

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524981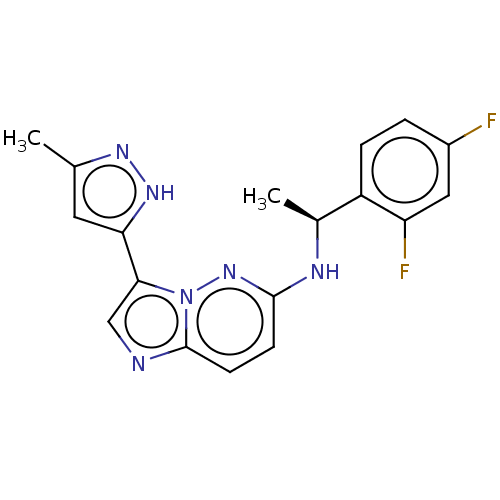 (CHEMBL4562879) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267928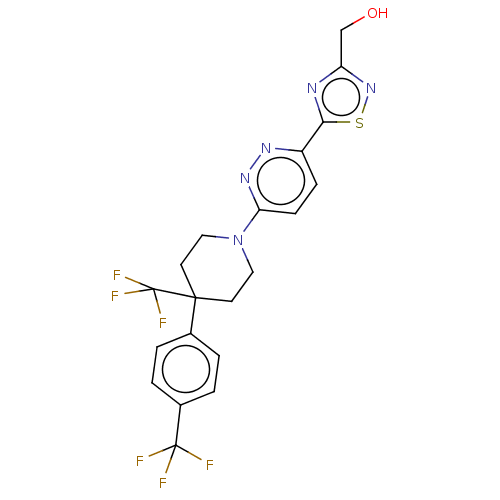 (CHEMBL4066506) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524985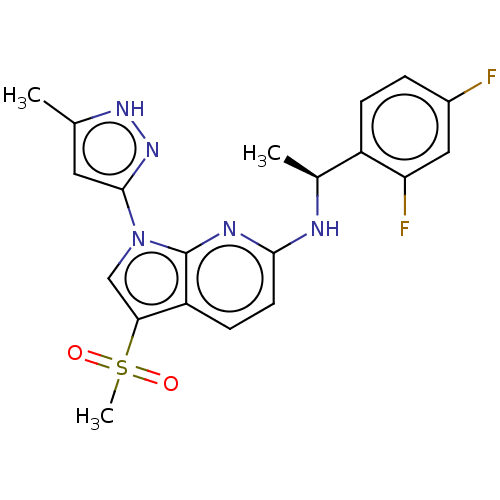 (CHEMBL4460367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524983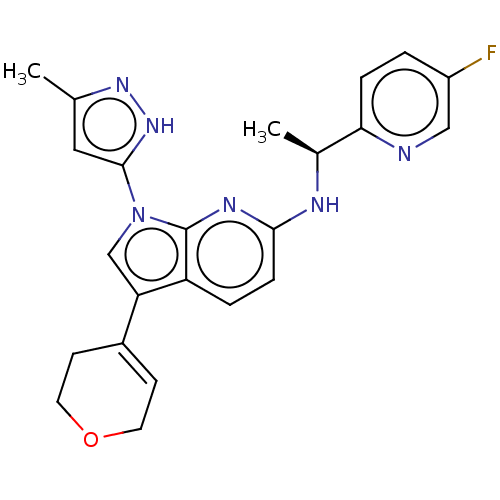 (CHEMBL4458269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524981 (CHEMBL4562879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267930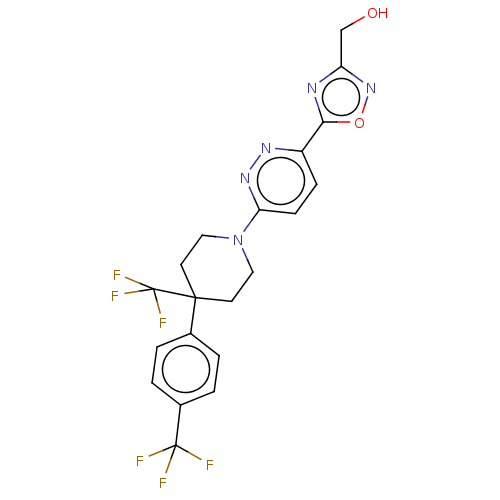 (CHEMBL4104190) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267945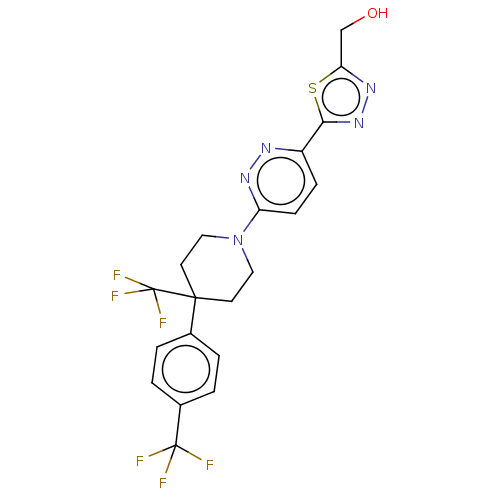 (CHEMBL4094042) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524980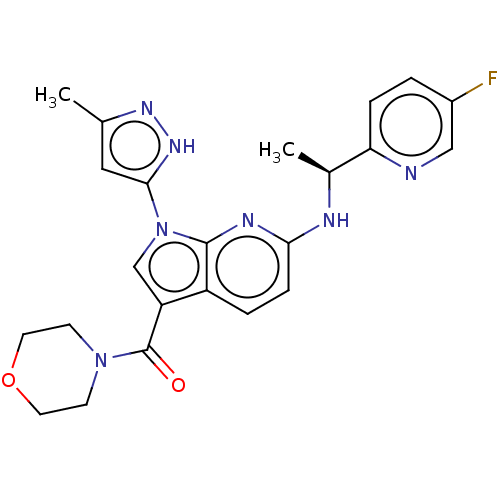 (CHEMBL4437605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50299971 (4-Fluoro-N-{[(+)-(4S)-2-oxo-6-(1H-pyrazol-5-yl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in Pichia pastoris using palmitoyl-CoA by long chain fatty acyl-CoA elongation assay | J Med Chem 52: 7289-300 (2009) Article DOI: 10.1021/jm900915x BindingDB Entry DOI: 10.7270/Q2XK8FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524983 (CHEMBL4458269) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524979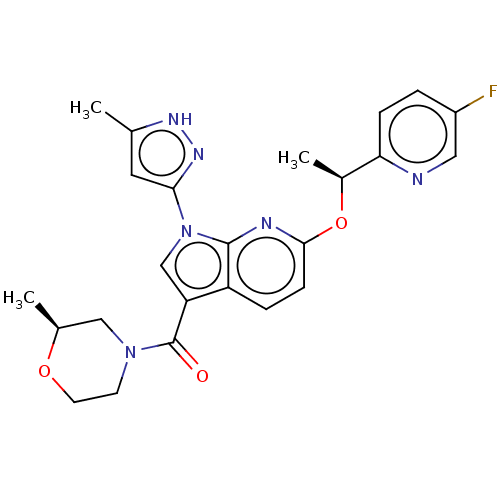 (CHEMBL4440381) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524984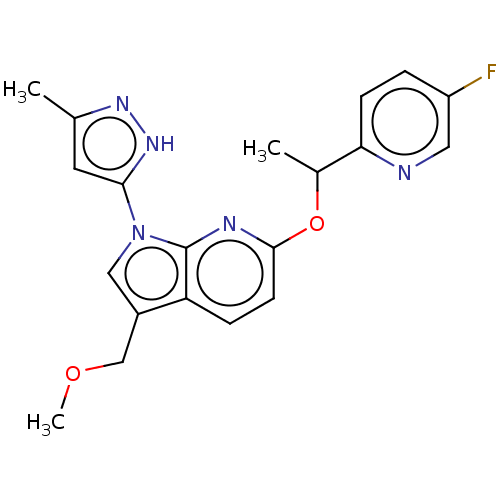 (CHEMBL4434659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524989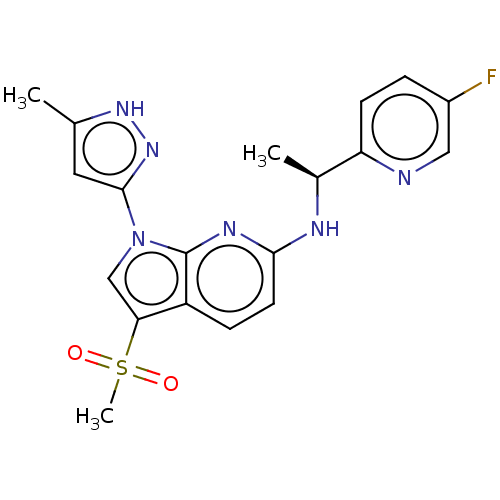 (CHEMBL4535072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Mus musculus) | BDBM50299971 (4-Fluoro-N-{[(+)-(4S)-2-oxo-6-(1H-pyrazol-5-yl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of ELOVL6 in mouse H2.35 cells assessed as elongation index | J Med Chem 52: 7289-300 (2009) Article DOI: 10.1021/jm900915x BindingDB Entry DOI: 10.7270/Q2XK8FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50299964 (4-Fluoro-N-{[2-oxo-6-(1H-pyrazol-5-yl)-4-(trifluor...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in Pichia pastoris using palmitoyl-CoA by long chain fatty acyl-CoA elongation assay | J Med Chem 52: 7289-300 (2009) Article DOI: 10.1021/jm900915x BindingDB Entry DOI: 10.7270/Q2XK8FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524978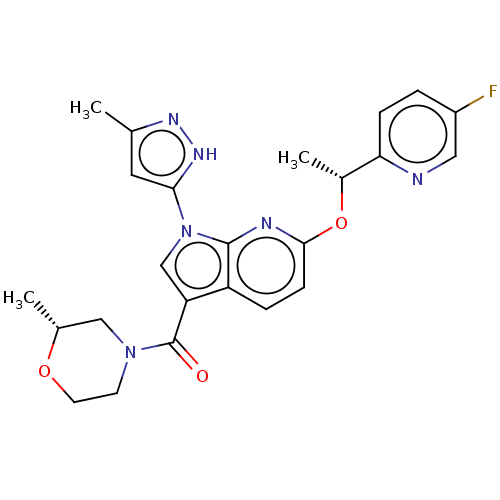 (CHEMBL4573505) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267929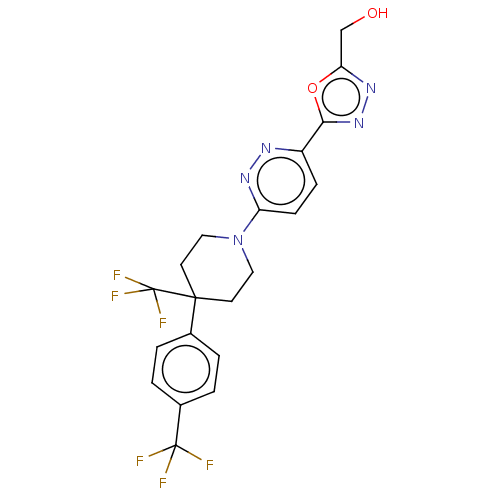 (CHEMBL4086275) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524981 (CHEMBL4562879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524988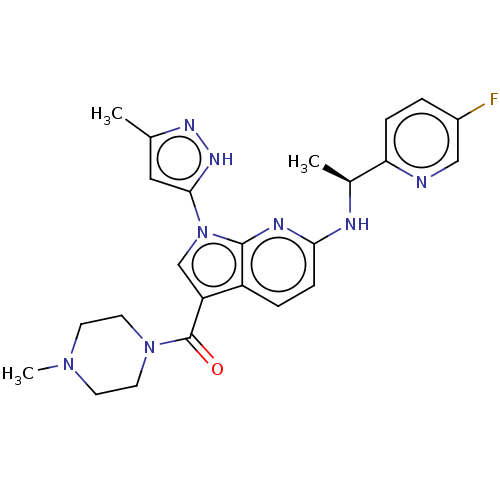 (CHEMBL4586773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524975 (CHEMBL4476859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267946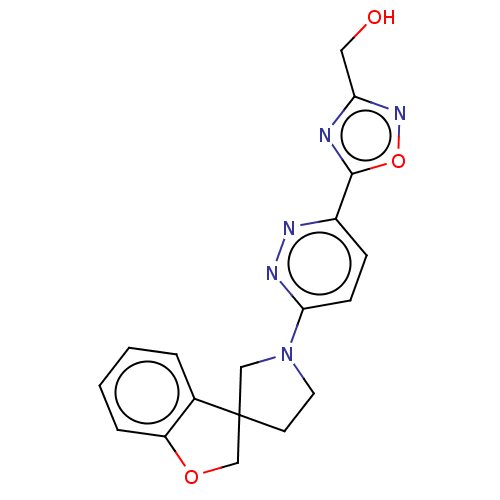 (CHEMBL4075104) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Inhibition of SCD1 (unknown origin) | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50249891 (3-[2-(4-methyllphenyl)-5-methyl-3-oxo-2,3-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in african green monkey COS7 cells assessed as palmitoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50249892 (6,6-dimethyl-3-{5-methyl-3-oxo-2-[4-(trifluorometh...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in african green monkey COS7 cells assessed as palmitoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524983 (CHEMBL4458269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267931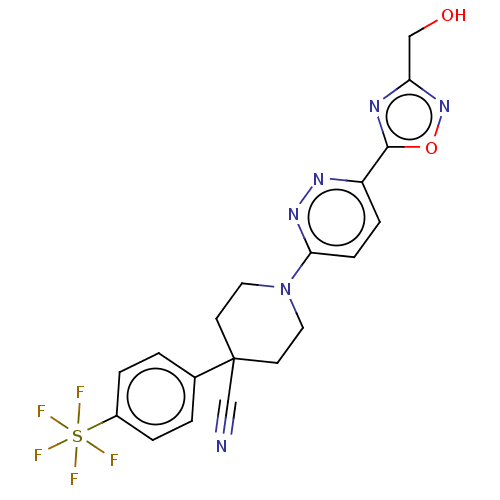 (CHEMBL4070522) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524977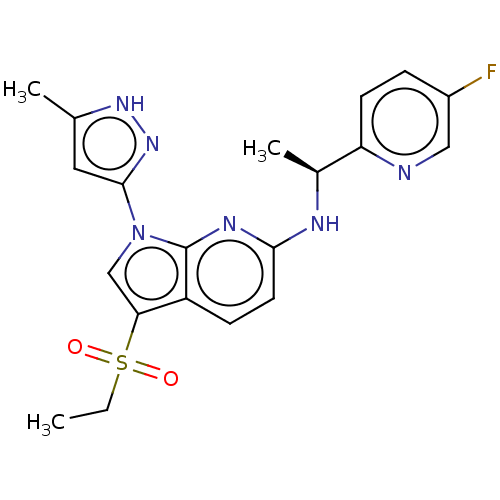 (CHEMBL4443254) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524982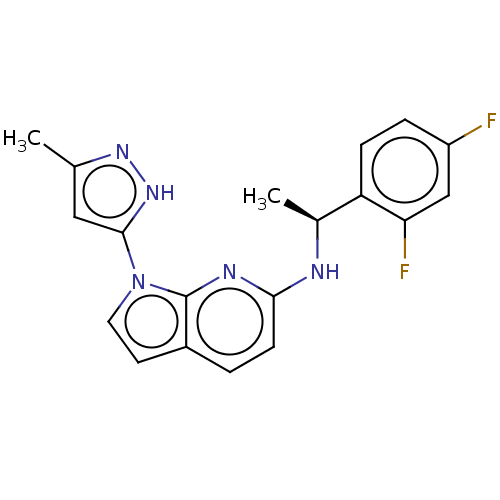 (CHEMBL4448434) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 3 (Homo sapiens (Human)) | BDBM50250164 (6,6-dimethyl-3-(5-methyl-3-oxo-2-phenyl-2,3-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL3 expressed in african green monkey COS7 cells assessed as stearoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50249844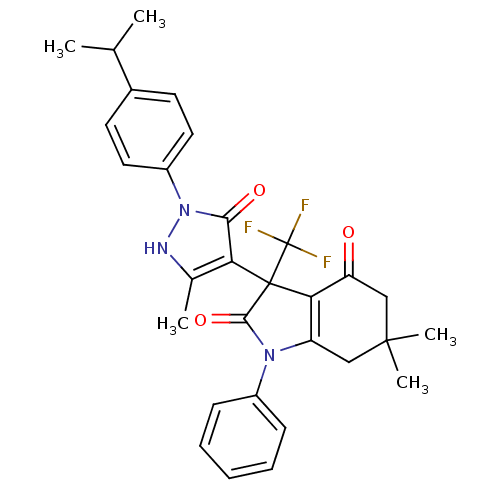 (3-[2-(4-isopropylphenyl)-5-methyl-3-oxo-2,3-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in african green monkey COS7 cells assessed as palmitoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267933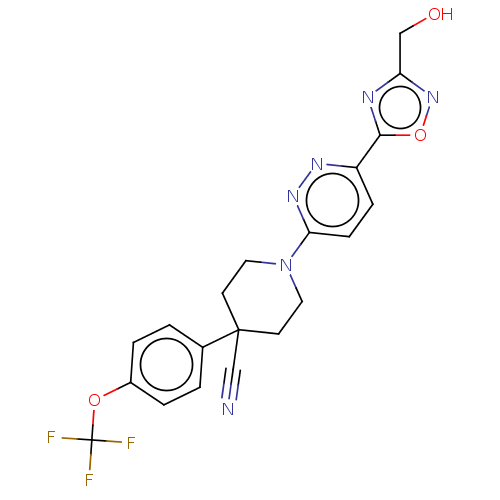 (CHEMBL4092350) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110994 (4-[2-Methyl-4-(5-methyl-thiophen-2-yl)-oxazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50249791 (3-[2-(4-chlorophenyl)-5-methyl-3-oxo-2,3-dihydro-1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in african green monkey COS7 cells assessed as palmitoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524989 (CHEMBL4535072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524982 (CHEMBL4448434) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Mus musculus) | BDBM50299971 (4-Fluoro-N-{[(+)-(4S)-2-oxo-6-(1H-pyrazol-5-yl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse ELOVL6 expressed in Pichia pastoris using palmitoyl-CoA by long chain fatty acyl-CoA elongation assay | J Med Chem 52: 7289-300 (2009) Article DOI: 10.1021/jm900915x BindingDB Entry DOI: 10.7270/Q2XK8FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50249843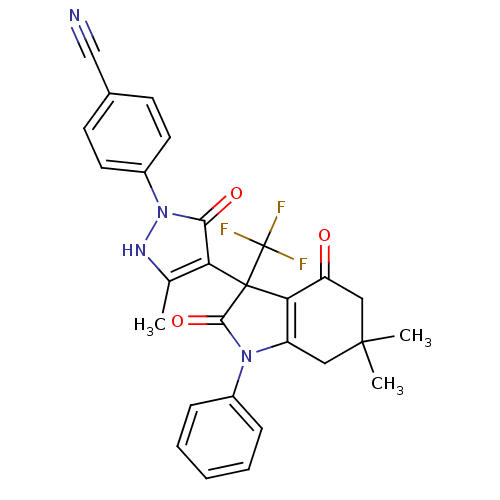 (3-[2-(4-cyanophenyl)-5-methyl-3-oxo-2,3-dihydro-1H...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in african green monkey COS7 cells assessed as palmitoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267936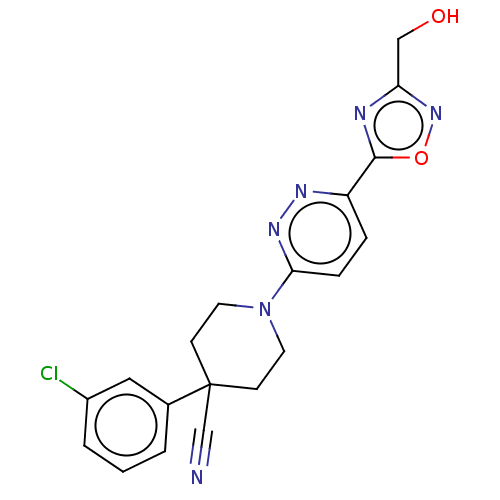 (CHEMBL4074320) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524985 (CHEMBL4460367) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Homo sapiens (Human)) | BDBM50249815 (3-[2-(4-methoxyphenyl)-5-methyl-3-oxo-2,3-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human ELOVL6 expressed in african green monkey COS7 cells assessed as palmitoyl-CoA elongation | J Med Chem 52: 3142-5 (2009) Article DOI: 10.1021/jm900391x BindingDB Entry DOI: 10.7270/Q20P0ZX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524976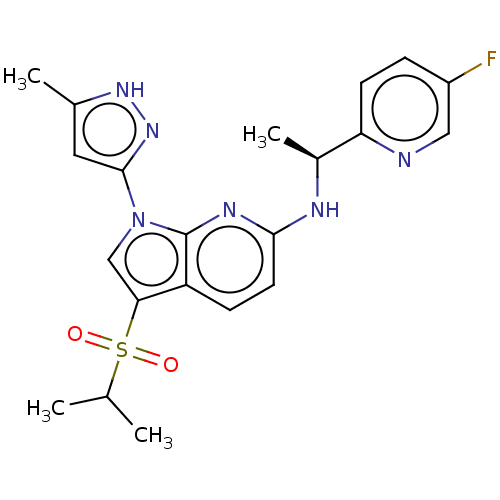 (CHEMBL4435574) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 3 (Mus musculus) | BDBM50299971 (4-Fluoro-N-{[(+)-(4S)-2-oxo-6-(1H-pyrazol-5-yl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse ELOVL3 | J Med Chem 52: 7289-300 (2009) Article DOI: 10.1021/jm900915x BindingDB Entry DOI: 10.7270/Q2XK8FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524986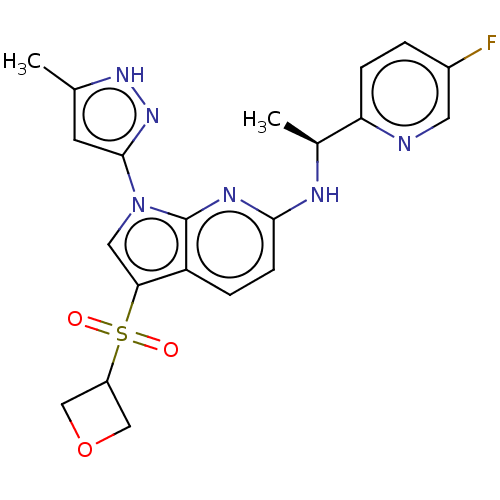 (CHEMBL4516124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267934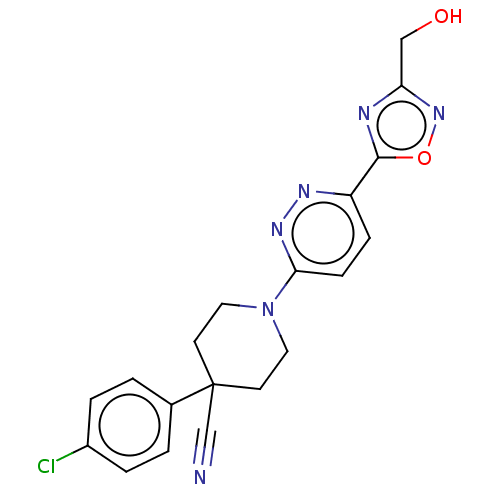 (CHEMBL4082092) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50107528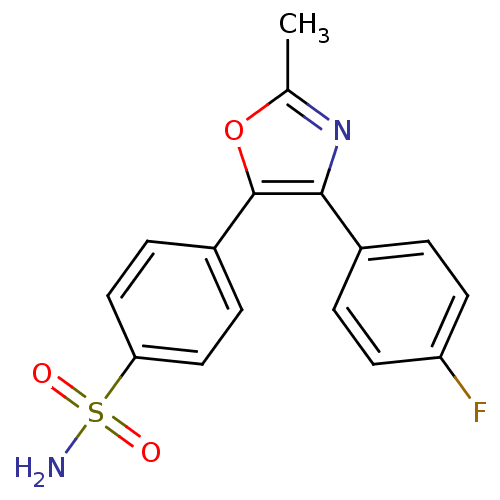 (4-[4-(4-Fluoro-phenyl)-2-methyl-oxazol-5-yl]-benze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elongation of very long chain fatty acids protein 6 (Mus musculus) | BDBM50299963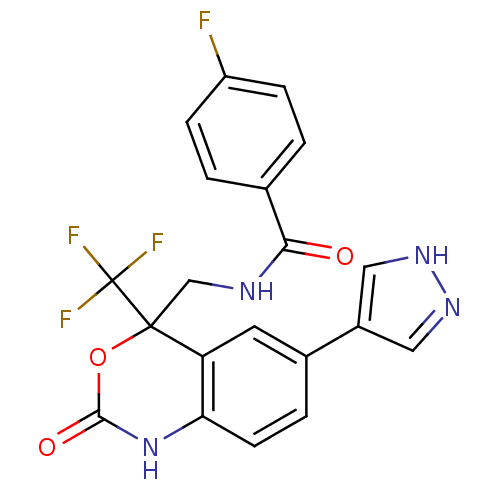 (4-Fluoro-N-{[(+)-2-oxo-6-(1H-pyrazol-4-yl)-4-(trif...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse ELOVL6 expressed in Pichia pastoris using palmitoyl-CoA by long chain fatty acyl-CoA elongation assay | J Med Chem 52: 7289-300 (2009) Article DOI: 10.1021/jm900915x BindingDB Entry DOI: 10.7270/Q2XK8FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 326 total ) | Next | Last >> |