 Found 3439 hits with Last Name = 'imoto' and Initial = 'h'
Found 3439 hits with Last Name = 'imoto' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50105033
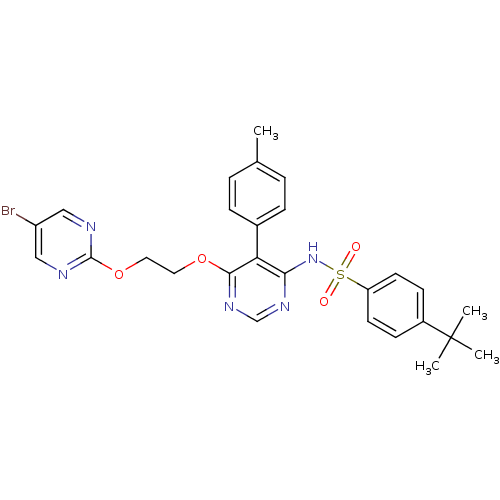
(CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...)Show SMILES Cc1ccc(cc1)-c1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)ncnc1OCCOc1ncc(Br)cn1 Show InChI InChI=1S/C27H28BrN5O4S/c1-18-5-7-19(8-6-18)23-24(33-38(34,35)22-11-9-20(10-12-22)27(2,3)4)31-17-32-25(23)36-13-14-37-26-29-15-21(28)16-30-26/h5-12,15-17H,13-14H2,1-4H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells |
J Med Chem 44: 3369-77 (2001)
BindingDB Entry DOI: 10.7270/Q27M08P8 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369953
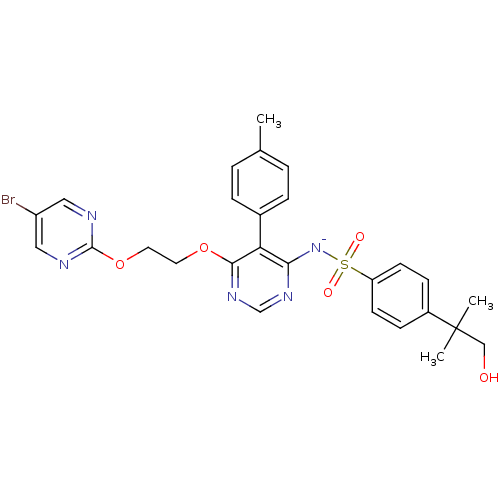
(CHEMBL1627022)Show SMILES Cc1ccc(cc1)-c1c([N-]S(=O)(=O)c2ccc(cc2)C(C)(C)CO)ncnc1OCCOc1ncc(Br)cn1 Show InChI InChI=1S/C27H27BrN5O5S/c1-18-4-6-19(7-5-18)23-24(33-39(35,36)22-10-8-20(9-11-22)27(2,3)16-34)31-17-32-25(23)37-12-13-38-26-29-14-21(28)15-30-26/h4-11,14-15,17,34H,12-13,16H2,1-3H3/q-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells |
J Med Chem 44: 3369-77 (2001)
BindingDB Entry DOI: 10.7270/Q27M08P8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260712

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-iso...)Show SMILES CC(C)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O3/c1-12(2)10-11-22-13-8-6-7-9-14(13)23(18(22)26)17(25)21-15(16(20)24)19(3,4)5/h6-9,12,15H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50088846

(CHEMBL353678 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-e...)Show InChI InChI=1S/C21H27N3O/c1-18(25)22-17-20-9-7-19(8-10-20)11-12-23-13-15-24(16-14-23)21-5-3-2-4-6-21/h2-10H,11-17H2,1H3,(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50088844
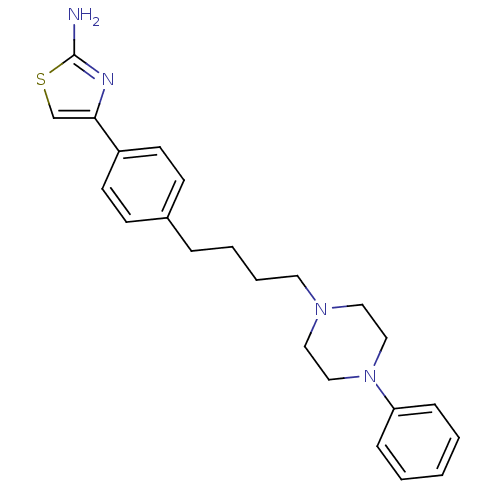
(4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...)Show SMILES Nc1nc(cs1)-c1ccc(CCCCN2CCN(CC2)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N4S/c24-23-25-22(18-28-23)20-11-9-19(10-12-20)6-4-5-13-26-14-16-27(17-15-26)21-7-2-1-3-8-21/h1-3,7-12,18H,4-6,13-17H2,(H2,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377918

(CHEMBL1162994)Show SMILES CC(C)(C)C(NC(=O)n1c2ccccc2n(CCC2CCOCC2)c1=O)C(N)=O Show InChI InChI=1S/C21H30N4O4/c1-21(2,3)17(18(22)26)23-19(27)25-16-7-5-4-6-15(16)24(20(25)28)11-8-14-9-12-29-13-10-14/h4-7,14,17H,8-13H2,1-3H3,(H2,22,26)(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088839
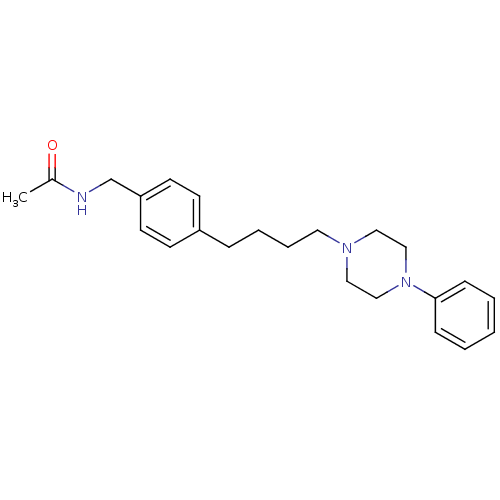
(CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...)Show InChI InChI=1S/C23H31N3O/c1-20(27)24-19-22-12-10-21(11-13-22)7-5-6-14-25-15-17-26(18-16-25)23-8-3-2-4-9-23/h2-4,8-13H,5-7,14-19H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260672
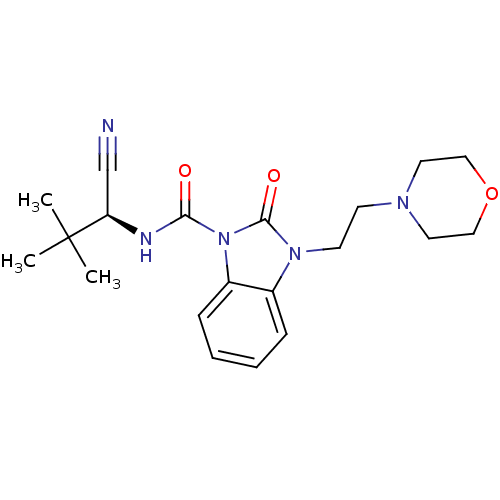
((S)-N-(1-cyano-2,2-dimethylpropyl)-3-(2-morpholino...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C#N |r| Show InChI InChI=1S/C20H27N5O3/c1-20(2,3)17(14-21)22-18(26)25-16-7-5-4-6-15(16)24(19(25)27)9-8-23-10-12-28-13-11-23/h4-7,17H,8-13H2,1-3H3,(H,22,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260673

((S)-N-(2,2-dimethyl-1-(2-methyl-2H-tetrazol-5-yl)p...)Show SMILES Cn1nnc(n1)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C21H30N8O3/c1-21(2,3)17(18-23-25-26(4)24-18)22-19(30)29-16-8-6-5-7-15(16)28(20(29)31)10-9-27-11-13-32-14-12-27/h5-8,17H,9-14H2,1-4H3,(H,22,30)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260627

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-(2-...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H29N5O4/c1-20(2,3)16(17(21)26)22-18(27)25-15-7-5-4-6-14(15)24(19(25)28)9-8-23-10-12-29-13-11-23/h4-7,16H,8-13H2,1-3H3,(H2,21,26)(H,22,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50088844

(4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...)Show SMILES Nc1nc(cs1)-c1ccc(CCCCN2CCN(CC2)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N4S/c24-23-25-22(18-28-23)20-11-9-19(10-12-20)6-4-5-13-26-14-16-27(17-15-26)21-7-2-1-3-8-21/h1-3,7-12,18H,4-6,13-17H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards Dopamine receptor D2 |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260709
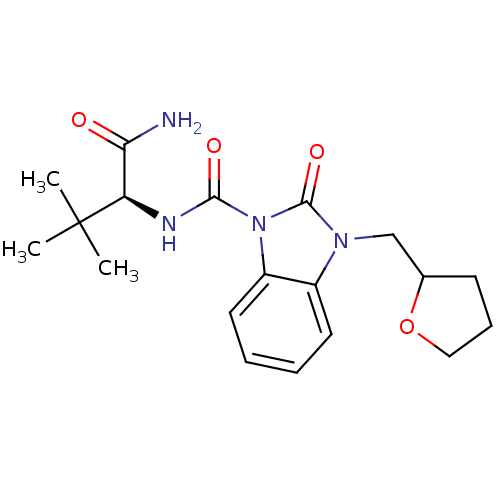
(CHEMBL496497 | N-((S)-1-amino-3,3-dimethyl-1-oxobu...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CC2CCCO2)c1=O)C(N)=O |r| Show InChI InChI=1S/C19H26N4O4/c1-19(2,3)15(16(20)24)21-17(25)23-14-9-5-4-8-13(14)22(18(23)26)11-12-7-6-10-27-12/h4-5,8-9,12,15H,6-7,10-11H2,1-3H3,(H2,20,24)(H,21,25)/t12?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50088839

(CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...)Show InChI InChI=1S/C23H31N3O/c1-20(27)24-19-22-12-10-21(11-13-22)7-5-6-14-25-15-17-26(18-16-25)23-8-3-2-4-9-23/h2-4,8-13H,5-7,14-19H2,1H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088841
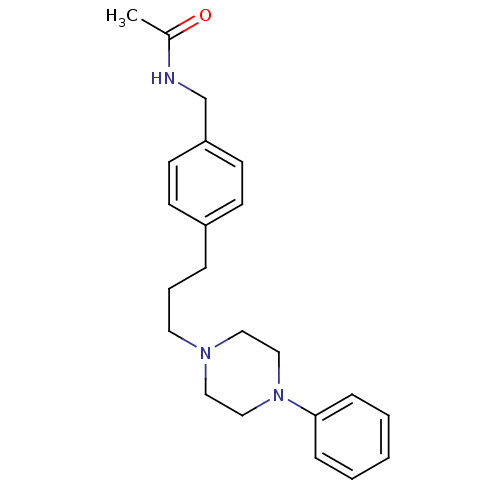
(CHEMBL352617 | N-{4-[3-(4-Phenyl-piperazin-1-yl)-p...)Show InChI InChI=1S/C22H29N3O/c1-19(26)23-18-21-11-9-20(10-12-21)6-5-13-24-14-16-25(17-15-24)22-7-3-2-4-8-22/h2-4,7-12H,5-6,13-18H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261052
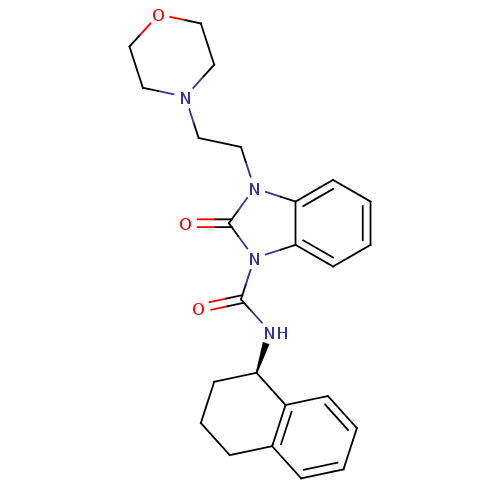
((R)-3-(2-morpholinoethyl)-2-oxo-N-(1,2,3,4-tetrahy...)Show SMILES O=C(N[C@@H]1CCCc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O |r| Show InChI InChI=1S/C24H28N4O3/c29-23(25-20-9-5-7-18-6-1-2-8-19(18)20)28-22-11-4-3-10-21(22)27(24(28)30)13-12-26-14-16-31-17-15-26/h1-4,6,8,10-11,20H,5,7,9,12-17H2,(H,25,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088844

(4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...)Show SMILES Nc1nc(cs1)-c1ccc(CCCCN2CCN(CC2)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N4S/c24-23-25-22(18-28-23)20-11-9-19(10-12-20)6-4-5-13-26-14-16-27(17-15-26)21-7-2-1-3-8-21/h1-3,7-12,18H,4-6,13-17H2,(H2,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of class C beta-lactamase derived from Enterobacter cloacae P99 |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260753
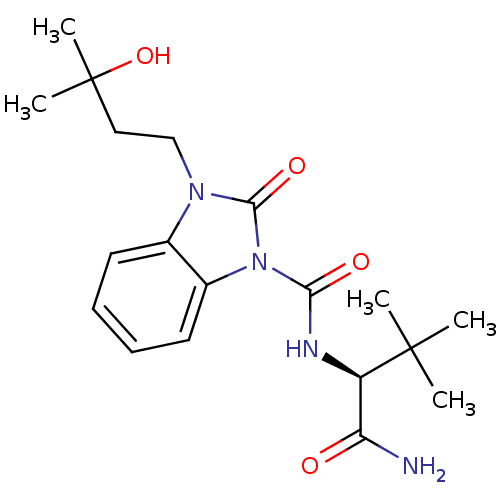
((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-(3-...)Show SMILES CC(C)(O)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O4/c1-18(2,3)14(15(20)24)21-16(25)23-13-9-7-6-8-12(13)22(17(23)26)11-10-19(4,5)27/h6-9,14,27H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261051

(3-(2-morpholinoethyl)-N-(naphthalen-1-yl)-2-oxo-2,...)Show SMILES O=C(Nc1cccc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C24H24N4O3/c29-23(25-20-9-5-7-18-6-1-2-8-19(18)20)28-22-11-4-3-10-21(22)27(24(28)30)13-12-26-14-16-31-17-15-26/h1-11H,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50032379
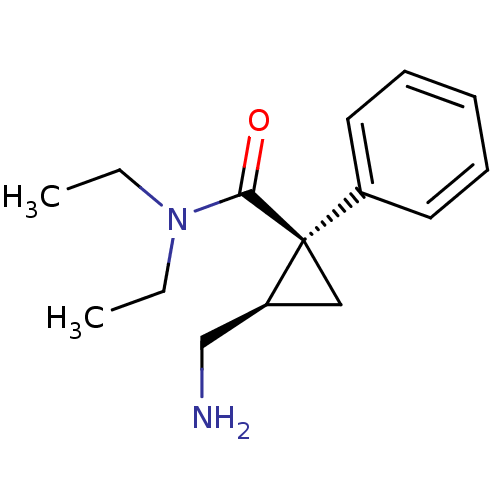
((1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclop...)Show InChI InChI=1S/C15H22N2O/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11,16H2,1-2H3/t13-,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of Serotonin transporter in cerebral cortical synaptic membrane of rats using [3H]-paroxetine |
J Med Chem 41: 3507-14 (1998)
Article DOI: 10.1021/jm980238m
BindingDB Entry DOI: 10.7270/Q2000175 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261099

((R)-1-(2-morpholinoethyl)-2-oxo-N-(1,2,3,4-tetrahy...)Show SMILES O=C(N[C@@H]1CCCc2ccccc12)N1Cc2ccccc2N(CCN2CCOCC2)C1=O |r| Show InChI InChI=1S/C25H30N4O3/c30-24(26-22-10-5-8-19-6-1-3-9-21(19)22)29-18-20-7-2-4-11-23(20)28(25(29)31)13-12-27-14-16-32-17-15-27/h1-4,6-7,9,11,22H,5,8,10,12-18H2,(H,26,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179
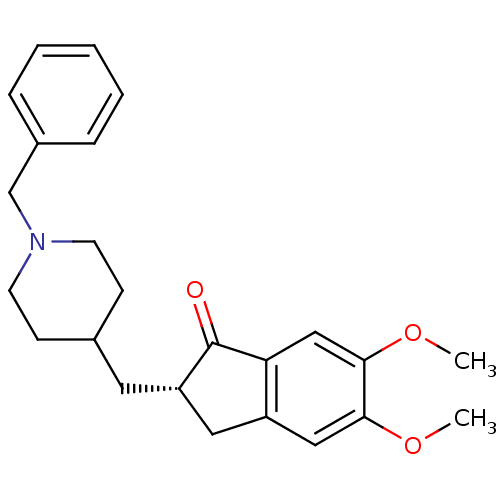
(CHEMBL2093912)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC |r| Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179

(CHEMBL2093912)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC |r| Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260514
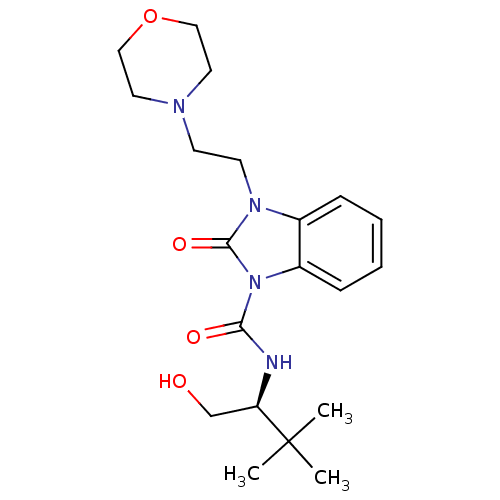
((S)-N-(1-hydroxy-3,3-dimethylbutan-2-yl)-3-(2-morp...)Show SMILES CC(C)(C)[C@@H](CO)NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O |r| Show InChI InChI=1S/C20H30N4O4/c1-20(2,3)17(14-25)21-18(26)24-16-7-5-4-6-15(16)23(19(24)27)9-8-22-10-12-28-13-11-22/h4-7,17,25H,8-14H2,1-3H3,(H,21,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260674
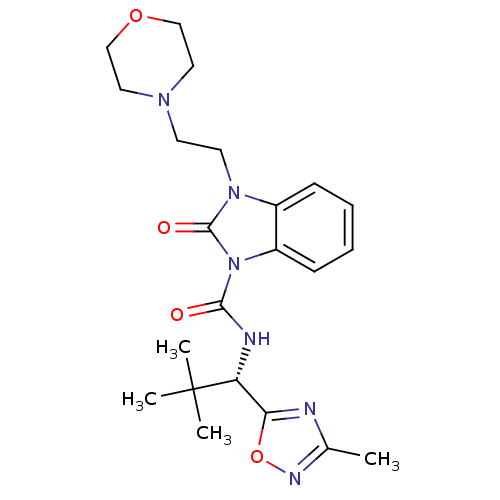
((S)-N-(2,2-dimethyl-1-(3-methyl-1,2,4-oxadiazol-5-...)Show SMILES Cc1noc(n1)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C22H30N6O4/c1-15-23-19(32-25-15)18(22(2,3)4)24-20(29)28-17-8-6-5-7-16(17)27(21(28)30)10-9-26-11-13-31-14-12-26/h5-8,18H,9-14H2,1-4H3,(H,24,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179

(CHEMBL2093912)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC |r| Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179

(CHEMBL2093912)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC |r| Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941
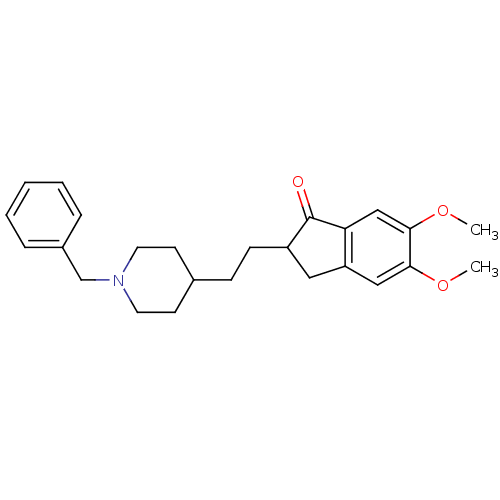
(2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...)Show SMILES COc1cc2CC(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C25H31NO3/c1-28-23-15-21-14-20(25(27)22(21)16-24(23)29-2)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,15-16,18,20H,8-14,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260626
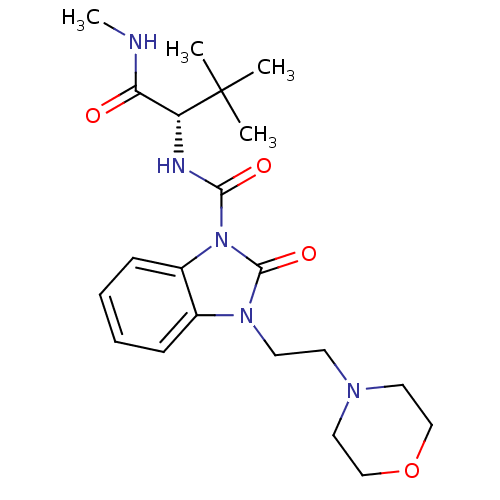
((S)-N'-(3,3-dimethyl-1-(methylamino)-1-oxobutan-2-...)Show SMILES CNC(=O)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C21H31N5O4/c1-21(2,3)17(18(27)22-4)23-19(28)26-16-8-6-5-7-15(16)25(20(26)29)10-9-24-11-13-30-14-12-24/h5-8,17H,9-14H2,1-4H3,(H,22,27)(H,23,28)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260538
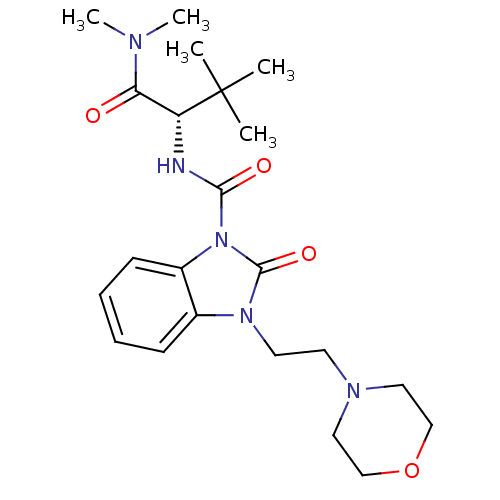
((S)-N-(1-(dimethylamino)-3,3-dimethyl-1-oxobutan-2...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C22H33N5O4/c1-22(2,3)18(19(28)24(4)5)23-20(29)27-17-9-7-6-8-16(17)26(21(27)30)11-10-25-12-14-31-15-13-25/h6-9,18H,10-15H2,1-5H3,(H,23,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941

(2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...)Show SMILES COc1cc2CC(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C25H31NO3/c1-28-23-15-21-14-20(25(27)22(21)16-24(23)29-2)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,15-16,18,20H,8-14,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50088841

(CHEMBL352617 | N-{4-[3-(4-Phenyl-piperazin-1-yl)-p...)Show InChI InChI=1S/C22H29N3O/c1-19(26)23-18-21-11-9-20(10-12-21)6-5-13-24-14-16-25(17-15-24)22-7-3-2-4-8-22/h2-4,7-12H,5-6,13-18H2,1H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM50088844

(4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...)Show SMILES Nc1nc(cs1)-c1ccc(CCCCN2CCN(CC2)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N4S/c24-23-25-22(18-28-23)20-11-9-19(10-12-20)6-4-5-13-26-14-16-27(17-15-26)21-7-2-1-3-8-21/h1-3,7-12,18H,4-6,13-17H2,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of GC1 extended spectrum class C beta-lactamase |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179

(CHEMBL2093912)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC |r| Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179

(CHEMBL2093912)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC |r| Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261053
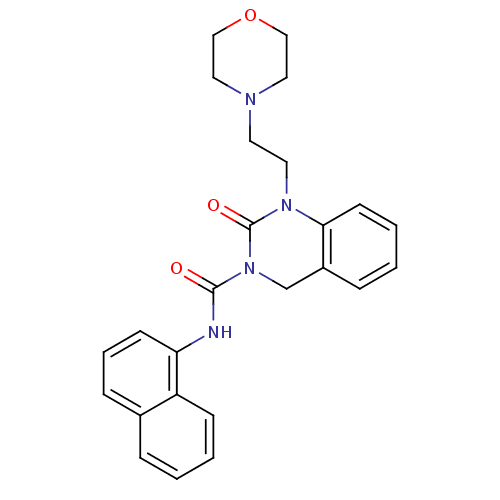
(1-(2-morpholinoethyl)-N-(naphthalen-1-yl)-2-oxo-1,...)Show SMILES O=C(Nc1cccc2ccccc12)N1Cc2ccccc2N(CCN2CCOCC2)C1=O Show InChI InChI=1S/C25H26N4O3/c30-24(26-22-10-5-8-19-6-1-3-9-21(19)22)29-18-20-7-2-4-11-23(20)28(25(29)31)13-12-27-14-16-32-17-15-27/h1-11H,12-18H2,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50369953

(CHEMBL1627022)Show SMILES Cc1ccc(cc1)-c1c([N-]S(=O)(=O)c2ccc(cc2)C(C)(C)CO)ncnc1OCCOc1ncc(Br)cn1 Show InChI InChI=1S/C27H27BrN5O5S/c1-18-4-6-19(7-5-18)23-24(33-39(35,36)22-10-8-20(9-11-22)27(2,3)16-34)31-17-32-25(23)37-12-13-38-26-29-14-21(28)15-30-26/h4-11,14-15,17,34H,12-13,16H2,1-3H3/q-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-ET-1 binding to human cloned ETB receptors expressed on CHO cells |
J Med Chem 44: 3369-77 (2001)
BindingDB Entry DOI: 10.7270/Q27M08P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941

(2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...)Show SMILES COc1cc2CC(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C25H31NO3/c1-28-23-15-21-14-20(25(27)22(21)16-24(23)29-2)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,15-16,18,20H,8-14,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM50088839

(CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...)Show InChI InChI=1S/C23H31N3O/c1-20(27)24-19-22-12-10-21(11-13-22)7-5-6-14-25-15-17-26(18-16-25)23-8-3-2-4-9-23/h2-4,8-13H,5-7,14-19H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of GC1 extended spectrum class C beta-lactamase |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50088839

(CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...)Show InChI InChI=1S/C23H31N3O/c1-20(27)24-19-22-12-10-21(11-13-22)7-5-6-14-25-15-17-26(18-16-25)23-8-3-2-4-9-23/h2-4,8-13H,5-7,14-19H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards Dopamine receptor D2 |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260512
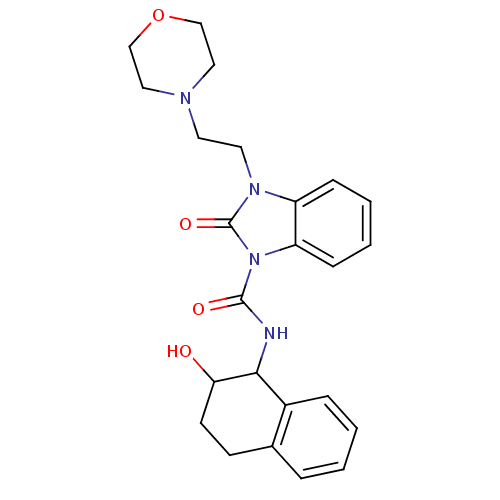
(CHEMBL494563 | N-(2-hydroxy-1,2,3,4-tetrahydronaph...)Show SMILES OC1CCc2ccccc2C1NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C24H28N4O4/c29-21-10-9-17-5-1-2-6-18(17)22(21)25-23(30)28-20-8-4-3-7-19(20)27(24(28)31)12-11-26-13-15-32-16-14-26/h1-8,21-22,29H,9-16H2,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912

(2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...)Show InChI InChI=1S/C23H27NO3/c1-26-21-13-18-12-19(23(25)20(18)14-22(21)27-2)17-8-10-24(11-9-17)15-16-6-4-3-5-7-16/h3-7,13-14,17,19H,8-12,15H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912

(2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...)Show InChI InChI=1S/C23H27NO3/c1-26-21-13-18-12-19(23(25)20(18)14-22(21)27-2)17-8-10-24(11-9-17)15-16-6-4-3-5-7-16/h3-7,13-14,17,19H,8-12,15H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261100
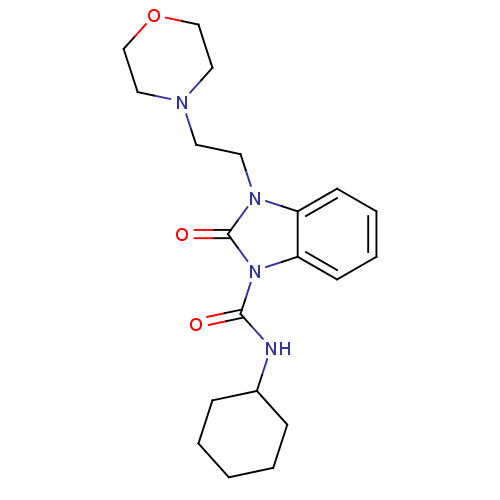
(CHEMBL494627 | N-cyclohexyl-3-(2-morpholinoethyl)-...)Show InChI InChI=1S/C20H28N4O3/c25-19(21-16-6-2-1-3-7-16)24-18-9-5-4-8-17(18)23(20(24)26)11-10-22-12-14-27-15-13-22/h4-5,8-9,16H,1-3,6-7,10-15H2,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260628
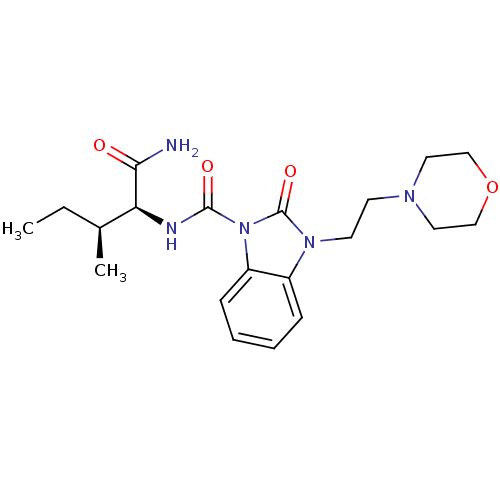
(CHEMBL495391 | N-((2S,3S)-1-amino-3-methyl-1-oxope...)Show SMILES CC[C@H](C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H29N5O4/c1-3-14(2)17(18(21)26)22-19(27)25-16-7-5-4-6-15(16)24(20(25)28)9-8-23-10-12-29-13-11-23/h4-7,14,17H,3,8-13H2,1-2H3,(H2,21,26)(H,22,27)/t14-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088846

(CHEMBL353678 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-e...)Show InChI InChI=1S/C21H27N3O/c1-18(25)22-17-20-9-7-19(8-10-20)11-12-23-13-15-24(16-14-23)21-5-3-2-4-6-21/h2-10H,11-17H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 875-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CZ36C7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260712

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-iso...)Show SMILES CC(C)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O3/c1-12(2)10-11-22-13-8-6-7-9-14(13)23(18(22)26)17(25)21-15(16(20)24)19(3,4)5/h6-9,12,15H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260511
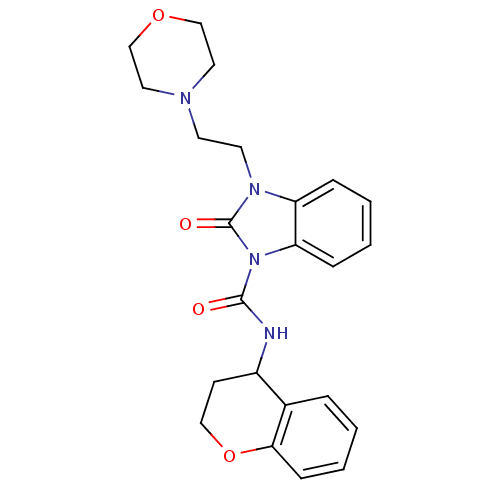
(CHEMBL495407 | N-(chroman-4-yl)-3-(2-morpholinoeth...)Show SMILES O=C(NC1CCOc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C23H26N4O4/c28-22(24-18-9-14-31-21-8-4-1-5-17(18)21)27-20-7-3-2-6-19(20)26(23(27)29)11-10-25-12-15-30-16-13-25/h1-8,18H,9-16H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50105000
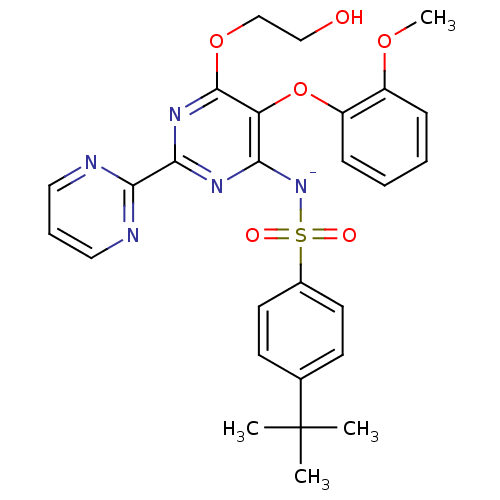
(CHEMBL175616 | sodium salt of 4-tert-Butyl-N-[6-(2...)Show SMILES COc1ccccc1Oc1c([N-]S(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCO)-c1ncccn1 Show InChI InChI=1S/C27H28N5O6S/c1-27(2,3)18-10-12-19(13-11-18)39(34,35)32-23-22(38-21-9-6-5-8-20(21)36-4)26(37-17-16-33)31-25(30-23)24-28-14-7-15-29-24/h5-15,33H,16-17H2,1-4H3/q-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells |
J Med Chem 44: 3369-77 (2001)
BindingDB Entry DOI: 10.7270/Q27M08P8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260750
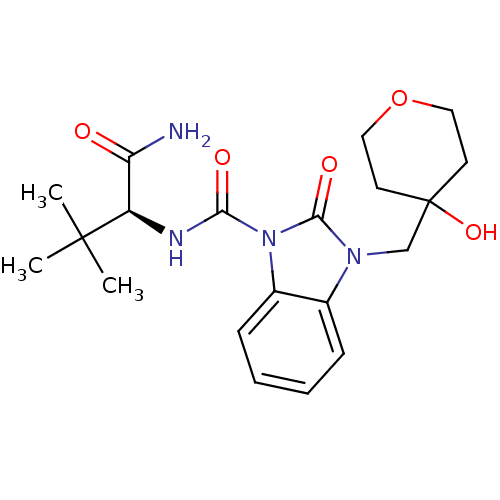
((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-((4...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CC2(O)CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H28N4O5/c1-19(2,3)15(16(21)25)22-17(26)24-14-7-5-4-6-13(14)23(18(24)27)12-20(28)8-10-29-11-9-20/h4-7,15,28H,8-12H2,1-3H3,(H2,21,25)(H,22,26)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912

(2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...)Show InChI InChI=1S/C23H27NO3/c1-26-21-13-18-12-19(23(25)20(18)14-22(21)27-2)17-8-10-24(11-9-17)15-16-6-4-3-5-7-16/h3-7,13-14,17,19H,8-12,15H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) |
J Med Chem 39: 4460-70 (1996)
Article DOI: 10.1021/jm950596e
BindingDB Entry DOI: 10.7270/Q2HD7WB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data