

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50434903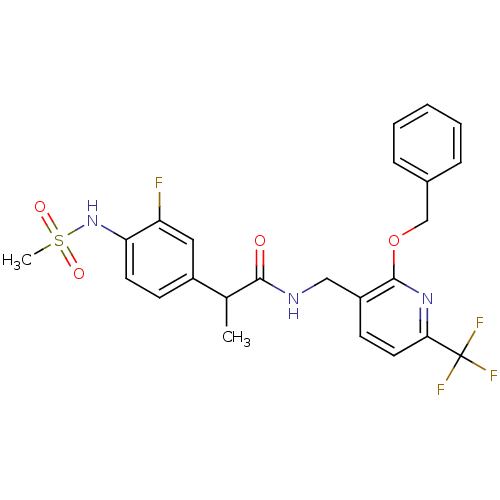 (CHEMBL2385223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... | Eur J Med Chem 64: 589-602 (2013) Article DOI: 10.1016/j.ejmech.2013.04.003 BindingDB Entry DOI: 10.7270/Q2BZ67FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417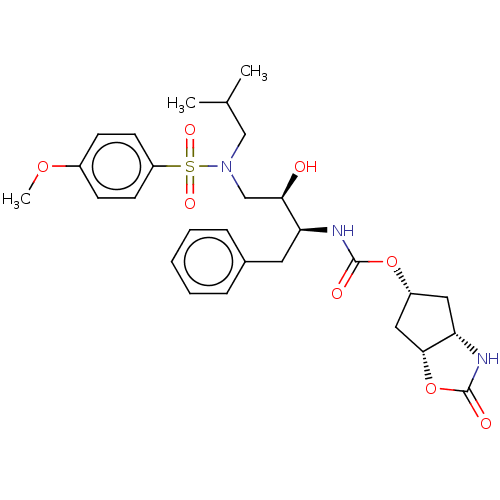 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033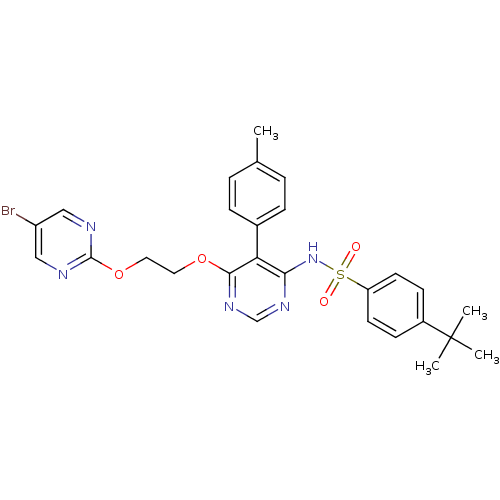 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50214974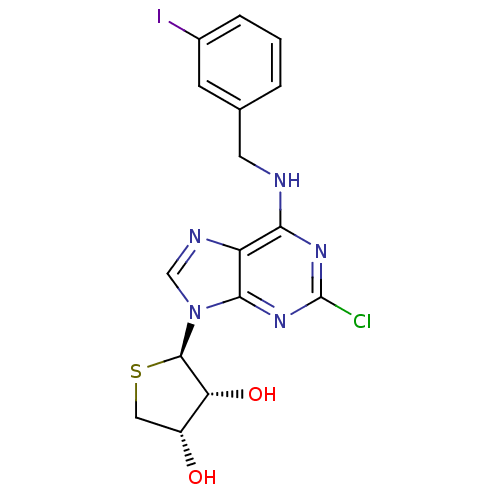 ((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human CD73 | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500526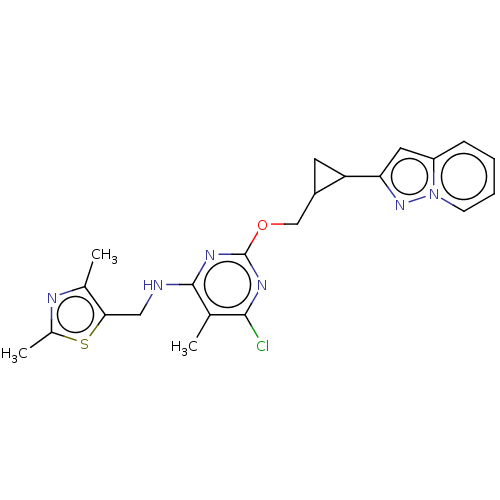 (CHEMBL3747517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520752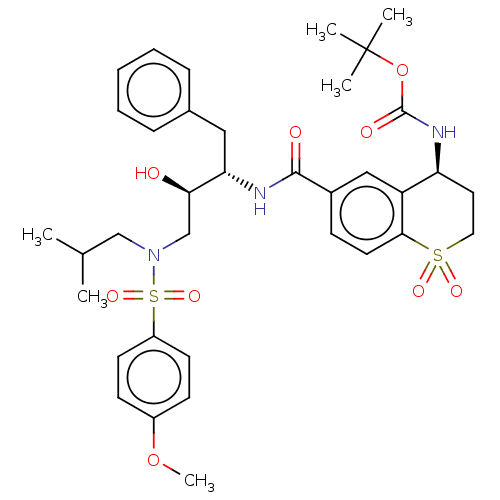 (CHEMBL4474261) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | Bioorg Med Chem 21: 6657-64 (2013) Article DOI: 10.1016/j.bmc.2013.08.015 BindingDB Entry DOI: 10.7270/Q26Q1ZPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500520 (CHEMBL3746993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500538 (CHEMBL3745790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | J Med Chem 55: 8392-408 (2012) Article DOI: 10.1021/jm300780p BindingDB Entry DOI: 10.7270/Q2TX3GH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500521 (CHEMBL3746277) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50214981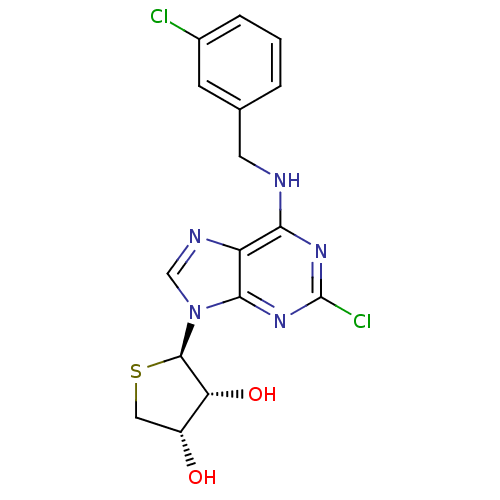 ((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953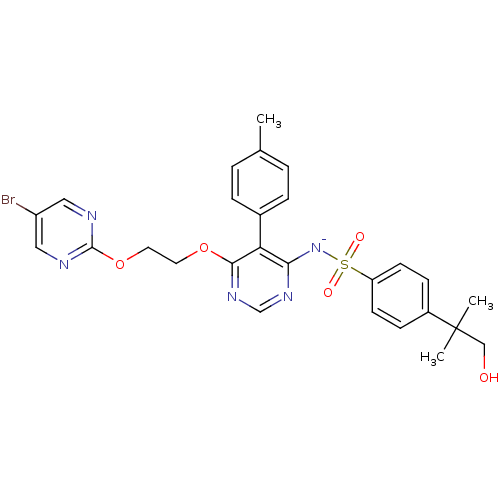 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469407 (CHEMBL4286714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469413 (CHEMBL4286231) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220995 (CHEMBL77788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144757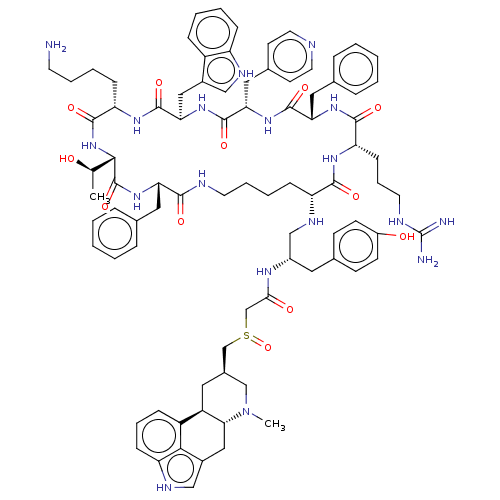 (US8952128, 15) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144761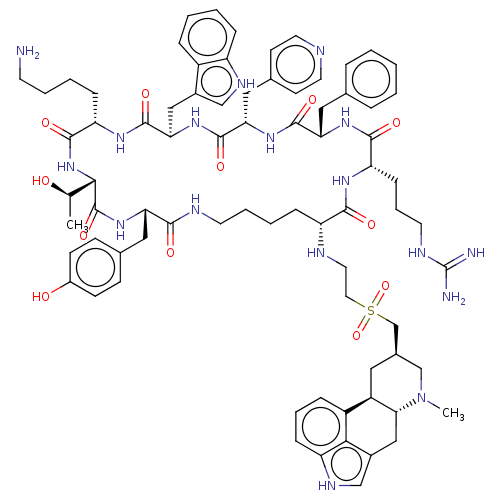 (US8952128, 20) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144762 (US8952128, 21) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50061298 (CHEMBL3393837) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay | Bioorg Med Chem Lett 25: 803-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.086 BindingDB Entry DOI: 10.7270/Q2JD4ZG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500519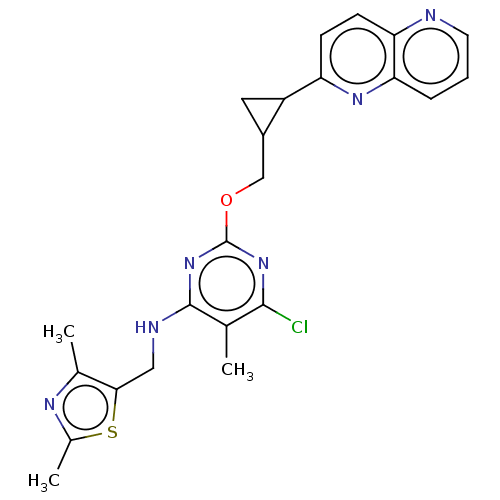 (CHEMBL3746917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500535 (CHEMBL3747450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553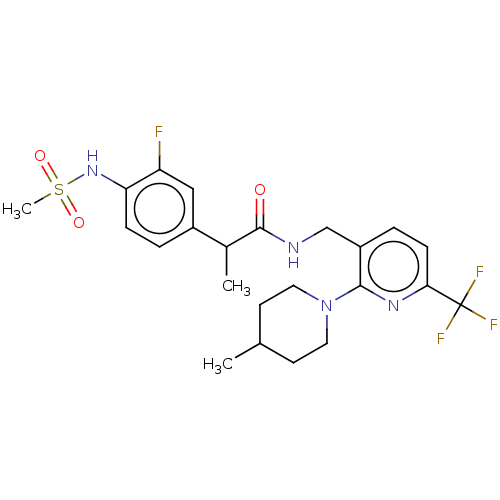 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 60 mins by activity based 100 fold dilution assay | Bioorg Med Chem 19: 5342-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.002 BindingDB Entry DOI: 10.7270/Q21R6QW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351401 (CHEMBL1819091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50327046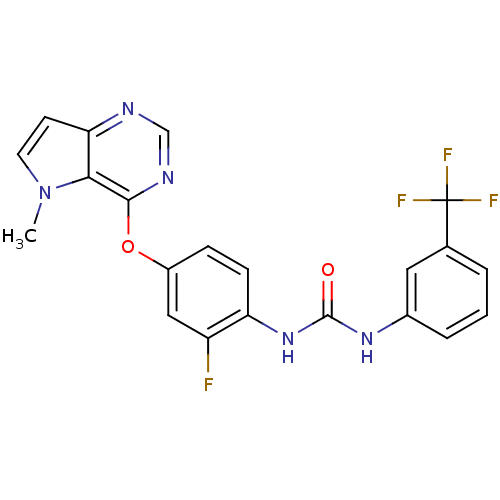 (1-(2-fluoro-4-(5-methyl-5H-pyrrolo[3,2-d]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 60 mins by activity based 100 fold dilution assay | Bioorg Med Chem 19: 5342-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.002 BindingDB Entry DOI: 10.7270/Q21R6QW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523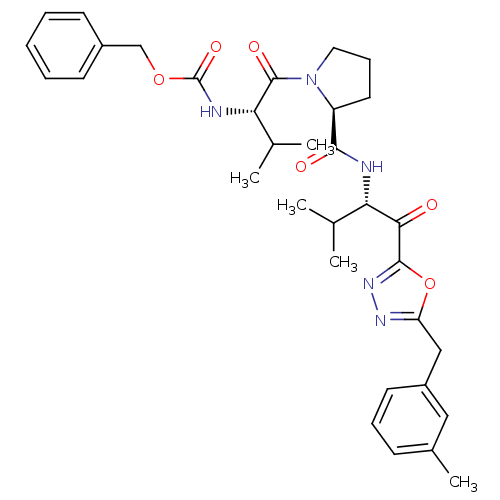 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351399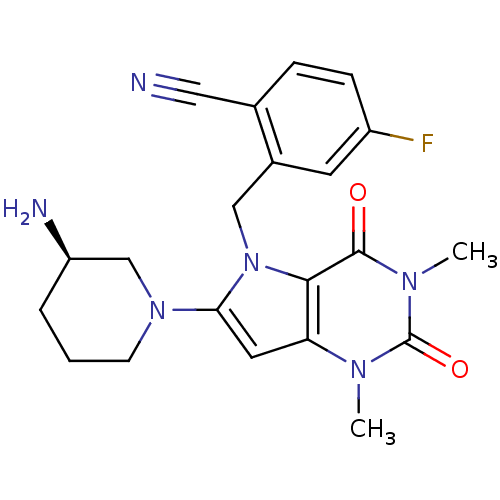 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102777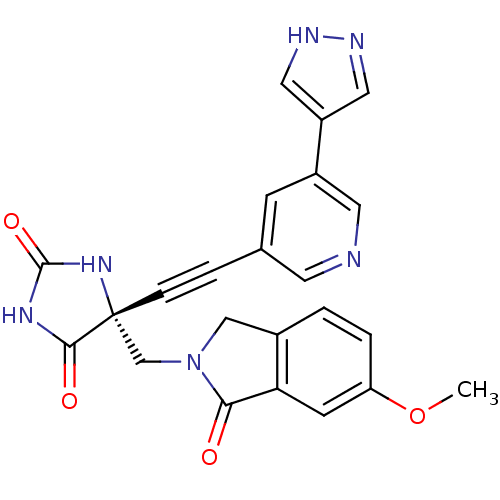 (US8541572, 2207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102624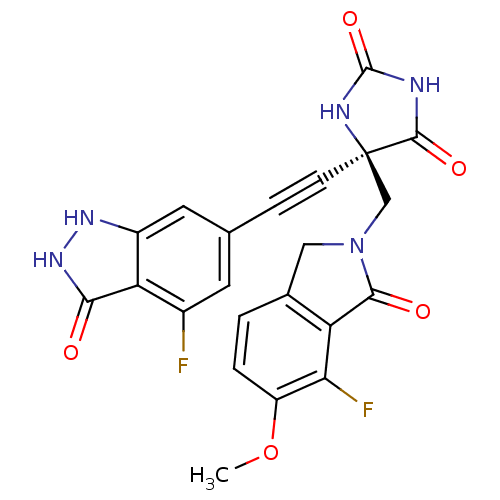 (US8541572, 303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | 383 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126823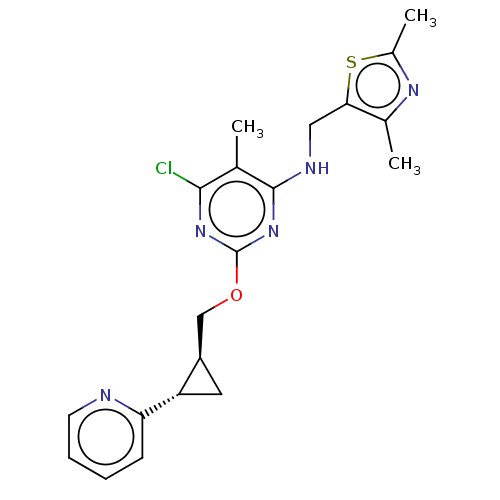 (US8785467, 1-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126823 (US8785467, 1-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469410 (CHEMBL4277886) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50128922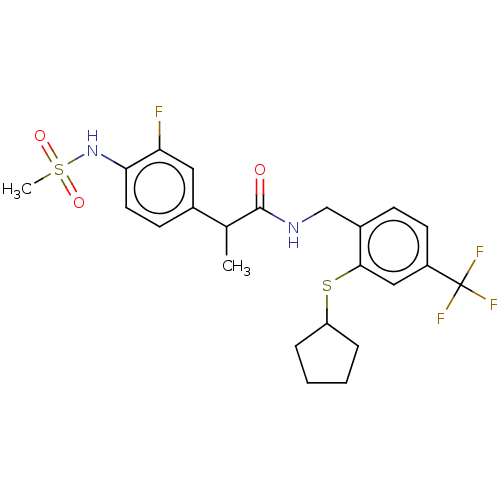 (CHEMBL3627950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay | Bioorg Med Chem 23: 6844-54 (2015) Article DOI: 10.1016/j.bmc.2015.10.001 BindingDB Entry DOI: 10.7270/Q2DZ0B4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50128911 (CHEMBL3627723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay | Bioorg Med Chem 23: 6844-54 (2015) Article DOI: 10.1016/j.bmc.2015.10.001 BindingDB Entry DOI: 10.7270/Q2DZ0B4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144770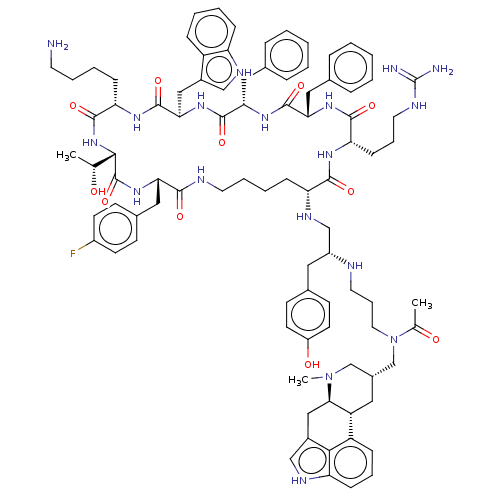 (US8952128, 28A) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144737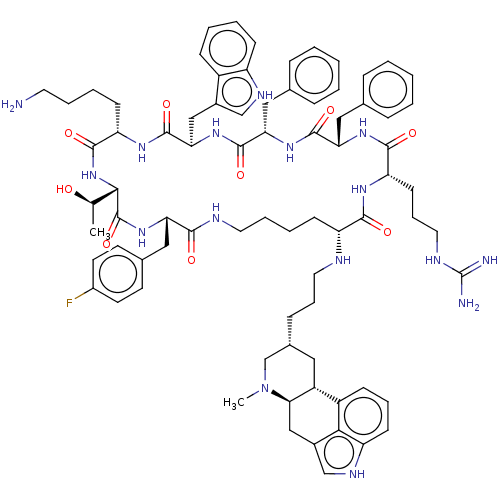 (US8952128, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144755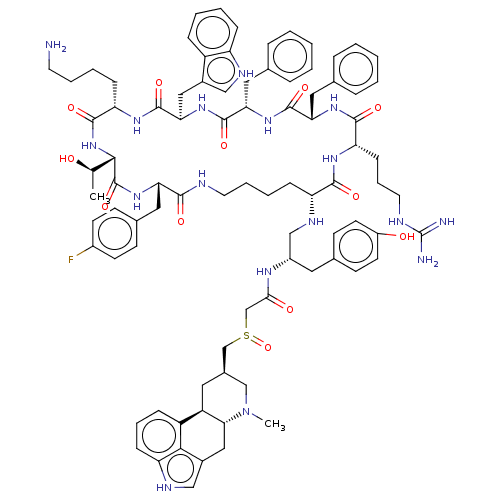 (US8952128, 13) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144758 (US8952128, 16) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469405 (CHEMBL4292006) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221005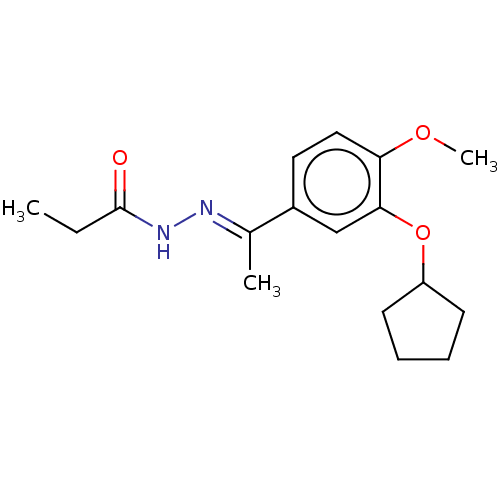 (CHEMBL75684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 50519 total ) | Next | Last >> |