 Found 151 hits with Last Name = 'ishihara' and Initial = 's'
Found 151 hits with Last Name = 'ishihara' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50024714

(2-(4-Carboxymethyl-5-oxo-3-thiophen-2-yl-[1,4]thia...)Show SMILES OC(=O)CN1C(CSC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1cccs1 Show InChI InChI=1S/C21H24N2O5S2/c24-19(25)11-23-17(18-7-4-10-30-18)13-29-12-16(20(23)26)22-15(21(27)28)9-8-14-5-2-1-3-6-14/h1-7,10,15-17,22H,8-9,11-13H2,(H,24,25)(H,27,28)/t15-,16-,17?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287241
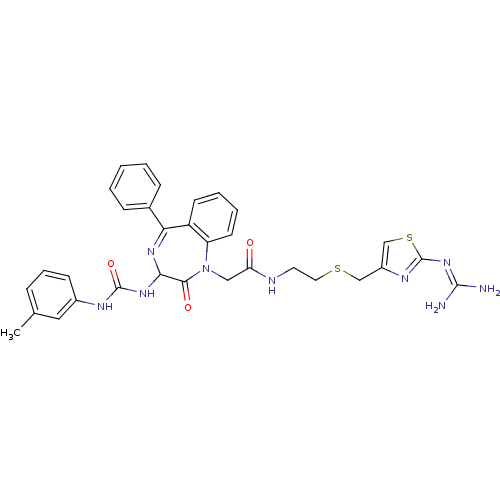
(CHEMBL285746 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C32H33N9O3S2/c1-20-8-7-11-22(16-20)36-31(44)39-28-29(43)41(25-13-6-5-12-24(25)27(38-28)21-9-3-2-4-10-21)17-26(42)35-14-15-45-18-23-19-46-32(37-23)40-30(33)34/h2-13,16,19,28H,14-15,17-18H2,1H3,(H,35,42)(H2,36,39,44)(H4,33,34,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023299
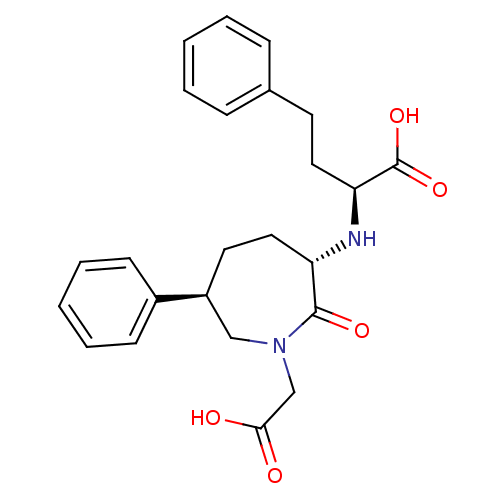
(2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...)Show SMILES OC(=O)CN1C[C@H](CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C24H28N2O5/c27-22(28)16-26-15-19(18-9-5-2-6-10-18)12-14-20(23(26)29)25-21(24(30)31)13-11-17-7-3-1-4-8-17/h1-10,19-21,25H,11-16H2,(H,27,28)(H,30,31)/t19-,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023298

(2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1[C@@H](CSC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)13-25-20(17-9-5-2-6-10-17)15-31-14-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023298

(2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1[C@@H](CSC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)13-25-20(17-9-5-2-6-10-17)15-31-14-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50024710

(2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...)Show SMILES OC(=O)CN1C[C@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1cccs1 Show InChI InChI=1S/C21H24N2O5S2/c24-19(25)12-23-11-18(17-7-4-10-29-17)30-13-16(20(23)26)22-15(21(27)28)9-8-14-5-2-1-3-6-14/h1-7,10,15-16,18,22H,8-9,11-13H2,(H,24,25)(H,27,28)/t15-,16-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023296

(2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...)Show SMILES OC(=O)CN1[C@@H](CCC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C24H28N2O5/c27-22(28)16-26-21(18-10-5-2-6-11-18)13-7-12-19(23(26)29)25-20(24(30)31)15-14-17-8-3-1-4-9-17/h1-6,8-11,19-21,25H,7,12-16H2,(H,27,28)(H,30,31)/t19-,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023295
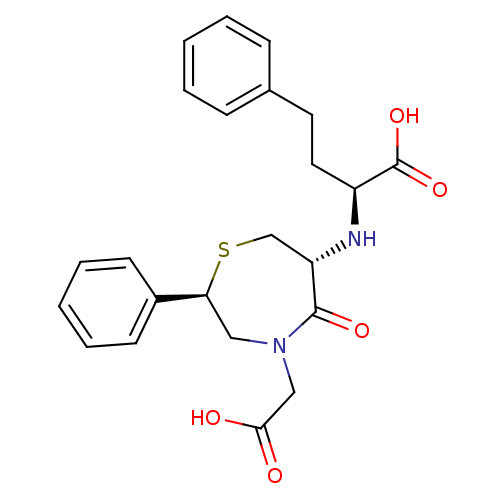
(2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1C[C@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)14-25-13-20(17-9-5-2-6-10-17)31-15-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023295

(2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1C[C@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)14-25-13-20(17-9-5-2-6-10-17)31-15-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287270

(CHEMBL432671 | [(2-{2-[2-(2,2-Diamino-vinyl)-thiaz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCNC(=O)CC(=O)NCCSCc3csc(CC(N)=N)n3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C37H40N10O6S2/c1-47-28-13-6-5-12-27(28)33(24-9-3-2-4-10-24)45-34(35(47)50)46-36(51)44-25-11-7-8-23(16-25)19-53-37(52)42-22-41-31(49)18-30(48)40-14-15-54-20-26-21-55-32(43-26)17-29(38)39/h2-13,16,21,34H,14-15,17-20,22H2,1H3,(H3,38,39)(H,40,48)(H,41,49)(H,42,52)(H2,44,46,51)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50024713

(2-(4-Carboxymethyl-5-oxo-2-thiophen-3-yl-[1,4]thia...)Show SMILES OC(=O)CN1C[C@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccsc1 Show InChI InChI=1S/C21H24N2O5S2/c24-19(25)11-23-10-18(15-8-9-29-12-15)30-13-17(20(23)26)22-16(21(27)28)7-6-14-4-2-1-3-5-14/h1-5,8-9,12,16-18,22H,6-7,10-11,13H2,(H,24,25)(H,27,28)/t16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023300
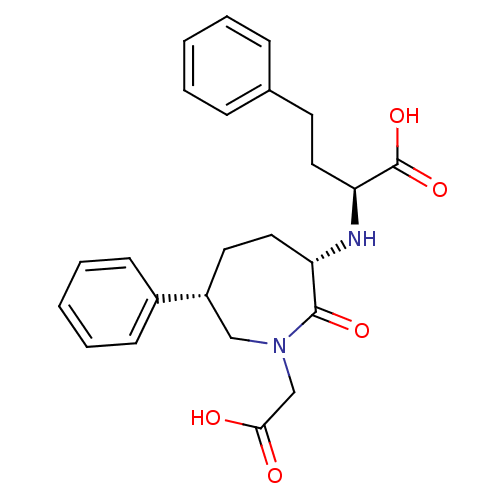
(2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...)Show SMILES OC(=O)CN1C[C@@H](CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C24H28N2O5/c27-22(28)16-26-15-19(18-9-5-2-6-10-18)12-14-20(23(26)29)25-21(24(30)31)13-11-17-7-3-1-4-8-17/h1-10,19-21,25H,11-16H2,(H,27,28)(H,30,31)/t19-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50011192

((R)-1-[(S)-2-((S)-1-Carboxy-3-phenyl-propylamino)-...)Show SMILES CC(NC(CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12?,14?,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287272

(CHEMBL33412 | [2-(4-{2-[3-(3-Piperidin-1-ylmethyl-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCN3CCN(CC3)C(=O)CNCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C48H59N9O6/c1-54-42-20-7-6-19-41(42)44(38-15-4-2-5-16-38)52-45(46(54)59)53-47(60)51-39-17-10-14-37(31-39)35-63-48(61)50-22-25-55-26-28-57(29-27-55)43(58)33-49-21-12-30-62-40-18-11-13-36(32-40)34-56-23-8-3-9-24-56/h2,4-7,10-11,13-20,31-32,45,49H,3,8-9,12,21-30,33-35H2,1H3,(H,50,61)(H2,51,53,60)/t45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287273
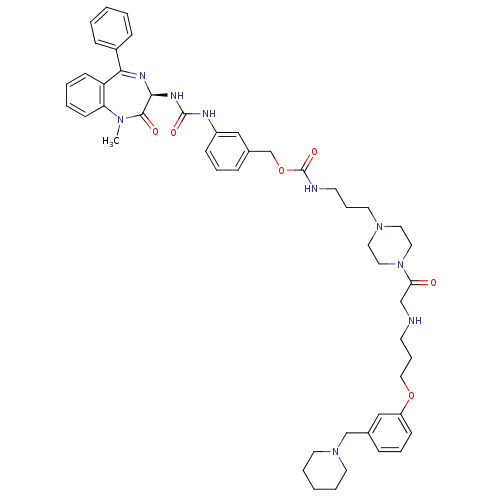
(CHEMBL285719 | [3-(4-{2-[3-(3-Piperidin-1-ylmethyl...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCCN3CCN(CC3)C(=O)CNCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C49H61N9O6/c1-55-43-21-7-6-20-42(43)45(39-16-4-2-5-17-39)53-46(47(55)60)54-48(61)52-40-18-10-15-38(32-40)36-64-49(62)51-23-12-26-56-27-29-58(30-28-56)44(59)34-50-22-13-31-63-41-19-11-14-37(33-41)35-57-24-8-3-9-25-57/h2,4-7,10-11,14-21,32-33,46,50H,3,8-9,12-13,22-31,34-36H2,1H3,(H,51,62)(H2,52,54,61)/t46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287268
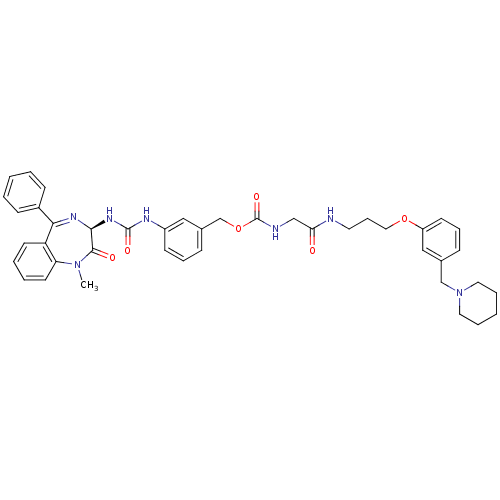
(CHEMBL284976 | {[3-(3-Piperidin-1-ylmethyl-phenoxy...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C42H47N7O6/c1-48-36-20-7-6-19-35(36)38(32-15-4-2-5-16-32)46-39(40(48)51)47-41(52)45-33-17-10-14-31(25-33)29-55-42(53)44-27-37(50)43-21-12-24-54-34-18-11-13-30(26-34)28-49-22-8-3-9-23-49/h2,4-7,10-11,13-20,25-26,39H,3,8-9,12,21-24,27-29H2,1H3,(H,43,50)(H,44,53)(H2,45,47,52)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287239
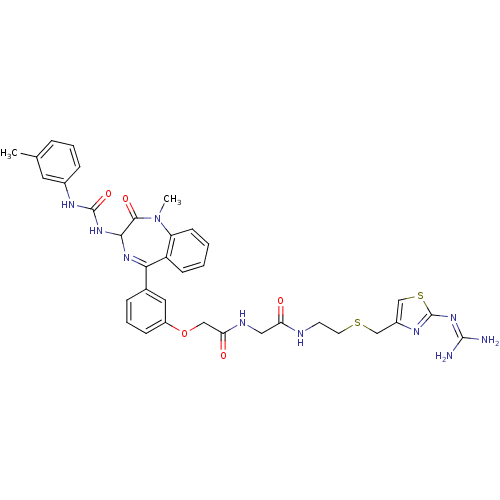
(CHEMBL30656 | N-{[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](/[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C35H38N10O5S2/c1-21-7-5-9-23(15-21)40-34(49)43-31-32(48)45(2)27-12-4-3-11-26(27)30(42-31)22-8-6-10-25(16-22)50-18-29(47)39-17-28(46)38-13-14-51-19-24-20-52-35(41-24)44-33(36)37/h3-12,15-16,20,31H,13-14,17-19H2,1-2H3,(H,38,46)(H,39,47)(H2,40,43,49)(H4,36,37,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287232
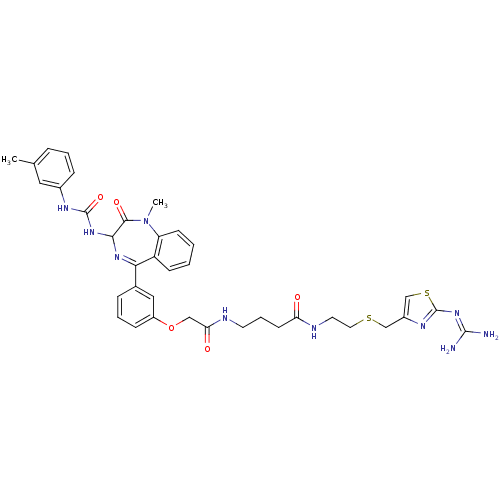
(CHEMBL31726 | N-[2-(2-Guanidino-thiazol-4-ylmethyl...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](\[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C37H42N10O5S2/c1-23-8-5-10-25(18-23)42-36(51)45-33-34(50)47(2)29-13-4-3-12-28(29)32(44-33)24-9-6-11-27(19-24)52-20-31(49)40-15-7-14-30(48)41-16-17-53-21-26-22-54-37(43-26)46-35(38)39/h3-6,8-13,18-19,22,33H,7,14-17,20-21H2,1-2H3,(H,40,49)(H,41,48)(H2,42,45,51)(H4,38,39,43,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023302
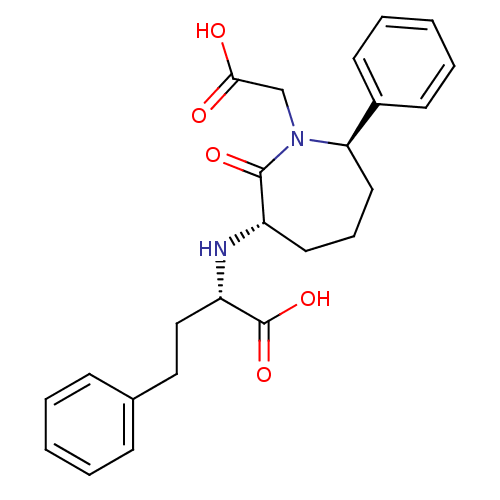
(2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...)Show SMILES OC(=O)CN1[C@H](CCC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C24H28N2O5/c27-22(28)16-26-21(18-10-5-2-6-11-18)13-7-12-19(23(26)29)25-20(24(30)31)15-14-17-8-3-1-4-9-17/h1-6,8-11,19-21,25H,7,12-16H2,(H,27,28)(H,30,31)/t19-,20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287238
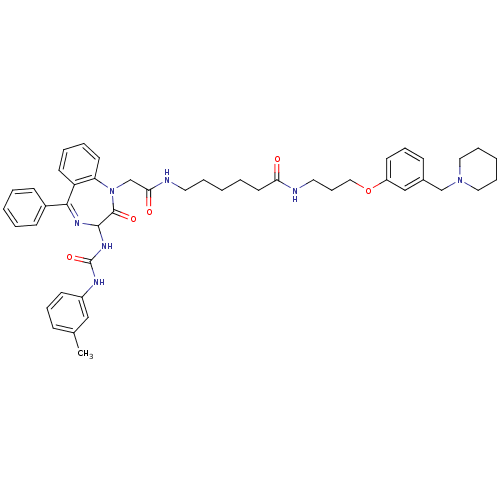
(6-{2-[2-Oxo-5-phenyl-3-(3-m-tolyl-ureido)-2,3-dihy...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3)c3ccccc3N(CC(=O)NCCCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)C2=O)c1 |t:11| Show InChI InChI=1S/C46H55N7O5/c1-34-16-13-20-37(30-34)49-46(57)51-44-45(56)53(40-23-9-8-22-39(40)43(50-44)36-18-5-2-6-19-36)33-42(55)48-25-10-3-7-24-41(54)47-26-15-29-58-38-21-14-17-35(31-38)32-52-27-11-4-12-28-52/h2,5-6,8-9,13-14,16-23,30-31,44H,3-4,7,10-12,15,24-29,32-33H2,1H3,(H,47,54)(H,48,55)(H2,49,51,57) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287260
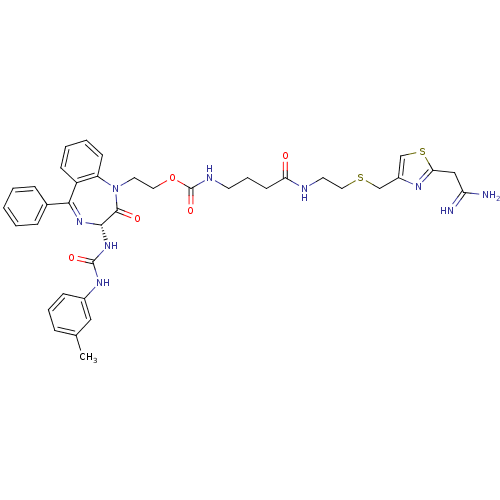
((3-{2-[2-(2,2-Diamino-vinyl)-thiazol-4-ylmethylsul...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CCOC(=O)NCCCC(=O)NCCSCc3csc(CC(N)=N)n3)C2=O)c1 |t:11| Show InChI InChI=1S/C38H43N9O5S2/c1-25-9-7-12-27(21-25)44-37(50)46-35-36(49)47(30-14-6-5-13-29(30)34(45-35)26-10-3-2-4-11-26)18-19-52-38(51)42-16-8-15-32(48)41-17-20-53-23-28-24-54-33(43-28)22-31(39)40/h2-7,9-14,21,24,35H,8,15-20,22-23H2,1H3,(H3,39,40)(H,41,48)(H,42,51)(H2,44,46,50)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287263
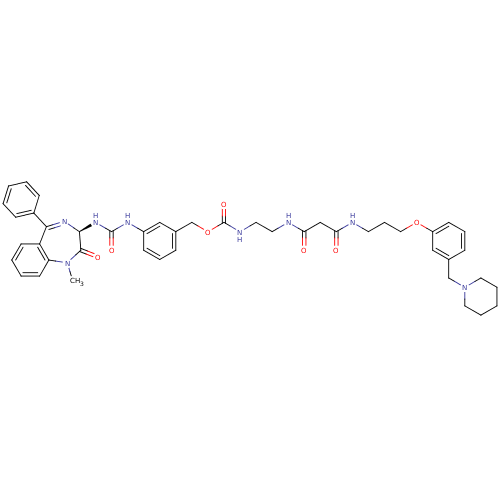
((2-{2-[3-(3-Piperidin-1-ylmethyl-phenoxy)-propylca...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCNC(=O)CC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C45H52N8O7/c1-52-38-20-7-6-19-37(38)41(34-15-4-2-5-16-34)50-42(43(52)56)51-44(57)49-35-17-10-14-33(27-35)31-60-45(58)48-23-22-47-40(55)29-39(54)46-21-12-26-59-36-18-11-13-32(28-36)30-53-24-8-3-9-25-53/h2,4-7,10-11,13-20,27-28,42H,3,8-9,12,21-26,29-31H2,1H3,(H,46,54)(H,47,55)(H,48,58)(H2,49,51,57)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
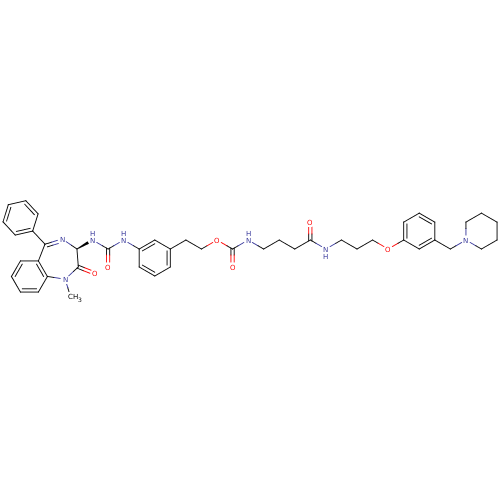
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287266
(CHEMBL290122 | {3-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C45H53N7O6/c1-51-39-21-7-6-20-38(39)41(35-16-4-2-5-17-35)49-42(43(51)54)50-44(55)48-36-18-10-14-33(30-36)23-29-58-45(56)47-24-12-22-40(53)46-25-13-28-57-37-19-11-15-34(31-37)32-52-26-8-3-9-27-52/h2,4-7,10-11,14-21,30-31,42H,3,8-9,12-13,22-29,32H2,1H3,(H,46,53)(H,47,56)(H2,48,50,55)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287245

(CHEMBL285586 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](/[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C36H40N10O5S2/c1-22-7-5-9-24(17-22)41-35(50)44-32-33(49)46(2)28-12-4-3-11-27(28)31(43-32)23-8-6-10-26(18-23)51-19-30(48)39-14-13-29(47)40-15-16-52-20-25-21-53-36(42-25)45-34(37)38/h3-12,17-18,21,32H,13-16,19-20H2,1-2H3,(H,39,48)(H,40,47)(H2,41,44,50)(H4,37,38,42,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287269
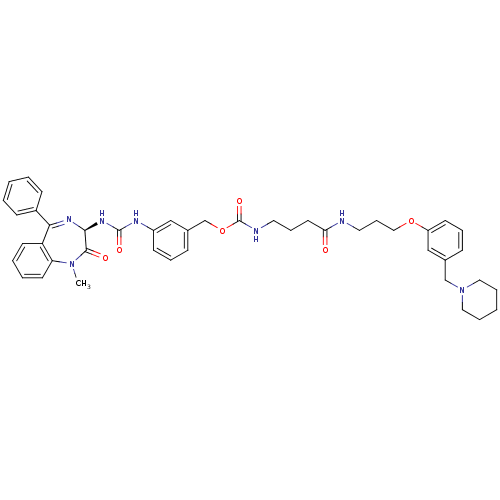
(CHEMBL33698 | {3-[3-(3-Piperidin-1-ylmethyl-phenox...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C44H51N7O6/c1-50-38-21-7-6-20-37(38)40(34-16-4-2-5-17-34)48-41(42(50)53)49-43(54)47-35-18-10-15-33(28-35)31-57-44(55)46-23-12-22-39(52)45-24-13-27-56-36-19-11-14-32(29-36)30-51-25-8-3-9-26-51/h2,4-7,10-11,14-21,28-29,41H,3,8-9,12-13,22-27,30-31H2,1H3,(H,45,52)(H,46,55)(H2,47,49,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287267

(CHEMBL284975 | {2-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C44H51N7O6/c1-50-38-20-7-6-19-37(38)40(34-15-4-2-5-16-34)48-41(42(50)53)49-43(54)47-35-17-10-13-32(29-35)22-28-57-44(55)46-24-21-39(52)45-23-12-27-56-36-18-11-14-33(30-36)31-51-25-8-3-9-26-51/h2,4-7,10-11,13-20,29-30,41H,3,8-9,12,21-28,31H2,1H3,(H,45,52)(H,46,55)(H2,47,49,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287240
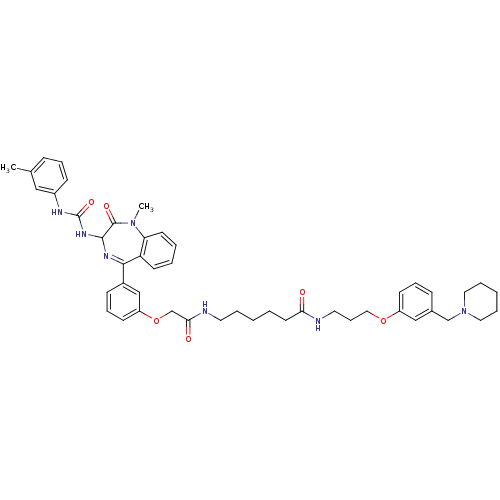
(6-(2-{3-[1-Methyl-2-oxo-3-(3-m-tolyl-ureido)-2,3-d...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OCC(=O)NCCCCCC(=O)NCCCOc2cccc(CN3CCCCC3)c2)c1 |c:9| Show InChI InChI=1S/C47H57N7O6/c1-34-15-11-18-37(29-34)50-47(58)52-45-46(57)53(2)41-22-7-6-21-40(41)44(51-45)36-17-13-20-39(31-36)60-33-43(56)49-24-8-3-5-23-42(55)48-25-14-28-59-38-19-12-16-35(30-38)32-54-26-9-4-10-27-54/h6-7,11-13,15-22,29-31,45H,3-5,8-10,14,23-28,32-33H2,1-2H3,(H,48,55)(H,49,56)(H2,50,52,58) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50287241

(CHEMBL285746 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C32H33N9O3S2/c1-20-8-7-11-22(16-20)36-31(44)39-28-29(43)41(25-13-6-5-12-24(25)27(38-28)21-9-3-2-4-10-21)17-26(42)35-14-15-45-18-23-19-46-32(37-23)40-30(33)34/h2-13,16,19,28H,14-15,17-18H2,1H3,(H,35,42)(H2,36,39,44)(H4,33,34,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023303
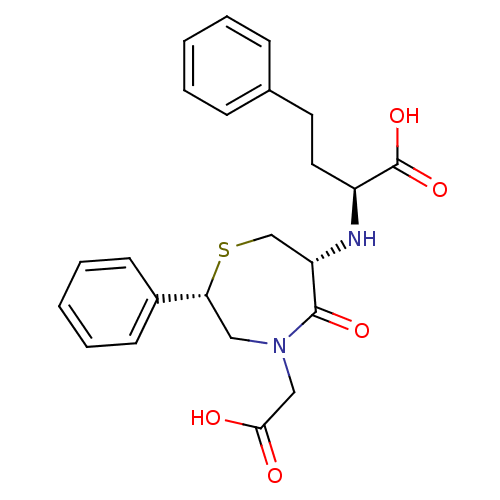
(2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1C[C@@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)14-25-13-20(17-9-5-2-6-10-17)31-15-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287265
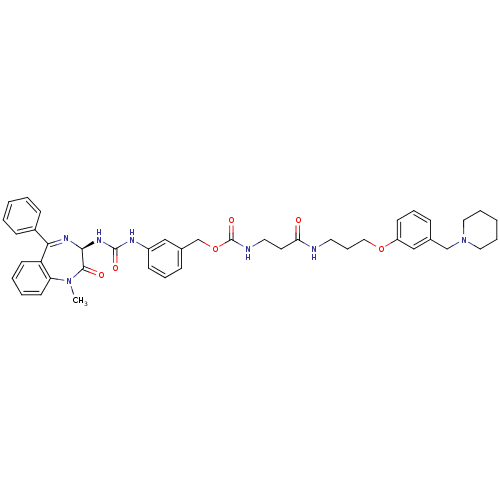
(CHEMBL406844 | {2-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C43H49N7O6/c1-49-37-20-7-6-19-36(37)39(33-15-4-2-5-16-33)47-40(41(49)52)48-42(53)46-34-17-10-14-32(27-34)30-56-43(54)45-23-21-38(51)44-22-12-26-55-35-18-11-13-31(28-35)29-50-24-8-3-9-25-50/h2,4-7,10-11,13-20,27-28,40H,3,8-9,12,21-26,29-30H2,1H3,(H,44,51)(H,45,54)(H2,46,48,53)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023303

(2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1C[C@@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)14-25-13-20(17-9-5-2-6-10-17)31-15-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287261
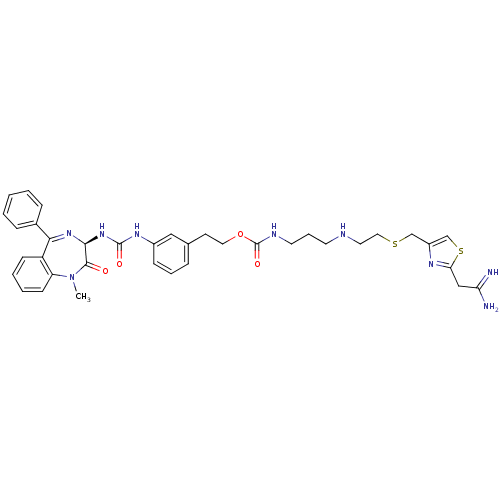
((3-{2-[2-(2,2-Diamino-vinyl)-thiazol-4-ylmethylsul...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCCNCCSCc3csc(CC(N)=N)n3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C37H43N9O4S2/c1-46-30-14-6-5-13-29(30)33(26-10-3-2-4-11-26)44-34(35(46)47)45-36(48)43-27-12-7-9-25(21-27)15-19-50-37(49)41-17-8-16-40-18-20-51-23-28-24-52-32(42-28)22-31(38)39/h2-7,9-14,21,24,34,40H,8,15-20,22-23H2,1H3,(H3,38,39)(H,41,49)(H2,43,45,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor. |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50287272

(CHEMBL33412 | [2-(4-{2-[3-(3-Piperidin-1-ylmethyl-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCN3CCN(CC3)C(=O)CNCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C48H59N9O6/c1-54-42-20-7-6-19-41(42)44(38-15-4-2-5-16-38)52-45(46(54)59)53-47(60)51-39-17-10-14-37(31-39)35-63-48(61)50-22-25-55-26-28-57(29-27-55)43(58)33-49-21-12-30-62-40-18-11-13-36(32-40)34-56-23-8-3-9-24-56/h2,4-7,10-11,13-20,31-32,45,49H,3,8-9,12,21-30,33-35H2,1H3,(H,50,61)(H2,51,53,60)/t45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287253
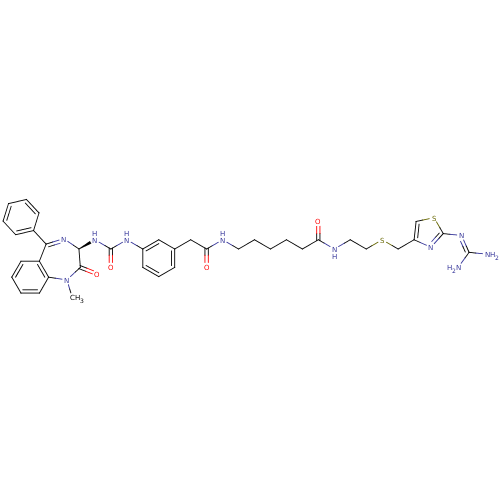
(6-(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydr...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](/[#7])-[#7])n3)c2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C38H44N10O4S2/c1-48-30-16-8-7-15-29(30)33(26-12-4-2-5-13-26)45-34(35(48)51)46-37(52)43-27-14-10-11-25(21-27)22-32(50)41-18-9-3-6-17-31(49)42-19-20-53-23-28-24-54-38(44-28)47-36(39)40/h2,4-5,7-8,10-16,21,24,34H,3,6,9,17-20,22-23H2,1H3,(H,41,50)(H,42,49)(H2,43,46,52)(H4,39,40,44,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287235

(4-(2-{3-[1-Methyl-2-oxo-3-(3-m-tolyl-ureido)-2,3-d...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OCC(=O)NCCCC(=O)NCCCOc2cccc(CN3CCCCC3)c2)c1 |c:9| Show InChI InChI=1S/C45H53N7O6/c1-32-13-8-16-35(27-32)48-45(56)50-43-44(55)51(2)39-20-5-4-19-38(39)42(49-43)34-15-10-18-37(29-34)58-31-41(54)47-22-11-21-40(53)46-23-12-26-57-36-17-9-14-33(28-36)30-52-24-6-3-7-25-52/h4-5,8-10,13-20,27-29,43H,3,6-7,11-12,21-26,30-31H2,1-2H3,(H,46,53)(H,47,54)(H2,48,50,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287243
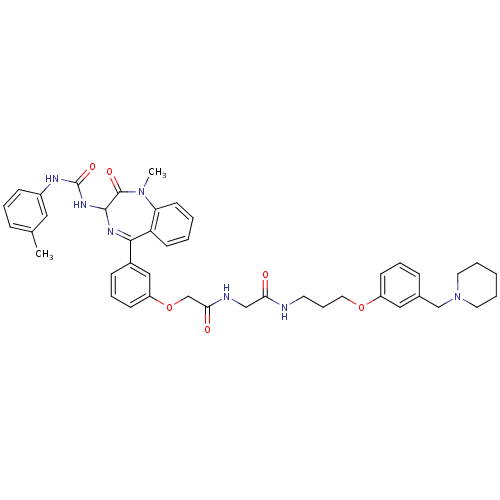
(2-{3-[1-Methyl-2-oxo-3-(3-m-tolyl-ureido)-2,3-dihy...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OCC(=O)NCC(=O)NCCCOc2cccc(CN3CCCCC3)c2)c1 |c:9| Show InChI InChI=1S/C43H49N7O6/c1-30-12-8-15-33(24-30)46-43(54)48-41-42(53)49(2)37-19-5-4-18-36(37)40(47-41)32-14-10-17-35(26-32)56-29-39(52)45-27-38(51)44-20-11-23-55-34-16-9-13-31(25-34)28-50-21-6-3-7-22-50/h4-5,8-10,12-19,24-26,41H,3,6-7,11,20-23,27-29H2,1-2H3,(H,44,51)(H,45,52)(H2,46,48,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50287269

(CHEMBL33698 | {3-[3-(3-Piperidin-1-ylmethyl-phenox...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C44H51N7O6/c1-50-38-21-7-6-20-37(38)40(34-16-4-2-5-17-34)48-41(42(50)53)49-43(54)47-35-18-10-15-33(28-35)31-57-44(55)46-23-12-22-39(52)45-24-13-27-56-36-19-11-14-32(29-36)30-51-25-8-3-9-26-51/h2,4-7,10-11,14-21,28-29,41H,3,8-9,12-13,22-27,30-31H2,1H3,(H,45,52)(H,46,55)(H2,47,49,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
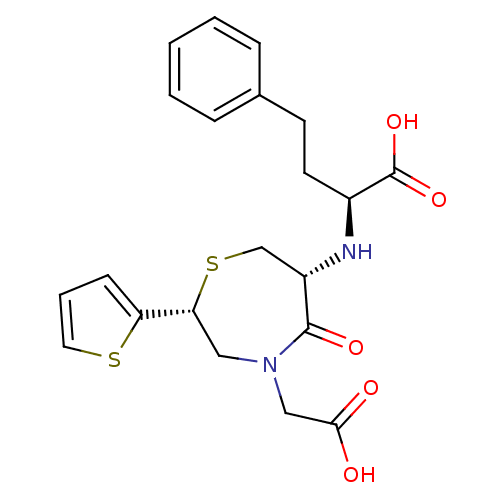
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50024712
(2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...)Show SMILES OC(=O)CN1C[C@@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1cccs1 Show InChI InChI=1S/C21H24N2O5S2/c24-19(25)12-23-11-18(17-7-4-10-29-17)30-13-16(20(23)26)22-15(21(27)28)9-8-14-5-2-1-3-6-14/h1-7,10,15-16,18,22H,8-9,11-13H2,(H,24,25)(H,27,28)/t15-,16-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287259

(CHEMBL286908 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C35H38N10O4S2/c1-22-8-7-11-24(18-22)40-34(49)43-31-32(48)45(27-13-6-5-12-26(27)30(42-31)23-9-3-2-4-10-23)19-29(47)38-15-14-28(46)39-16-17-50-20-25-21-51-35(41-25)44-33(36)37/h2-13,18,21,31H,14-17,19-20H2,1H3,(H,38,47)(H,39,46)(H2,40,43,49)(H4,36,37,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50287270

(CHEMBL432671 | [(2-{2-[2-(2,2-Diamino-vinyl)-thiaz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCNC(=O)CC(=O)NCCSCc3csc(CC(N)=N)n3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C37H40N10O6S2/c1-47-28-13-6-5-12-27(28)33(24-9-3-2-4-10-24)45-34(35(47)50)46-36(51)44-25-11-7-8-23(16-25)19-53-37(52)42-22-41-31(49)18-30(48)40-14-15-54-20-26-21-55-32(43-26)17-29(38)39/h2-13,16,21,34H,14-15,17-20,22H2,1H3,(H3,38,39)(H,40,48)(H,41,49)(H,42,52)(H2,44,46,51)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
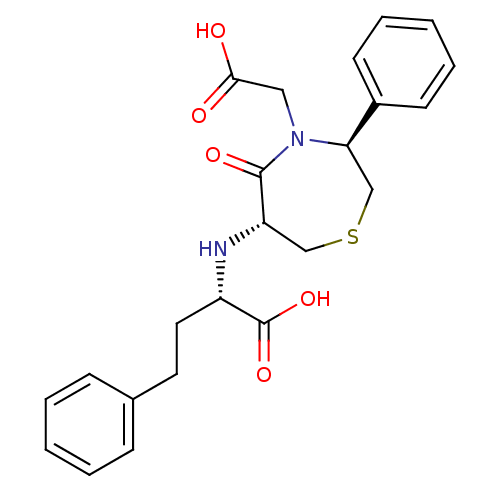
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023301
(2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1[C@H](CSC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)13-25-20(17-9-5-2-6-10-17)15-31-14-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50023301

(2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...)Show SMILES OC(=O)CN1[C@H](CSC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1 Show InChI InChI=1S/C23H26N2O5S/c26-21(27)13-25-20(17-9-5-2-6-10-17)15-31-14-19(22(25)28)24-18(23(29)30)12-11-16-7-3-1-4-8-16/h1-10,18-20,24H,11-15H2,(H,26,27)(H,29,30)/t18-,19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate |
J Med Chem 31: 422-8 (1988)
BindingDB Entry DOI: 10.7270/Q2S75GWH |
More data for this
Ligand-Target Pair | |
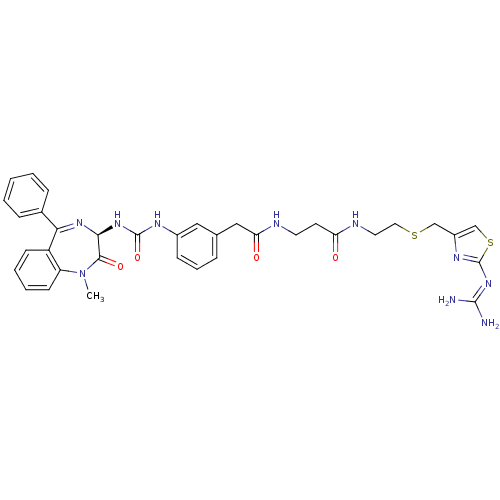
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50287255
(CHEMBL30821 | N-[2-(2-Guanidino-thiazol-4-ylmethyl...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)c2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C35H38N10O4S2/c1-45-27-13-6-5-12-26(27)30(23-9-3-2-4-10-23)42-31(32(45)48)43-34(49)40-24-11-7-8-22(18-24)19-29(47)38-15-14-28(46)39-16-17-50-20-25-21-51-35(41-25)44-33(36)37/h2-13,18,21,31H,14-17,19-20H2,1H3,(H,38,47)(H,39,46)(H2,40,43,49)(H4,36,37,41,44)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against gastrin receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
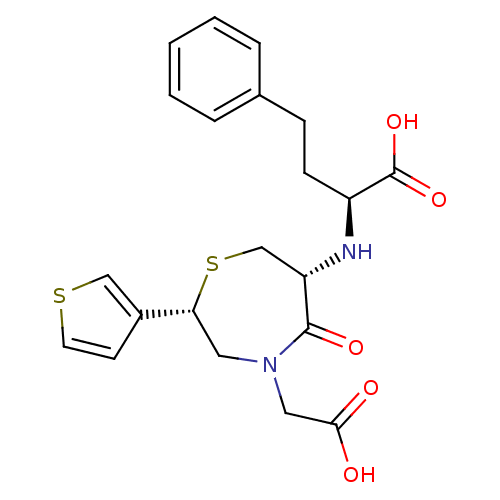
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50024711
(2-(4-Carboxymethyl-5-oxo-2-thiophen-3-yl-[1,4]thia...)Show SMILES OC(=O)CN1C[C@@H](SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccsc1 Show InChI InChI=1S/C21H24N2O5S2/c24-19(25)11-23-10-18(15-8-9-29-12-15)30-13-17(20(23)26)22-16(21(27)28)7-6-14-4-2-1-3-5-14/h1-5,8-9,12,16-18,22H,6-7,10-11,13H2,(H,24,25)(H,27,28)/t16-,17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate |
J Med Chem 30: 1984-91 (1987)
BindingDB Entry DOI: 10.7270/Q2Q52NMJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50287256

(6-(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydr...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CC(=O)NCCCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C46H55N7O5/c1-52-40-23-9-8-22-39(40)43(36-18-5-2-6-19-36)50-44(45(52)56)51-46(57)49-37-20-13-16-34(30-37)32-42(55)48-25-10-3-7-24-41(54)47-26-15-29-58-38-21-14-17-35(31-38)33-53-27-11-4-12-28-53/h2,5-6,8-9,13-14,16-23,30-31,44H,3-4,7,10-12,15,24-29,32-33H2,1H3,(H,47,54)(H,48,55)(H2,49,51,57)/t44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type B receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data