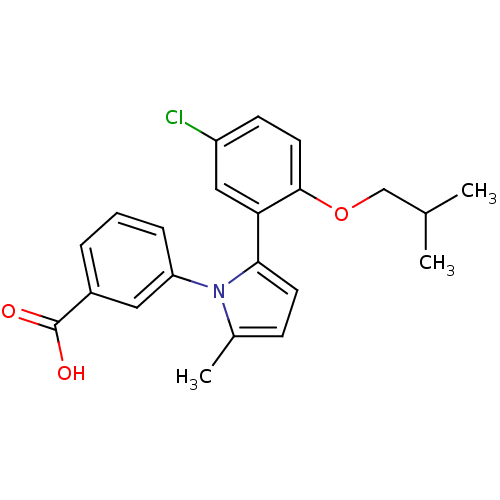
(CHEMBL209800)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccc(C)n1-c1cccc(c1)C(O)=O Show InChI InChI=1S/C22H22ClNO3/c1-14(2)13-27-21-10-8-17(23)12-19(21)20-9-7-15(3)24(20)18-6-4-5-16(11-18)22(25)26/h4-12,14H,13H2,1-3H3,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160917

(3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...)Show SMILES OC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClO3S/c25-19-9-10-22(28-15-16-5-2-1-3-6-16)21(14-19)20-11-12-29-23(20)17-7-4-8-18(13-17)24(26)27/h1-14H,15H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 16: 2666-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.014
BindingDB Entry DOI: 10.7270/Q2J102RM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413668

(CHEMBL457142)Show SMILES OC(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccc(F)cc1 Show InChI InChI=1S/C25H17ClFNO3/c26-17-10-13-24(31-15-16-8-11-18(27)12-9-16)21(14-17)19-4-1-2-5-20(19)22-6-3-7-23(28-22)25(29)30/h1-14H,15H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP1 receptor expressed in CHO-K1 cells after 30 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419403
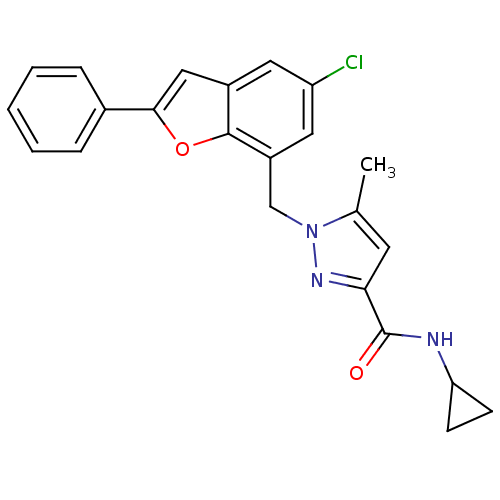
(CHEMBL1914467)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)NC1CC1 Show InChI InChI=1S/C23H20ClN3O2/c1-14-9-20(23(28)25-19-7-8-19)26-27(14)13-17-11-18(24)10-16-12-21(29-22(16)17)15-5-3-2-4-6-15/h2-6,9-12,19H,7-8,13H2,1H3,(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419417
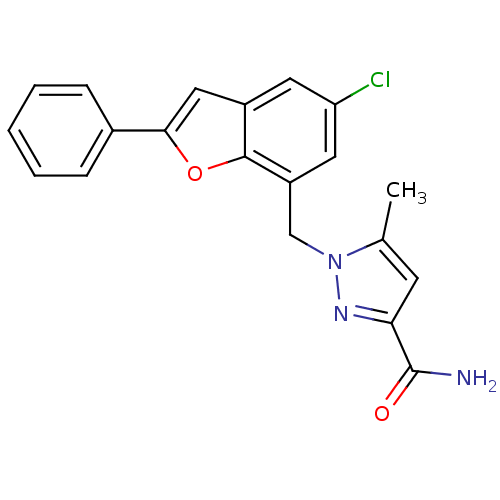
(CHEMBL1915254)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(N)=O Show InChI InChI=1S/C20H16ClN3O2/c1-12-7-17(20(22)25)23-24(12)11-15-9-16(21)8-14-10-18(26-19(14)15)13-5-3-2-4-6-13/h2-10H,11H2,1H3,(H2,22,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096727
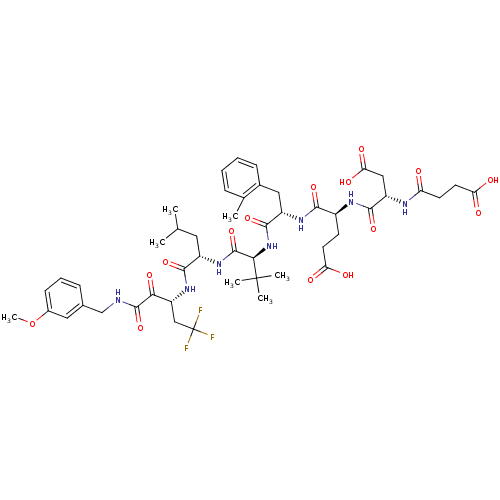
((S)-4-[(S)-3-Carboxy-2-(3-carboxy-propionylamino)-...)Show SMILES COc1cccc(CNC(=O)C(=O)[C@@H](CC(F)(F)F)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)c1 Show InChI InChI=1S/C48H64F3N7O15/c1-25(2)19-31(42(68)57-34(23-48(49,50)51)39(66)45(71)52-24-27-12-10-14-29(20-27)73-7)56-46(72)40(47(4,5)6)58-44(70)32(21-28-13-9-8-11-26(28)3)55-41(67)30(15-17-36(60)61)54-43(69)33(22-38(64)65)53-35(59)16-18-37(62)63/h8-14,20,25,30-34,40H,15-19,21-24H2,1-7H3,(H,52,71)(H,53,59)(H,54,69)(H,55,67)(H,56,72)(H,57,68)(H,58,70)(H,60,61)(H,62,63)(H,64,65)/t30-,31-,32-,33-,34+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50423574
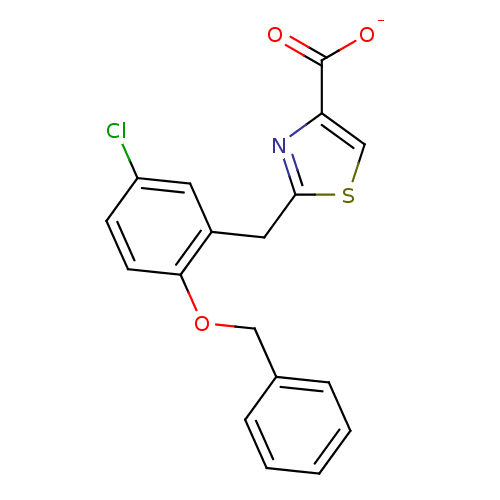
(CHEMBL255652)Show InChI InChI=1S/C18H14ClNO3S/c19-14-6-7-16(23-10-12-4-2-1-3-5-12)13(8-14)9-17-20-15(11-24-17)18(21)22/h1-8,11H,9-10H2,(H,21,22)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50191163

(1-(2-(benzyloxy)-5-bromobenzyl)-5-methyl-1H-pyrazo...)Show InChI InChI=1S/C19H17BrN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50423563
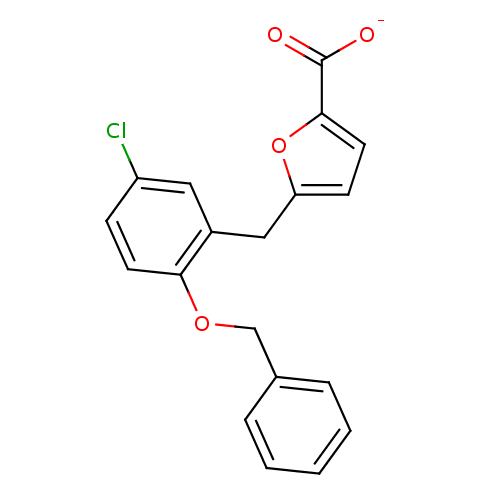
(CHEMBL272793)Show InChI InChI=1S/C19H15ClO4/c20-15-6-8-17(23-12-13-4-2-1-3-5-13)14(10-15)11-16-7-9-18(24-16)19(21)22/h1-10H,11-12H2,(H,21,22)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50376788

(CHEMBL257997)Show SMILES CC(C)Cc1cn(-c2nc(cs2)C([O-])=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C16H15ClN2O2S/c1-9(2)5-10-7-19(14-6-11(17)3-4-12(10)14)16-18-13(8-22-16)15(20)21/h3-4,6-9H,5H2,1-2H3,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 2684-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.018
BindingDB Entry DOI: 10.7270/Q2PC338K |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419419
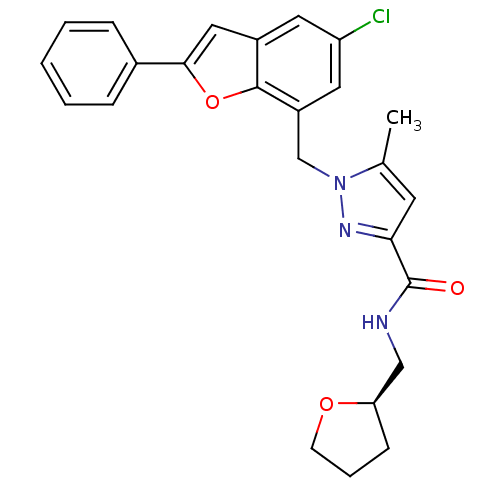
(CHEMBL1915262)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)NC[C@H]1CCCO1 |r| Show InChI InChI=1S/C25H24ClN3O3/c1-16-10-22(25(30)27-14-21-8-5-9-31-21)28-29(16)15-19-12-20(26)11-18-13-23(32-24(18)19)17-6-3-2-4-7-17/h2-4,6-7,10-13,21H,5,8-9,14-15H2,1H3,(H,27,30)/t21-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413673

(CHEMBL456496)Show SMILES CCC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C29H24ClNO4/c1-2-28(32)31-23-15-20(14-21(16-23)29(33)34)24-10-6-7-11-25(24)26-17-22(30)12-13-27(26)35-18-19-8-4-3-5-9-19/h3-17H,2,18H2,1H3,(H,31,32)(H,33,34) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096733
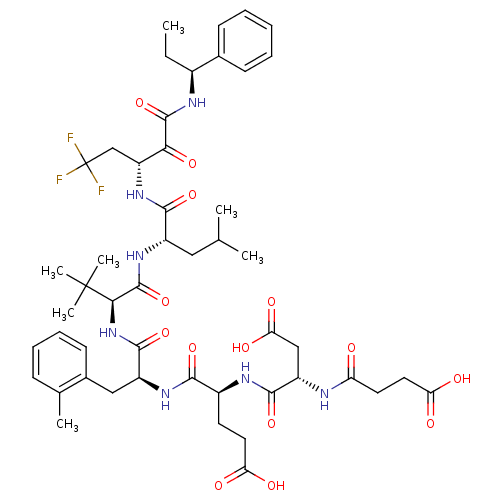
(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC[C@H](NC(=O)C(=O)[C@@H](CC(F)(F)F)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C49H66F3N7O14/c1-8-30(28-15-10-9-11-16-28)54-46(72)40(67)35(25-49(50,51)52)58-43(69)32(22-26(2)3)57-47(73)41(48(5,6)7)59-45(71)33(23-29-17-13-12-14-27(29)4)56-42(68)31(18-20-37(61)62)55-44(70)34(24-39(65)66)53-36(60)19-21-38(63)64/h9-17,26,30-35,41H,8,18-25H2,1-7H3,(H,53,60)(H,54,72)(H,55,70)(H,56,68)(H,57,73)(H,58,69)(H,59,71)(H,61,62)(H,63,64)(H,65,66)/t30-,31-,32-,33-,34-,35+,41+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096728

((S)-4-((S)-1-{(S)-1-[(S)-1-((S)-1-Amino-2-carbamoy...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](N)C(=O)C(N)=O Show InChI InChI=1S/C38H56N8O14/c1-18(2)15-22(36(59)46-31(39)29(54)32(40)55)44-37(60)30(38(4,5)6)45-35(58)23(16-20-10-8-7-9-19(20)3)43-33(56)21(11-13-26(48)49)42-34(57)24(17-28(52)53)41-25(47)12-14-27(50)51/h7-10,18,21-24,30-31H,11-17,39H2,1-6H3,(H2,40,55)(H,41,47)(H,42,57)(H,43,56)(H,44,60)(H,45,58)(H,46,59)(H,48,49)(H,50,51)(H,52,53)/t21-,22-,23-,24-,30+,31-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096725
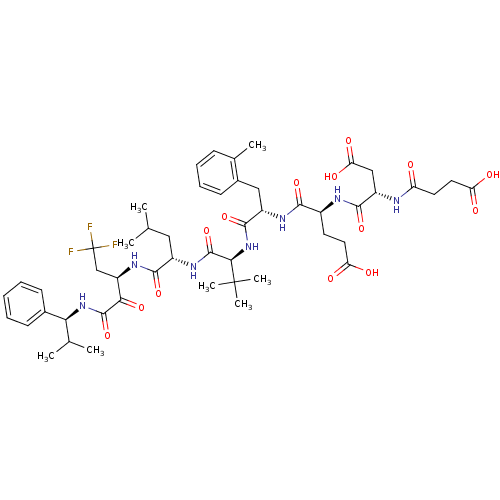
(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@@H](C(C)C)c1ccccc1 Show InChI InChI=1S/C50H68F3N7O14/c1-26(2)22-32(44(70)58-35(25-50(51,52)53)41(68)47(73)59-40(27(3)4)29-15-10-9-11-16-29)57-48(74)42(49(6,7)8)60-46(72)33(23-30-17-13-12-14-28(30)5)56-43(69)31(18-20-37(62)63)55-45(71)34(24-39(66)67)54-36(61)19-21-38(64)65/h9-17,26-27,31-35,40,42H,18-25H2,1-8H3,(H,54,61)(H,55,71)(H,56,69)(H,57,74)(H,58,70)(H,59,73)(H,60,72)(H,62,63)(H,64,65)(H,66,67)/t31-,32-,33-,34-,35+,40-,42+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against delta receptor of (endomorphin 2) in mouse vas deferens was determined |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50183185

(3-(2-(2-(benzyloxy)-5-chlorophenyl)-5-methyl-1H-py...)Show SMILES Cc1ccc(-c2cc(Cl)ccc2OCc2ccccc2)n1-c1cccc(c1)C(O)=O Show InChI InChI=1S/C25H20ClNO3/c1-17-10-12-23(27(17)21-9-5-8-19(14-21)25(28)29)22-15-20(26)11-13-24(22)30-16-18-6-3-2-4-7-18/h2-15H,16H2,1H3,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50423558
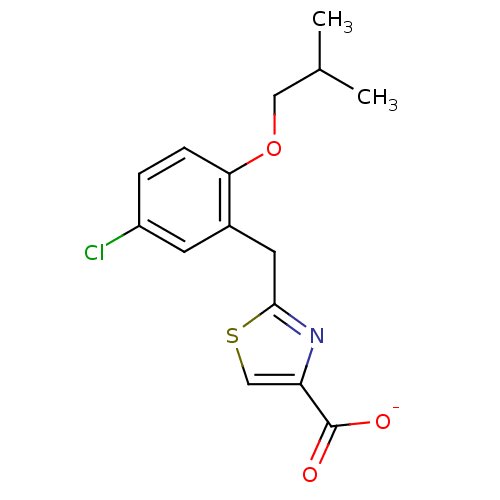
(CHEMBL255009)Show InChI InChI=1S/C15H16ClNO3S/c1-9(2)7-20-13-4-3-11(16)5-10(13)6-14-17-12(8-21-14)15(18)19/h3-5,8-9H,6-7H2,1-2H3,(H,18,19)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419408
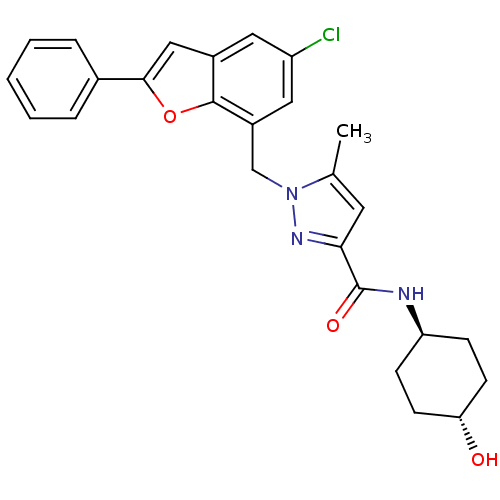
(CHEMBL1915261)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:26.29,wD:29.33,(19.51,-3.15,;19.53,-1.54,;21.04,-1.85,;21.81,-.51,;20.77,.63,;19.36,-.01,;18.02,.76,;16.69,-.02,;15.36,.75,;14.03,-.02,;12.69,.75,;14.03,-1.57,;15.36,-2.34,;15.68,-3.85,;17.21,-4.01,;17.84,-2.6,;16.7,-1.57,;17.98,-5.35,;17.2,-6.67,;17.96,-8.01,;19.51,-8.01,;20.28,-6.67,;19.51,-5.34,;23.34,-.35,;23.96,1.06,;24.25,-1.59,;25.78,-1.59,;26.55,-.26,;28.09,-.26,;28.85,-1.59,;30.39,-1.6,;28.08,-2.92,;26.55,-2.92,)| Show InChI InChI=1S/C26H26ClN3O3/c1-16-11-23(26(32)28-21-7-9-22(31)10-8-21)29-30(16)15-19-13-20(27)12-18-14-24(33-25(18)19)17-5-3-2-4-6-17/h2-6,11-14,21-22,31H,7-10,15H2,1H3,(H,28,32)/t21-,22- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096732

(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC[C@H](NC(=O)C(=O)[C@@H](CC(F)(F)F)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)c1ccc2ccccc2c1 Show InChI InChI=1S/C53H68F3N7O14/c1-8-34(33-18-17-30-14-11-12-16-32(30)24-33)58-50(76)44(71)39(27-53(54,55)56)62-47(73)36(23-28(2)3)61-51(77)45(52(5,6)7)63-49(75)37(25-31-15-10-9-13-29(31)4)60-46(72)35(19-21-41(65)66)59-48(74)38(26-43(69)70)57-40(64)20-22-42(67)68/h9-18,24,28,34-39,45H,8,19-23,25-27H2,1-7H3,(H,57,64)(H,58,76)(H,59,74)(H,60,72)(H,61,77)(H,62,73)(H,63,75)(H,65,66)(H,67,68)(H,69,70)/t34-,35-,36-,37-,38-,39+,45+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419416
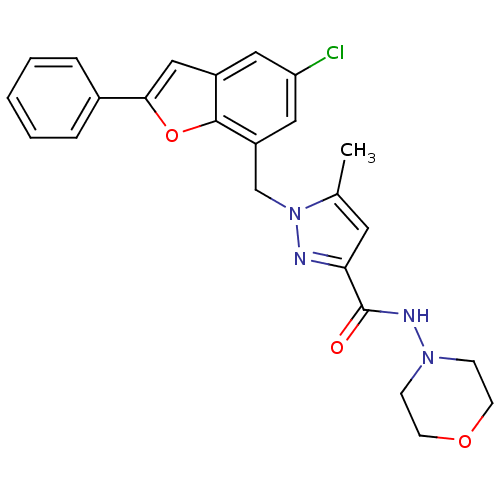
(CHEMBL1915264)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)NN1CCOCC1 Show InChI InChI=1S/C24H23ClN4O3/c1-16-11-21(24(30)27-28-7-9-31-10-8-28)26-29(16)15-19-13-20(25)12-18-14-22(32-23(18)19)17-5-3-2-4-6-17/h2-6,11-14H,7-10,15H2,1H3,(H,27,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
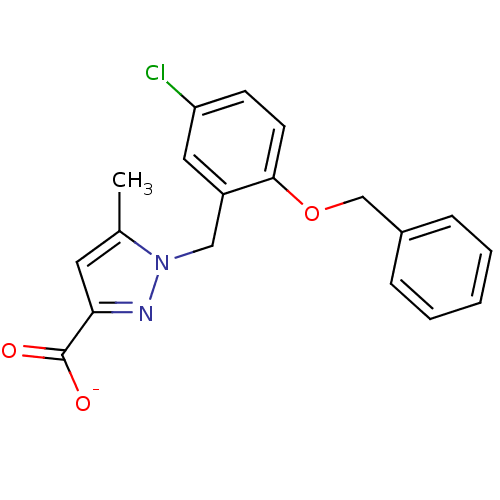
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50412226
(CHEMBL404525)Show InChI InChI=1S/C19H17ClN2O3/c1-13-9-17(19(23)24)21-22(13)11-15-10-16(20)7-8-18(15)25-12-14-5-3-2-4-6-14/h2-10H,11-12H2,1H3,(H,23,24)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |
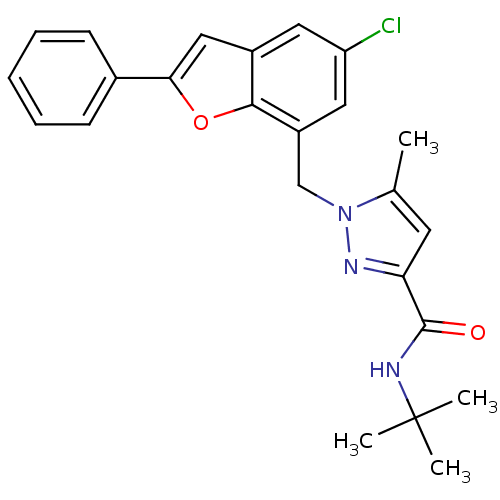
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419402
(CHEMBL1915255)Show SMILES Cc1cc(nn1Cc1cc(Cl)cc2cc(oc12)-c1ccccc1)C(=O)NC(C)(C)C Show InChI InChI=1S/C24H24ClN3O2/c1-15-10-20(23(29)26-24(2,3)4)27-28(15)14-18-12-19(25)11-17-13-21(30-22(17)18)16-8-6-5-7-9-16/h5-13H,14H2,1-4H3,(H,26,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419401
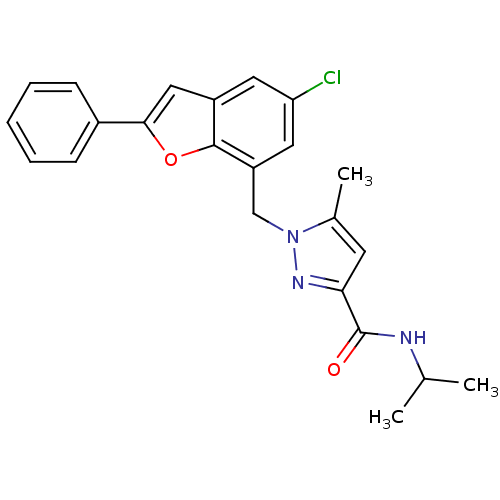
(CHEMBL1915253)Show SMILES CC(C)NC(=O)c1cc(C)n(Cc2cc(Cl)cc3cc(oc23)-c2ccccc2)n1 Show InChI InChI=1S/C23H22ClN3O2/c1-14(2)25-23(28)20-9-15(3)27(26-20)13-18-11-19(24)10-17-12-21(29-22(17)18)16-7-5-4-6-8-16/h4-12,14H,13H2,1-3H3,(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50423571
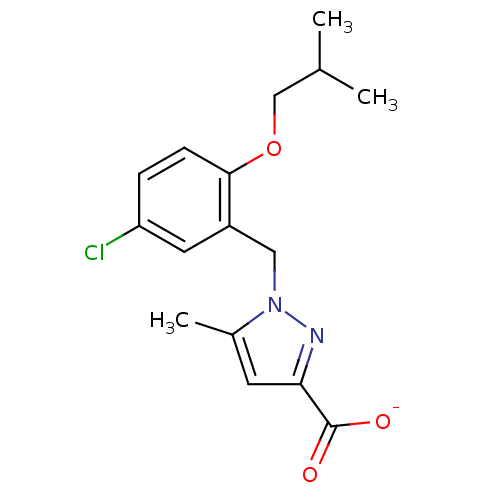
(CHEMBL270208)Show InChI InChI=1S/C16H19ClN2O3/c1-10(2)9-22-15-5-4-13(17)7-12(15)8-19-11(3)6-14(18-19)16(20)21/h4-7,10H,8-9H2,1-3H3,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 18: 1592-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.071
BindingDB Entry DOI: 10.7270/Q2N58NNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data