 Found 3681 hits with Last Name = 'iguchi' and Initial = 't'
Found 3681 hits with Last Name = 'iguchi' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586369

(CHEMBL5094265)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586368

(CHEMBL5073848)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240130
(CHEMBL4060961)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C90H165N35O27S/c1-44(2)39-60(70(97)135)120-78(143)57(26-28-63(95)131)116-74(139)52(20-10-13-32-92)114-76(141)55(23-16-35-105-89(100)101)118-83(148)62-25-18-37-125(62)87(152)46(4)110-73(138)51(19-9-12-31-91)111-66(134)41-107-65(133)40-108-84(149)67(47(5)128)122-82(147)61(43-127)121-77(142)53(21-11-14-33-93)115-75(140)54(22-15-34-104-88(98)99)112-71(136)45(3)109-85(150)68(48(6)129)123-81(146)58(27-29-64(96)132)117-79(144)59(30-38-153-8)119-86(151)69(49(7)130)124-80(145)56(24-17-36-106-90(102)103)113-72(137)50(94)42-126/h44-62,67-69,126-130H,9-43,91-94H2,1-8H3,(H2,95,131)(H2,96,132)(H2,97,135)(H,107,133)(H,108,149)(H,109,150)(H,110,138)(H,111,134)(H,112,136)(H,113,137)(H,114,141)(H,115,140)(H,116,139)(H,117,144)(H,118,148)(H,119,151)(H,120,143)(H,121,142)(H,122,147)(H,123,146)(H,124,145)(H4,98,99,104)(H4,100,101,105)(H4,102,103,106)/t45-,46-,47+,48+,49+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,67-,68-,69-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50004519

(CHEMBL2181929)Show SMILES CC1(C)Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H17FN2O4S/c1-17(2)16(21)20(13-7-4-11(18)5-8-13)14-9-6-12(10-15(14)24-17)19-25(3,22)23/h4-10,19H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240124
(CHEMBL4090728)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)N)[C@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C93H170N36O27S/c1-45(2)41-62(84(150)112-47(4)72(100)138)124-80(146)59(28-30-65(98)134)120-76(142)54(22-12-15-34-95)118-78(144)57(25-18-37-108-92(103)104)122-86(152)64-27-20-39-129(64)90(156)49(6)114-75(141)53(21-11-14-33-94)115-68(137)43-110-67(136)42-111-87(153)69(50(7)131)126-85(151)63(44-130)125-79(145)55(23-13-16-35-96)119-77(143)56(24-17-36-107-91(101)102)117-74(140)48(5)113-88(154)70(51(8)132)127-83(149)60(29-31-66(99)135)121-81(147)61(32-40-157-10)123-89(155)71(52(9)133)128-82(148)58(116-73(139)46(3)97)26-19-38-109-93(105)106/h45-64,69-71,130-133H,11-44,94-97H2,1-10H3,(H2,98,134)(H2,99,135)(H2,100,138)(H,110,136)(H,111,153)(H,112,150)(H,113,154)(H,114,141)(H,115,137)(H,116,139)(H,117,140)(H,118,144)(H,119,143)(H,120,142)(H,121,147)(H,122,152)(H,123,155)(H,124,146)(H,125,145)(H,126,151)(H,127,149)(H,128,148)(H4,101,102,107)(H4,103,104,108)(H4,105,106,109)/t46-,47+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,62+,63+,64+,69+,70+,71+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 assessed as reduction in H2O2 production using pLys4Met H3 peptide as substrate by peroxidase coupled UV-visible spectrophot... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50318300
(CHEMBL1095097 | EPLERENONE | SC-66110)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]23O[C@@H]2C[C@@]2(C)[C@@H](CC[C@@]22CCC(=O)O2)[C@H]13 |r,t:6| Show InChI InChI=1S/C24H30O6/c1-21-7-4-14(25)10-13(21)11-15(20(27)28-3)19-16-5-8-23(9-6-18(26)30-23)22(16,2)12-17-24(19,21)29-17/h10,15-17,19H,4-9,11-12H2,1-3H3/t15-,16+,17-,19+,21+,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586370
(CHEMBL5084197)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240122

(CHEMBL4103690)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(=O)[C@@H](N)CO)C(N)=O |r| Show InChI InChI=1S/C99H182N38O30S/c1-49(2)43-67(90(161)123-57(77(107)148)21-12-16-37-114-79(150)55(103)46-138)132-86(157)64(29-31-70(105)144)128-82(153)59(23-10-14-35-101)126-84(155)62(26-18-39-116-98(110)111)130-92(163)69-28-20-41-137(69)96(167)51(4)121-81(152)58(22-9-13-34-100)122-73(147)45-118-72(146)44-119-93(164)74(52(5)141)134-91(162)68(48-140)133-85(156)60(24-11-15-36-102)127-83(154)61(25-17-38-115-97(108)109)124-78(149)50(3)120-94(165)75(53(6)142)135-89(160)65(30-32-71(106)145)129-87(158)66(33-42-168-8)131-95(166)76(54(7)143)136-88(159)63(27-19-40-117-99(112)113)125-80(151)56(104)47-139/h49-69,74-76,138-143H,9-48,100-104H2,1-8H3,(H2,105,144)(H2,106,145)(H2,107,148)(H,114,150)(H,118,146)(H,119,164)(H,120,165)(H,121,152)(H,122,147)(H,123,161)(H,124,149)(H,125,151)(H,126,155)(H,127,154)(H,128,153)(H,129,158)(H,130,163)(H,131,166)(H,132,157)(H,133,156)(H,134,162)(H,135,160)(H,136,159)(H4,108,109,115)(H4,110,111,116)(H4,112,113,117)/t50-,51-,52+,53+,54+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,74-,75-,76-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586371
(CHEMBL5089876)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCOCCn2cc(COc3cc(OCc4cn(CCOCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240126
(CHEMBL4105288)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C90H165N35O26S/c1-44(2)40-60(70(97)134)120-78(142)57(27-29-63(95)130)116-74(138)52(21-11-14-33-92)114-76(140)55(24-17-36-105-89(100)101)118-83(147)62-26-19-38-125(62)87(151)47(5)110-73(137)51(20-10-13-32-91)111-66(133)42-107-65(132)41-108-84(148)67(48(6)127)122-82(146)61(43-126)121-77(141)53(22-12-15-34-93)115-75(139)54(23-16-35-104-88(98)99)113-72(136)46(4)109-85(149)68(49(7)128)123-81(145)58(28-30-64(96)131)117-79(143)59(31-39-152-9)119-86(150)69(50(8)129)124-80(144)56(112-71(135)45(3)94)25-18-37-106-90(102)103/h44-62,67-69,126-129H,10-43,91-94H2,1-9H3,(H2,95,130)(H2,96,131)(H2,97,134)(H,107,132)(H,108,148)(H,109,149)(H,110,137)(H,111,133)(H,112,135)(H,113,136)(H,114,140)(H,115,139)(H,116,138)(H,117,143)(H,118,147)(H,119,150)(H,120,142)(H,121,141)(H,122,146)(H,123,145)(H,124,144)(H4,98,99,104)(H4,100,101,105)(H4,102,103,106)/t45-,46-,47-,48+,49+,50+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,67-,68-,69-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586366

(CHEMBL5093950)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586367

(CHEMBL5089144)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50158884

(CHEMBL3785550)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C51H87N17O10/c1-29(2)26-36(46(75)67-40(30(3)4)48(77)62-34(19-12-24-60-51(56)57)43(72)63-35(16-8-9-21-52)49(78)68-25-13-20-39(68)41(53)70)64-45(74)37(27-31-14-6-5-7-15-31)65-47(76)38(28-69)66-44(73)33(18-11-23-59-50(54)55)61-42(71)32-17-10-22-58-32/h5-7,14-15,29-30,32-40,58,69H,8-13,16-28,52H2,1-4H3,(H2,53,70)(H,61,71)(H,62,77)(H,63,72)(H,64,74)(H,65,76)(H,66,73)(H,67,75)(H4,54,55,59)(H4,56,57,60)/t32-,33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged LSD1 (171 to 836 residues)/GST-tagged CoREST (308 to 440 residues) complex using H3K4 peptide substrate by... |
Bioorg Med Chem 25: 1227-1234 (2017)
Article DOI: 10.1016/j.bmc.2016.12.033
BindingDB Entry DOI: 10.7270/Q2X92D9V |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597078

(CHEMBL5181002) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597086
(CHEMBL5187656)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CCC3)Oc2c1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597084

(CHEMBL5174401)Show SMILES CC1Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597087
(CHEMBL5181278)Show SMILES CC1(C)CN(c2ccc(F)cc2)c2ccc(NS(C)(=O)=O)cc2O1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586373

(CHEMBL5075544)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(OCc2cn(CCO)nn2)cc(OCc2cn(CCO)nn2)c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586365

(CHEMBL5078239)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597084

(CHEMBL5174401)Show SMILES CC1Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597083
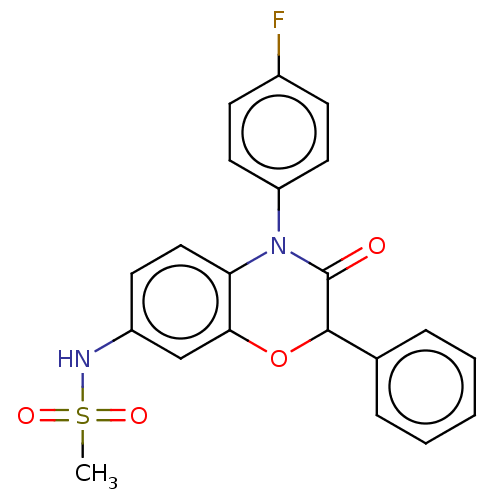
(CHEMBL5171957)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C(Oc2c1)c1ccccc1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240123
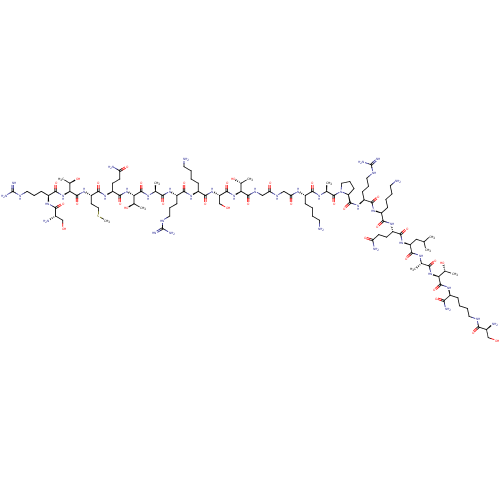
(CHEMBL4085763)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCNC(=O)[C@@H](N)CO)C(N)=O |r| Show InChI InChI=1S/C106H194N40O33S/c1-51(2)45-71(96(172)127-53(4)84(160)142-80(57(8)152)101(177)131-61(82(114)158)23-14-18-39-121-85(161)59(110)48-147)140-92(168)68(31-33-74(112)154)136-88(164)63(25-12-16-37-108)134-90(166)66(28-20-41-123-105(117)118)138-98(174)73-30-22-43-146(73)103(179)54(5)129-87(163)62(24-11-15-36-107)130-77(157)47-125-76(156)46-126-99(175)78(55(6)150)143-97(173)72(50-149)141-91(167)64(26-13-17-38-109)135-89(165)65(27-19-40-122-104(115)116)132-83(159)52(3)128-100(176)79(56(7)151)144-95(171)69(32-34-75(113)155)137-93(169)70(35-44-180-10)139-102(178)81(58(9)153)145-94(170)67(29-21-42-124-106(119)120)133-86(162)60(111)49-148/h51-73,78-81,147-153H,11-50,107-111H2,1-10H3,(H2,112,154)(H2,113,155)(H2,114,158)(H,121,161)(H,125,156)(H,126,175)(H,127,172)(H,128,176)(H,129,163)(H,130,157)(H,131,177)(H,132,159)(H,133,162)(H,134,166)(H,135,165)(H,136,164)(H,137,169)(H,138,174)(H,139,178)(H,140,168)(H,141,167)(H,142,160)(H,143,173)(H,144,171)(H,145,170)(H4,115,116,122)(H4,117,118,123)(H4,119,120,124)/t52-,53-,54-,55+,56+,57+,58+,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,78-,79-,80-,81-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597083

(CHEMBL5171957)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C(Oc2c1)c1ccccc1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597078

(CHEMBL5181002) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597087

(CHEMBL5181278)Show SMILES CC1(C)CN(c2ccc(F)cc2)c2ccc(NS(C)(=O)=O)cc2O1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240121
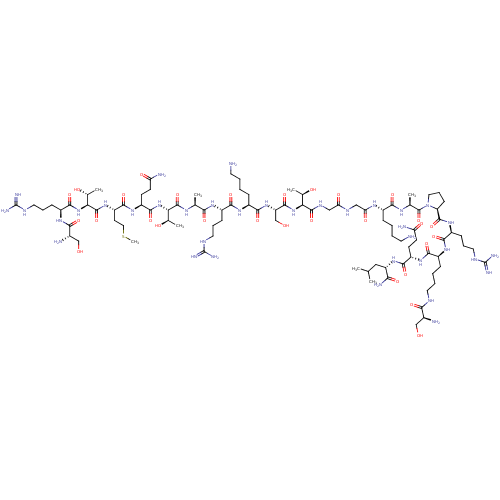
(CHEMBL4089148)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCNC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C93H170N36O29S/c1-45(2)39-62(72(100)140)124-81(149)59(26-28-65(98)136)120-77(145)55(21-11-14-33-107-74(142)51(96)42-130)119-79(147)57(23-16-35-109-92(103)104)122-86(154)64-25-18-37-129(64)90(158)47(4)114-76(144)53(19-9-12-31-94)115-68(139)41-111-67(138)40-112-87(155)69(48(5)133)126-85(153)63(44-132)125-80(148)54(20-10-13-32-95)118-78(146)56(22-15-34-108-91(101)102)116-73(141)46(3)113-88(156)70(49(6)134)127-84(152)60(27-29-66(99)137)121-82(150)61(30-38-159-8)123-89(157)71(50(7)135)128-83(151)58(24-17-36-110-93(105)106)117-75(143)52(97)43-131/h45-64,69-71,130-135H,9-44,94-97H2,1-8H3,(H2,98,136)(H2,99,137)(H2,100,140)(H,107,142)(H,111,138)(H,112,155)(H,113,156)(H,114,144)(H,115,139)(H,116,141)(H,117,143)(H,118,146)(H,119,147)(H,120,145)(H,121,150)(H,122,154)(H,123,157)(H,124,149)(H,125,148)(H,126,153)(H,127,152)(H,128,151)(H4,101,102,108)(H4,103,104,109)(H4,105,106,110)/t46-,47-,48+,49+,50+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597085
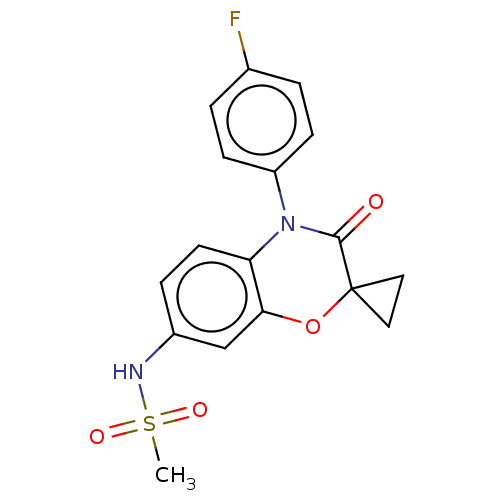
(CHEMBL5183454)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CC3)Oc2c1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597081
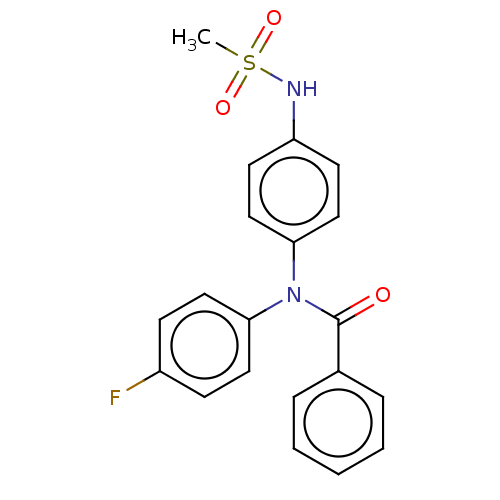
(CHEMBL5184647)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 596 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597077
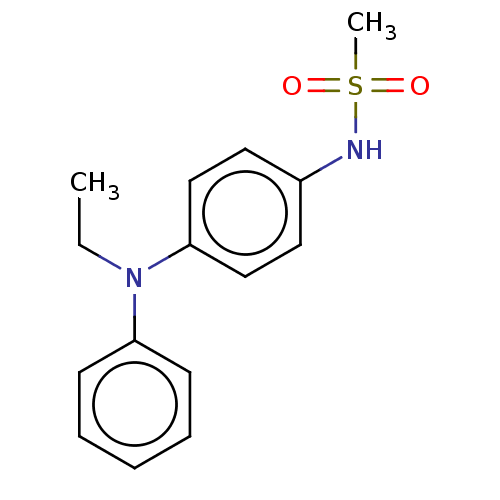
(CHEMBL5172350) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 607 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597081

(CHEMBL5184647)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 611 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597086

(CHEMBL5187656)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CCC3)Oc2c1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586372
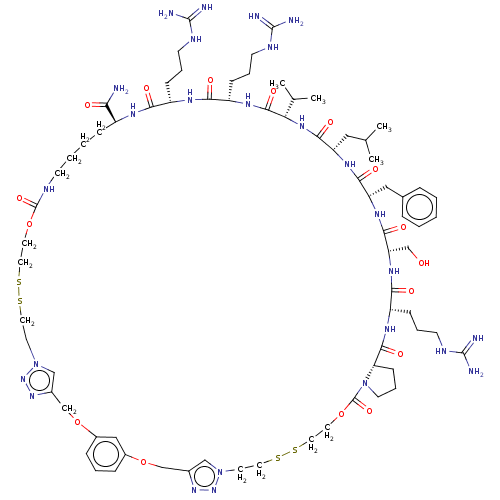
(CHEMBL5085737)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cccc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)c3)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597076

(CHEMBL5176336) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597068
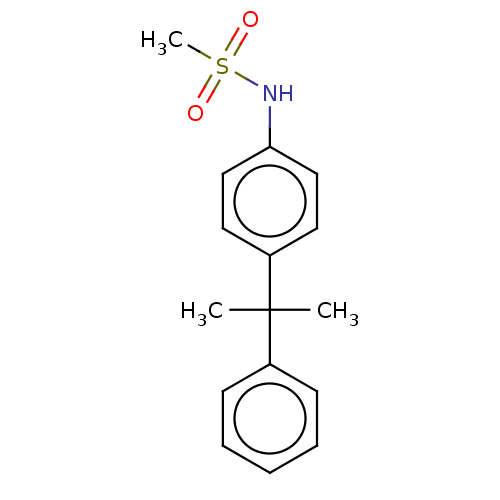
(CHEMBL5201004) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597085

(CHEMBL5183454)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CC3)Oc2c1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 808 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597076

(CHEMBL5176336) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586364
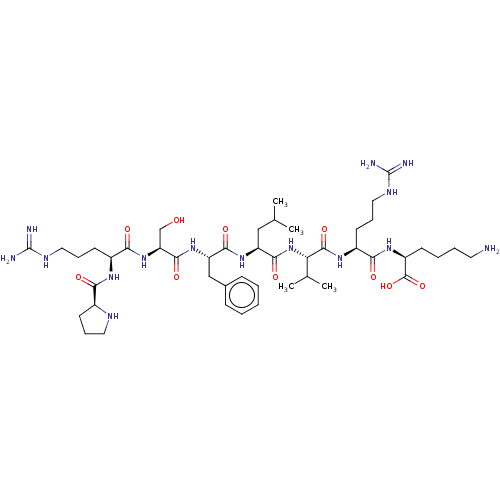
(CHEMBL5084292)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586363
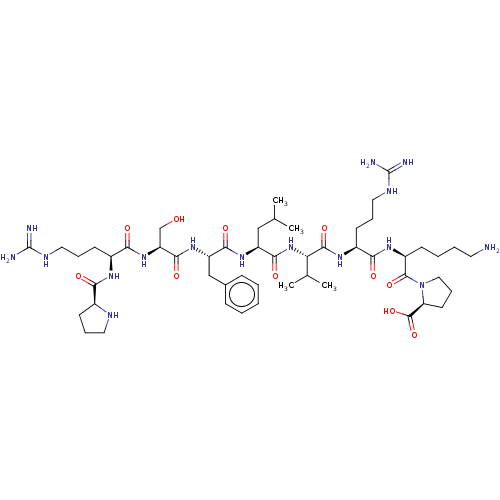
(CHEMBL5079374)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597080
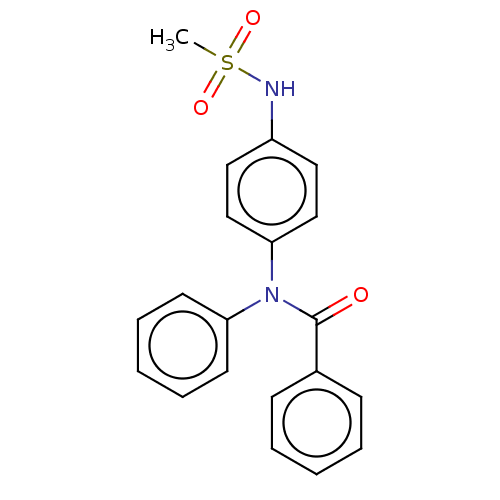
(CHEMBL5207249)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597080

(CHEMBL5207249)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597073
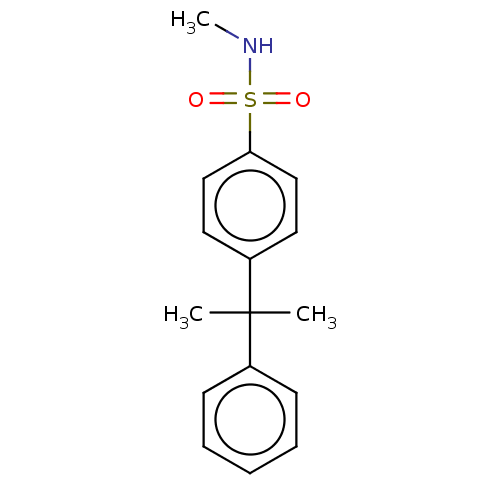
(CHEMBL5182687) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240129
(CHEMBL4080345)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCNC(=O)CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C92H167N35O28S/c1-45(2)40-61(72(98)138)122-80(146)58(27-29-64(96)133)118-76(142)54(22-12-15-34-105-68(137)44-129)117-78(144)56(24-17-36-107-91(101)102)120-85(151)63-26-19-38-127(63)89(155)48(5)112-75(141)52(20-10-13-32-93)113-67(136)42-109-66(135)41-110-86(152)69(49(6)130)124-84(150)62(43-128)123-79(145)53(21-11-14-33-94)116-77(143)55(23-16-35-106-90(99)100)115-74(140)47(4)111-87(153)70(50(7)131)125-83(149)59(28-30-65(97)134)119-81(147)60(31-39-156-9)121-88(154)71(51(8)132)126-82(148)57(114-73(139)46(3)95)25-18-37-108-92(103)104/h45-63,69-71,128-132H,10-44,93-95H2,1-9H3,(H2,96,133)(H2,97,134)(H2,98,138)(H,105,137)(H,109,135)(H,110,152)(H,111,153)(H,112,141)(H,113,136)(H,114,139)(H,115,140)(H,116,143)(H,117,144)(H,118,142)(H,119,147)(H,120,151)(H,121,154)(H,122,146)(H,123,145)(H,124,150)(H,125,149)(H,126,148)(H4,99,100,106)(H4,101,102,107)(H4,103,104,108)/t46-,47-,48-,49+,50+,51+,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu... |
Bioorg Med Chem 25: 2617-2624 (2017)
Article DOI: 10.1016/j.bmc.2017.03.016
BindingDB Entry DOI: 10.7270/Q2KH0QG1 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597077

(CHEMBL5172350) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50210048

(CHEMBL3884919)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C57H93N17O14S2/c1-33(2)30-39-49(80)72-44(34(3)4)52(83)67-37(18-11-23-64-55(61)62)46(77)68-38(53(84)73-24-12-19-42(73)45(58)76)16-8-9-21-65-56(85)87-26-28-89-90-29-27-88-57(86)74-25-13-20-43(74)51(82)66-36(17-10-22-63-54(59)60)47(78)71-41(32-75)50(81)70-40(48(79)69-39)31-35-14-6-5-7-15-35/h5-7,14-15,33-34,36-44,75H,8-13,16-32H2,1-4H3,(H2,58,76)(H,65,85)(H,66,82)(H,67,83)(H,68,77)(H,69,79)(H,70,81)(H,71,78)(H,72,80)(H4,59,60,63)(H4,61,62,64)/t36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll... |
Bioorg Med Chem 25: 1227-1234 (2017)
Article DOI: 10.1016/j.bmc.2016.12.033
BindingDB Entry DOI: 10.7270/Q2X92D9V |
More data for this
Ligand-Target Pair | |
Oxoeicosanoid receptor 1
(Homo sapiens (Human)) | BDBM50210259
((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O Show InChI InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd.
Curated by PDSP Ki Database
| |
J Biol Chem 277: 31459-65 (2002)
Article DOI: 10.1074/jbc.M203194200
BindingDB Entry DOI: 10.7270/Q2Z036QB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597082

(CHEMBL5204612) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158884

(CHEMBL3785550)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C51H87N17O10/c1-29(2)26-36(46(75)67-40(30(3)4)48(77)62-34(19-12-24-60-51(56)57)43(72)63-35(16-8-9-21-52)49(78)68-25-13-20-39(68)41(53)70)64-45(74)37(27-31-14-6-5-7-15-31)65-47(76)38(28-69)66-44(73)33(18-11-23-59-50(54)55)61-42(71)32-17-10-22-58-32/h5-7,14-15,29-30,32-40,58,69H,8-13,16-28,52H2,1-4H3,(H2,53,70)(H,61,71)(H,62,77)(H,63,72)(H,64,74)(H,65,76)(H,66,73)(H,67,75)(H4,54,55,59)(H4,56,57,60)/t32-,33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll... |
Bioorg Med Chem 25: 1227-1234 (2017)
Article DOI: 10.1016/j.bmc.2016.12.033
BindingDB Entry DOI: 10.7270/Q2X92D9V |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597070
(CHEMBL5186524) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597068

(CHEMBL5201004) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Oxoeicosanoid receptor 1
(Homo sapiens (Human)) | BDBM50269534
((8Z,11Z,14Z)-Icosatrienoic acid | (8Z,11Z,14Z)-ico...)Show InChI InChI=1S/C20H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13H,2-5,8,11,14-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd.
Curated by PDSP Ki Database
| |
J Biol Chem 277: 31459-65 (2002)
Article DOI: 10.1074/jbc.M203194200
BindingDB Entry DOI: 10.7270/Q2Z036QB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data