 Found 883 hits with Last Name = 'jakob' and Initial = 'c'
Found 883 hits with Last Name = 'jakob' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151078
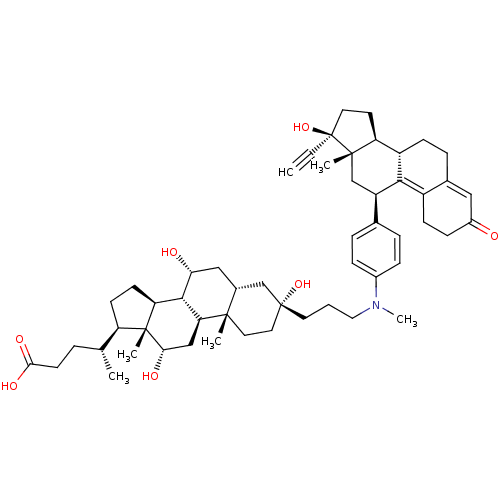
((4R)-4-[(1S,2S,5S,7R,9R,10R,11S,14R,15R,16S)-5-[3-...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@](O)(CCCN(C)c5ccc(cc5)[C@H]5C[C@@]6(C)[C@@H](CC[C@@]6(O)C#C)[C@@H]6CCC7=CC(=O)CCC7=C56)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C |t:46,53| Show InChI InChI=1S/C54H75NO7/c1-7-54(62)23-21-42-39-16-12-34-27-37(56)15-17-38(34)48(39)40(31-51(42,54)4)33-10-13-36(14-11-33)55(6)26-8-22-53(61)25-24-50(3)35(30-53)28-45(57)49-43-19-18-41(32(2)9-20-47(59)60)52(43,5)46(58)29-44(49)50/h1,10-11,13-14,27,32,35,39-46,49,57-58,61-62H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35-,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151076
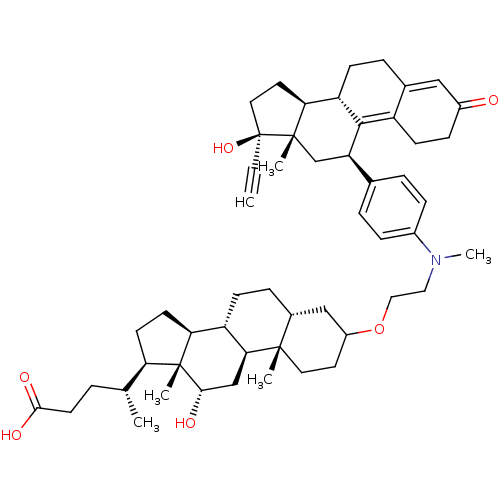
((4R)-4-[(1S,2S,7R,10R,11S,14R,15R,16S)-5-[2-({4-[(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-23-44-41-16-11-34-28-37(55)15-18-39(34)49(41)42(31-51(44,53)4)33-9-13-36(14-10-33)54(6)26-27-60-38-22-24-50(3)35(29-38)12-17-40-45-20-19-43(32(2)8-21-48(57)58)52(45,5)47(56)30-46(40)50/h1,9-10,13-14,28,32,35,38,40-47,56,59H,8,11-12,15-27,29-31H2,2-6H3,(H,57,58)/t32-,35-,38?,40+,41+,42-,43-,44+,45+,46+,47+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410188
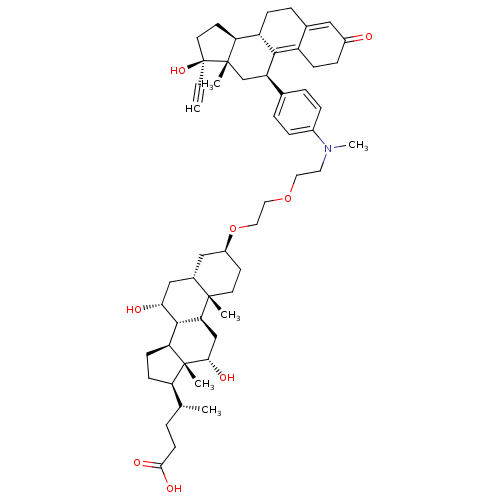
(CHEMBL2096708)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39+,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151077
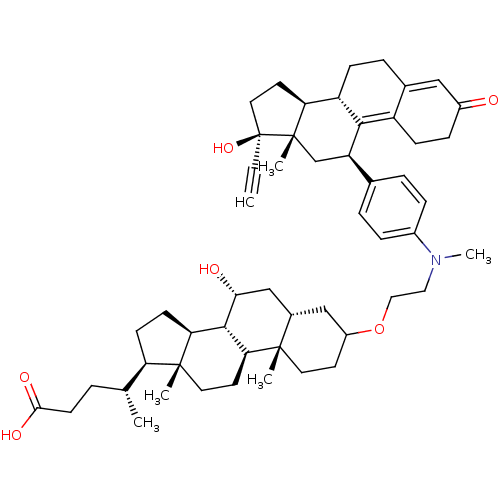
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R)-5-[2-({4-[(1...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-22-43-40-15-11-34-28-37(55)14-16-39(34)48(40)41(31-52(43,53)5)33-9-12-36(13-10-33)54(6)26-27-60-38-20-23-50(3)35(29-38)30-46(56)49-44-18-17-42(32(2)8-19-47(57)58)51(44,4)24-21-45(49)50/h1,9-10,12-13,28,32,35,38,40-46,49,56,59H,8,11,14-27,29-31H2,2-6H3,(H,57,58)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,49+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410187
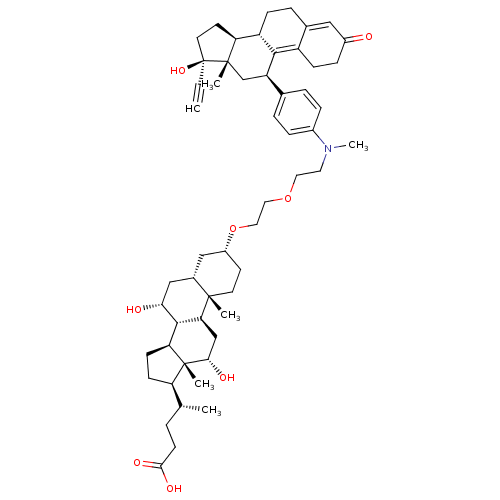
(CHEMBL2096804)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39-,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151064
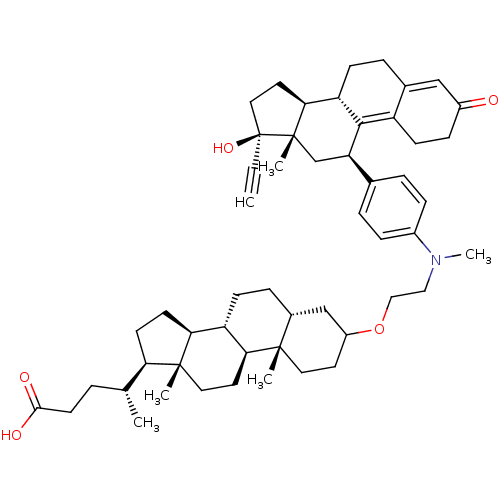
((4R)-4-[(1S,2S,7R,10R,11S,14R,15R)-5-[2-({4-[(10S,...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:57,64| Show InChI InChI=1S/C53H73NO5/c1-7-53(58)27-24-47-42-16-11-35-30-38(55)15-18-40(35)49(42)43(32-52(47,53)5)34-9-13-37(14-10-34)54(6)28-29-59-39-22-25-50(3)36(31-39)12-17-41-45-20-19-44(33(2)8-21-48(56)57)51(45,4)26-23-46(41)50/h1,9-10,13-14,30,33,36,39,41-47,58H,8,11-12,15-29,31-32H2,2-6H3,(H,56,57)/t33-,36-,39?,41+,42+,43-,44-,45+,46+,47+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151068
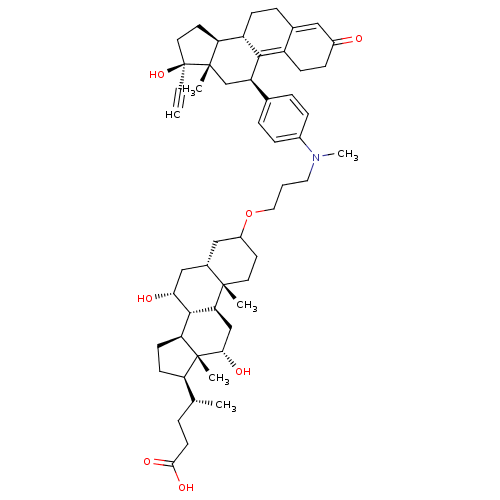
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[3-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:60,67| Show InChI InChI=1S/C54H75NO7/c1-7-54(61)24-22-43-40-16-12-34-27-37(56)15-17-39(34)49(40)41(31-52(43,54)4)33-10-13-36(14-11-33)55(6)25-8-26-62-38-21-23-51(3)35(28-38)29-46(57)50-44-19-18-42(32(2)9-20-48(59)60)53(44,5)47(58)30-45(50)51/h1,10-11,13-14,27,32,35,38,40-47,50,57-58,61H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151067
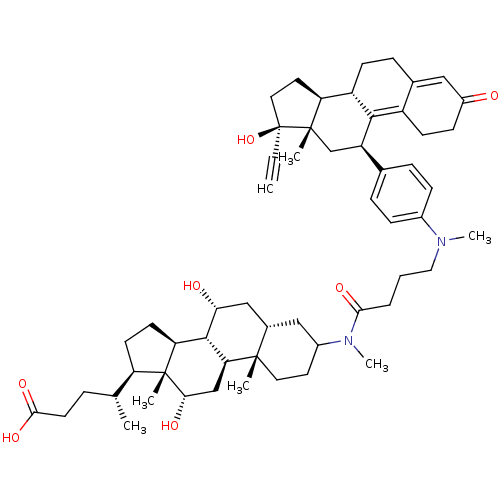
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-[4-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C56H78N2O7/c1-8-56(65)26-24-44-41-18-14-35-28-39(59)17-19-40(35)51(41)42(32-54(44,56)4)34-12-15-37(16-13-34)57(6)27-9-10-49(62)58(7)38-23-25-53(3)36(29-38)30-47(60)52-45-21-20-43(33(2)11-22-50(63)64)55(45,5)48(61)31-46(52)53/h1,12-13,15-16,28,33,36,38,41-48,52,60-61,65H,9-11,14,17-27,29-32H2,2-7H3,(H,63,64)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,52+,53+,54+,55-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151059
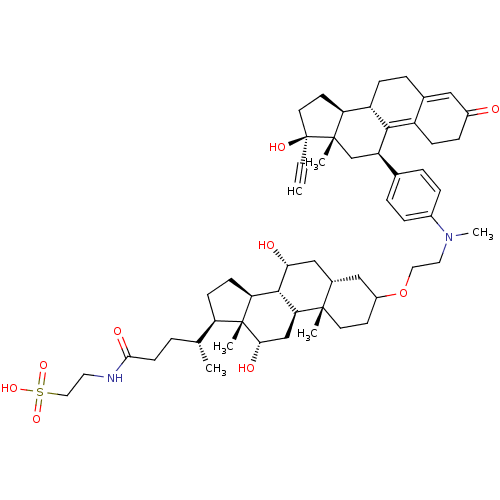
(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:65,72| Show InChI InChI=1S/C55H78N2O9S/c1-7-55(62)23-21-44-41-15-11-35-28-38(58)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)57(6)25-26-66-39-20-22-52(3)36(29-39)30-47(59)51-45-18-17-43(54(45,5)48(60)31-46(51)52)33(2)8-19-49(61)56-24-27-67(63,64)65/h1,9-10,12-13,28,33,36,39,41-48,51,59-60,62H,8,11,14-27,29-32H2,2-6H3,(H,56,61)(H,63,64,65)/t33-,36+,39?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235631
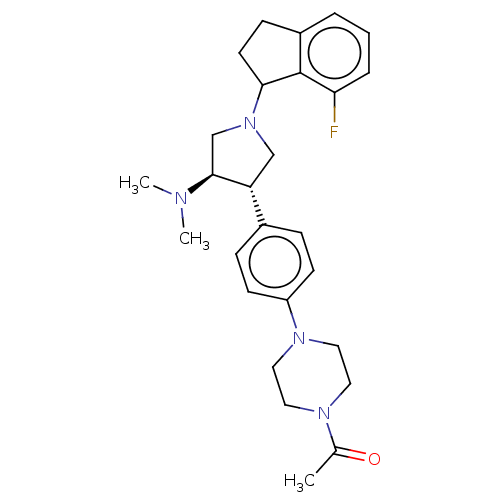
(CHEMBL4060827)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)C(C)=O)C1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C27H35FN4O/c1-19(33)30-13-15-31(16-14-30)22-10-7-20(8-11-22)23-17-32(18-26(23)29(2)3)25-12-9-21-5-4-6-24(28)27(21)25/h4-8,10-11,23,25-26H,9,12-18H2,1-3H3/t23-,25?,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay |
Bioorg Med Chem Lett 27: 1576-1583 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.030
BindingDB Entry DOI: 10.7270/Q22F7QQG |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151060
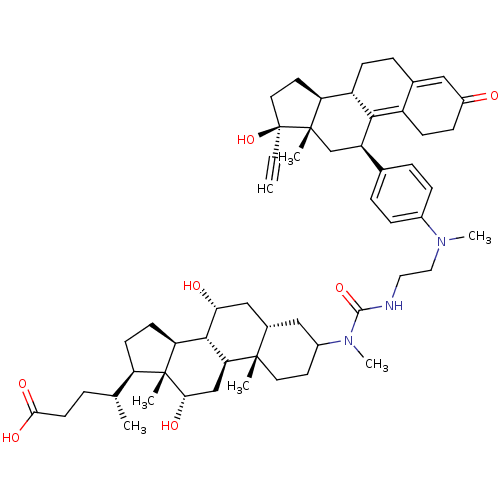
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C55H77N3O7/c1-8-55(65)24-22-43-40-16-12-34-27-38(59)15-17-39(34)49(40)41(31-53(43,55)4)33-10-13-36(14-11-33)57(6)26-25-56-51(64)58(7)37-21-23-52(3)35(28-37)29-46(60)50-44-19-18-42(32(2)9-20-48(62)63)54(44,5)47(61)30-45(50)52/h1,10-11,13-14,27,32,35,37,40-47,50,60-61,65H,9,12,15-26,28-31H2,2-7H3,(H,56,64)(H,62,63)/t32-,35+,37?,40+,41-,42-,43+,44+,45+,46-,47+,50+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235630
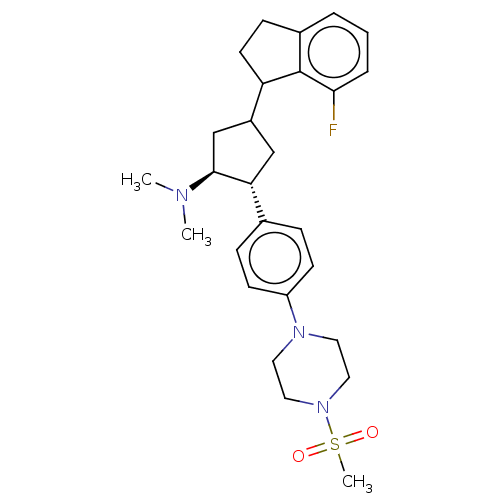
(CHEMBL4093096)Show SMILES CN(C)[C@H]1CC(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C27H36FN3O2S/c1-29(2)26-18-21(23-12-9-20-5-4-6-25(28)27(20)23)17-24(26)19-7-10-22(11-8-19)30-13-15-31(16-14-30)34(3,32)33/h4-8,10-11,21,23-24,26H,9,12-18H2,1-3H3/t21?,23?,24-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay |
Bioorg Med Chem Lett 27: 1576-1583 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.030
BindingDB Entry DOI: 10.7270/Q22F7QQG |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151076

((4R)-4-[(1S,2S,7R,10R,11S,14R,15R,16S)-5-[2-({4-[(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-23-44-41-16-11-34-28-37(55)15-18-39(34)49(41)42(31-51(44,53)4)33-9-13-36(14-10-33)54(6)26-27-60-38-22-24-50(3)35(29-38)12-17-40-45-20-19-43(32(2)8-21-48(57)58)52(45,5)47(56)30-46(40)50/h1,9-10,13-14,28,32,35,38,40-47,56,59H,8,11-12,15-27,29-31H2,2-6H3,(H,57,58)/t32-,35-,38?,40+,41+,42-,43-,44+,45+,46+,47+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151069
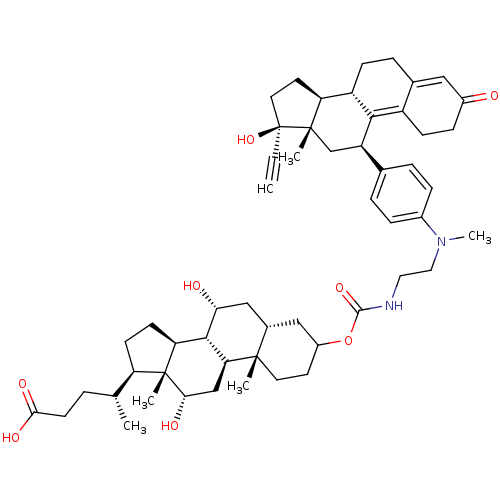
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151069

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151074
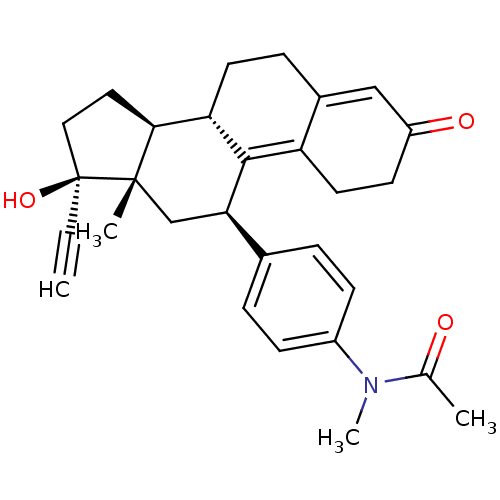
(CHEMBL364368 | N-[4-((2R,8S,13S,14S,17R)-17-Ethyny...)Show SMILES CN(C(C)=O)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:27,34| Show InChI InChI=1S/C29H33NO3/c1-5-29(33)15-14-26-24-12-8-20-16-22(32)11-13-23(20)27(24)25(17-28(26,29)3)19-6-9-21(10-7-19)30(4)18(2)31/h1,6-7,9-10,16,24-26,33H,8,11-15,17H2,2-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223987
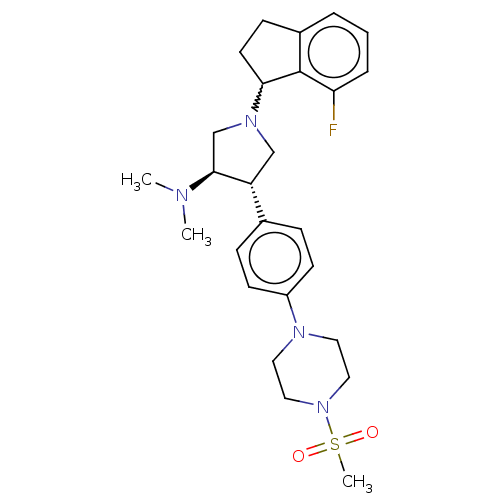
(A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r,w:24.26| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151063
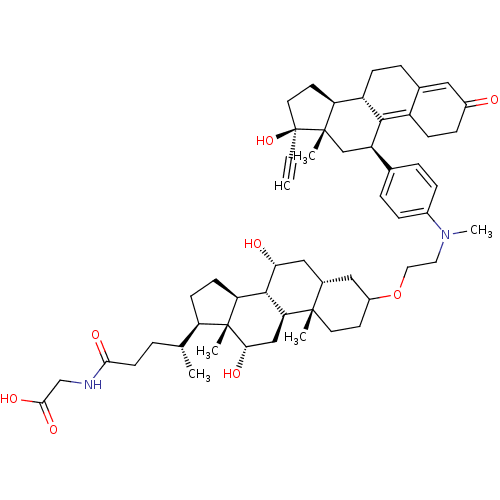
(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C55H76N2O8/c1-7-55(64)23-21-43-40-15-11-34-26-37(58)14-16-39(34)50(40)41(30-53(43,55)4)33-9-12-36(13-10-33)57(6)24-25-65-38-20-22-52(3)35(27-38)28-46(59)51-44-18-17-42(54(44,5)47(60)29-45(51)52)32(2)8-19-48(61)56-31-49(62)63/h1,9-10,12-13,26,32,35,38,40-47,51,59-60,64H,8,11,14-25,27-31H2,2-6H3,(H,56,61)(H,62,63)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151065
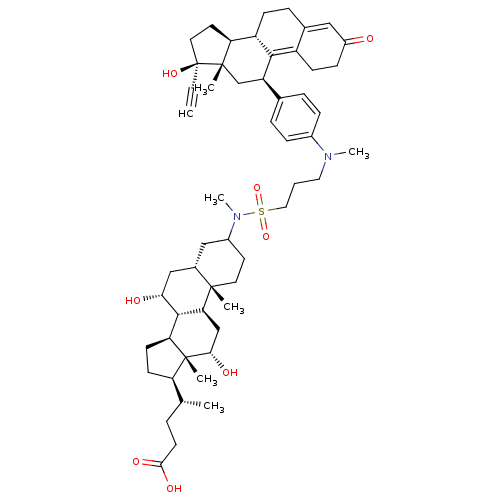
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9,16-dih...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)S(=O)(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:64,71| Show InChI InChI=1S/C55H78N2O8S/c1-8-55(63)25-23-44-41-17-13-35-28-39(58)16-18-40(35)50(41)42(32-53(44,55)4)34-11-14-37(15-12-34)56(6)26-9-27-66(64,65)57(7)38-22-24-52(3)36(29-38)30-47(59)51-45-20-19-43(33(2)10-21-49(61)62)54(45,5)48(60)31-46(51)52/h1,11-12,14-15,28,33,36,38,41-48,51,59-60,63H,9-10,13,16-27,29-32H2,2-7H3,(H,61,62)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235643
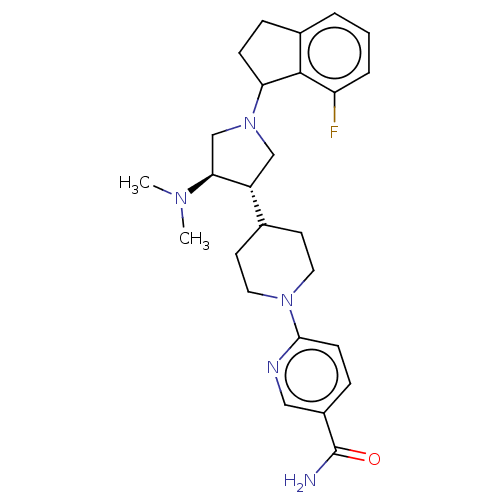
(CHEMBL4076017)Show SMILES CN(C)[C@H]1CN(C[C@@H]1C1CCN(CC1)c1ccc(cn1)C(N)=O)C1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C26H34FN5O/c1-30(2)23-16-32(22-8-6-18-4-3-5-21(27)25(18)22)15-20(23)17-10-12-31(13-11-17)24-9-7-19(14-29-24)26(28)33/h3-5,7,9,14,17,20,22-23H,6,8,10-13,15-16H2,1-2H3,(H2,28,33)/t20-,22?,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 27: 1576-1583 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.030
BindingDB Entry DOI: 10.7270/Q22F7QQG |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50410188

(CHEMBL2096708)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39+,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223987

(A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r,w:24.26| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151059

(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:65,72| Show InChI InChI=1S/C55H78N2O9S/c1-7-55(62)23-21-44-41-15-11-35-28-38(58)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)57(6)25-26-66-39-20-22-52(3)36(29-39)30-47(59)51-45-18-17-43(54(45,5)48(60)31-46(51)52)33(2)8-19-49(61)56-24-27-67(63,64)65/h1,9-10,12-13,28,33,36,39,41-48,51,59-60,62H,8,11,14-27,29-32H2,2-6H3,(H,56,61)(H,63,64,65)/t33-,36+,39?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235658
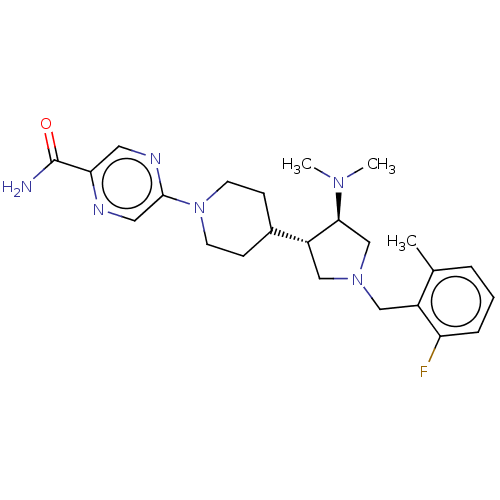
(CHEMBL4073166)Show SMILES CN(C)[C@H]1CN(Cc2c(C)cccc2F)C[C@@H]1C1CCN(CC1)c1cnc(cn1)C(N)=O |r| Show InChI InChI=1S/C24H33FN6O/c1-16-5-4-6-20(25)18(16)13-30-14-19(22(15-30)29(2)3)17-7-9-31(10-8-17)23-12-27-21(11-28-23)24(26)32/h4-6,11-12,17,19,22H,7-10,13-15H2,1-3H3,(H2,26,32)/t19-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 27: 1576-1583 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.030
BindingDB Entry DOI: 10.7270/Q22F7QQG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human androgen receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151061
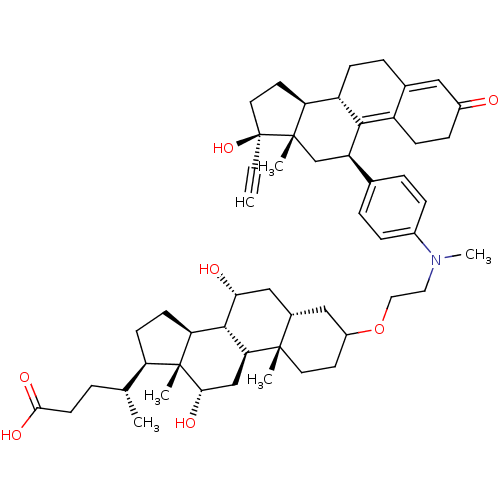
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:59,66| Show InChI InChI=1S/C53H73NO7/c1-7-53(60)23-21-42-39-15-11-33-26-36(55)14-16-38(33)48(39)40(30-51(42,53)4)32-9-12-35(13-10-32)54(6)24-25-61-37-20-22-50(3)34(27-37)28-45(56)49-43-18-17-41(31(2)8-19-47(58)59)52(43,5)46(57)29-44(49)50/h1,9-10,12-13,26,31,34,37,39-46,49,56-57,60H,8,11,14-25,27-30H2,2-6H3,(H,58,59)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151061

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:59,66| Show InChI InChI=1S/C53H73NO7/c1-7-53(60)23-21-42-39-15-11-33-26-36(55)14-16-38(33)48(39)40(30-51(42,53)4)32-9-12-35(13-10-32)54(6)24-25-61-37-20-22-50(3)34(27-37)28-45(56)49-43-18-17-41(31(2)8-19-47(58)59)52(43,5)46(57)29-44(49)50/h1,9-10,12-13,26,31,34,37,39-46,49,56-57,60H,8,11,14-25,27-30H2,2-6H3,(H,58,59)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235644
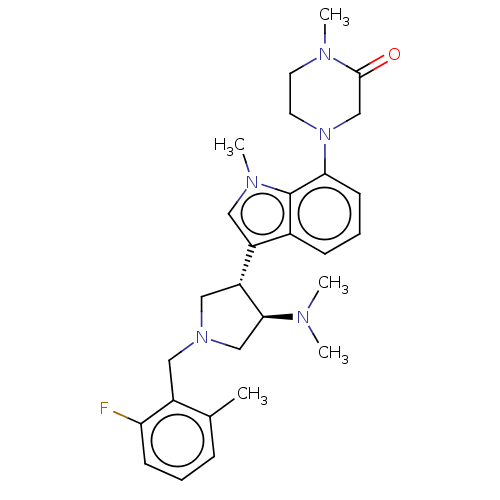
(CHEMBL4065766)Show SMILES CN(C)[C@H]1CN(Cc2c(C)cccc2F)C[C@@H]1c1cn(C)c2c(cccc12)N1CCN(C)C(=O)C1 |r| Show InChI InChI=1S/C28H36FN5O/c1-19-8-6-10-24(29)21(19)15-33-16-23(26(17-33)30(2)3)22-14-32(5)28-20(22)9-7-11-25(28)34-13-12-31(4)27(35)18-34/h6-11,14,23,26H,12-13,15-18H2,1-5H3/t23-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 27: 1576-1583 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.030
BindingDB Entry DOI: 10.7270/Q22F7QQG |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151077

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R)-5-[2-({4-[(1...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-22-43-40-15-11-34-28-37(55)14-16-39(34)48(40)41(31-52(43,53)5)33-9-12-36(13-10-33)54(6)26-27-60-38-20-23-50(3)35(29-38)30-46(56)49-44-18-17-42(32(2)8-19-47(57)58)51(44,4)24-21-45(49)50/h1,9-10,12-13,28,32,35,38,40-46,49,56,59H,8,11,14-27,29-31H2,2-6H3,(H,57,58)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,49+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151072
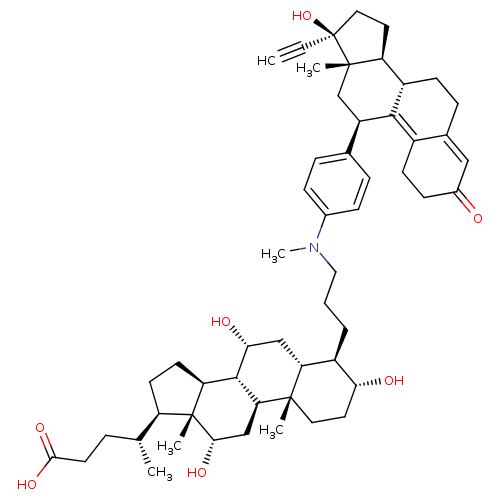
((4R)-4-[(1S,2S,5R,6R,7R,9R,10R,11S,14R,15R,16S)-6-...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4[C@@H](CCCN(C)c5ccc(cc5)[C@H]5C[C@@]6(C)[C@@H](CC[C@@]6(O)C#C)[C@@H]6CCC7=CC(=O)CCC7=C56)[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C |t:44,51| Show InChI InChI=1S/C54H75NO7/c1-7-54(62)25-22-41-38-17-13-33-27-35(56)16-18-36(33)49(38)39(30-52(41,54)4)32-11-14-34(15-12-32)55(6)26-8-9-37-43-28-46(58)50-42-20-19-40(31(2)10-21-48(60)61)53(42,5)47(59)29-44(50)51(43,3)24-23-45(37)57/h1,11-12,14-15,27,31,37-47,50,57-59,62H,8-10,13,16-26,28-30H2,2-6H3,(H,60,61)/t31-,37-,38+,39-,40-,41+,42+,43-,44+,45-,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151067

((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-[4-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C56H78N2O7/c1-8-56(65)26-24-44-41-18-14-35-28-39(59)17-19-40(35)51(41)42(32-54(44,56)4)34-12-15-37(16-13-34)57(6)27-9-10-49(62)58(7)38-23-25-53(3)36(29-38)30-47(60)52-45-21-20-43(33(2)11-22-50(63)64)55(45,5)48(61)31-46(52)53/h1,12-13,15-16,28,33,36,38,41-48,52,60-61,65H,9-11,14,17-27,29-32H2,2-7H3,(H,63,64)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,52+,53+,54+,55-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223986
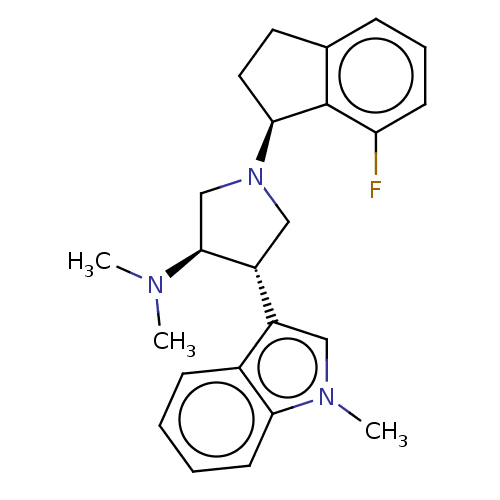
((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1cn(C)c2ccccc12)[C@H]1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C24H28FN3/c1-26(2)23-15-28(22-12-11-16-7-6-9-20(25)24(16)22)14-19(23)18-13-27(3)21-10-5-4-8-17(18)21/h4-10,13,19,22-23H,11-12,14-15H2,1-3H3/t19-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM11644
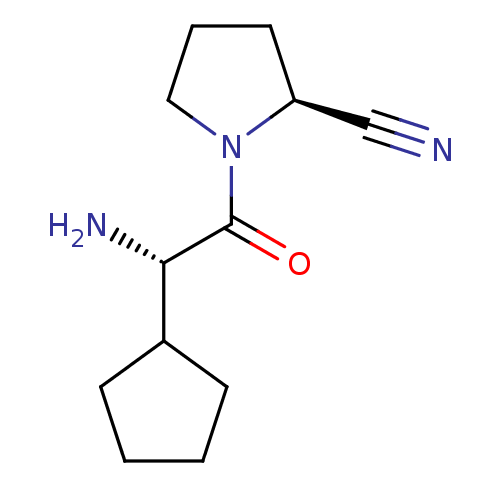
((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...)Show InChI InChI=1S/C12H19N3O/c13-8-10-6-3-7-15(10)12(16)11(14)9-4-1-2-5-9/h9-11H,1-7,14H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
Biochemistry 45: 7474-82 (2006)
Article DOI: 10.1021/bi060184f
BindingDB Entry DOI: 10.7270/Q2P26WCG |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235632
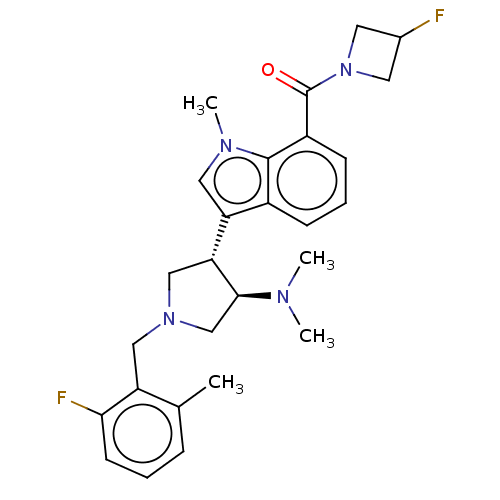
(CHEMBL4077363)Show SMILES CN(C)[C@H]1CN(Cc2c(C)cccc2F)C[C@@H]1c1cn(C)c2c(cccc12)C(=O)N1CC(F)C1 |r| Show InChI InChI=1S/C27H32F2N4O/c1-17-7-5-10-24(29)21(17)14-32-15-23(25(16-32)30(2)3)22-13-31(4)26-19(22)8-6-9-20(26)27(34)33-11-18(28)12-33/h5-10,13,18,23,25H,11-12,14-16H2,1-4H3/t23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 27: 1576-1583 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.030
BindingDB Entry DOI: 10.7270/Q22F7QQG |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151063

(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C55H76N2O8/c1-7-55(64)23-21-43-40-15-11-34-26-37(58)14-16-39(34)50(40)41(30-53(43,55)4)33-9-12-36(13-10-33)57(6)24-25-65-38-20-22-52(3)35(27-38)28-46(59)51-44-18-17-42(54(44,5)47(60)29-45(51)52)32(2)8-19-48(61)56-31-49(62)63/h1,9-10,12-13,26,32,35,38,40-47,51,59-60,64H,8,11,14-25,27-31H2,2-6H3,(H,56,61)(H,62,63)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11644

((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...)Show InChI InChI=1S/C12H19N3O/c13-8-10-6-3-7-15(10)12(16)11(14)9-4-1-2-5-9/h9-11H,1-7,14H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
Biochemistry 45: 7474-82 (2006)
Article DOI: 10.1021/bi060184f
BindingDB Entry DOI: 10.7270/Q2P26WCG |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410188

(CHEMBL2096708)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39+,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151068

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[3-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:60,67| Show InChI InChI=1S/C54H75NO7/c1-7-54(61)24-22-43-40-16-12-34-27-37(56)15-17-39(34)49(40)41(31-52(43,54)4)33-10-13-36(14-11-33)55(6)25-8-26-62-38-21-23-51(3)35(28-38)29-46(57)50-44-19-18-42(32(2)9-20-48(59)60)53(44,5)47(58)30-45(50)51/h1,10-11,13-14,27,32,35,38,40-47,50,57-58,61H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151070
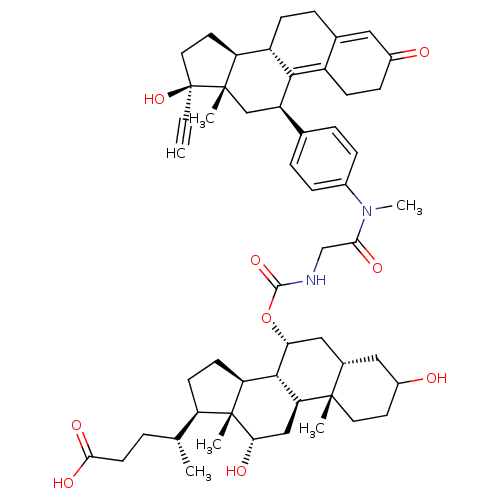
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9-({[({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](C[C@@H]4CC(O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCC(=O)N(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C54H72N2O9/c1-7-54(64)23-21-41-38-15-11-32-24-35(57)14-16-37(32)48(38)39(28-52(41,54)4)31-9-12-34(13-10-31)56(6)46(60)29-55-50(63)65-44-26-33-25-36(58)20-22-51(33,3)43-27-45(59)53(5)40(17-18-42(53)49(43)44)30(2)8-19-47(61)62/h1,9-10,12-13,24,30,33,36,38-45,49,58-59,64H,8,11,14-23,25-29H2,2-6H3,(H,55,63)(H,61,62)/t30-,33+,36?,38+,39-,40-,41+,42+,43+,44-,45+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151068

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[3-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:60,67| Show InChI InChI=1S/C54H75NO7/c1-7-54(61)24-22-43-40-16-12-34-27-37(56)15-17-39(34)49(40)41(31-52(43,54)4)33-10-13-36(14-11-33)55(6)25-8-26-62-38-21-23-51(3)35(28-38)29-46(57)50-44-19-18-42(32(2)9-20-48(59)60)53(44,5)47(58)30-45(50)51/h1,10-11,13-14,27,32,35,38,40-47,50,57-58,61H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151067

((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-[4-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C56H78N2O7/c1-8-56(65)26-24-44-41-18-14-35-28-39(59)17-19-40(35)51(41)42(32-54(44,56)4)34-12-15-37(16-13-34)57(6)27-9-10-49(62)58(7)38-23-25-53(3)36(29-38)30-47(60)52-45-21-20-43(33(2)11-22-50(63)64)55(45,5)48(61)31-46(52)53/h1,12-13,15-16,28,33,36,38,41-48,52,60-61,65H,9-11,14,17-27,29-32H2,2-7H3,(H,63,64)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,52+,53+,54+,55-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151071
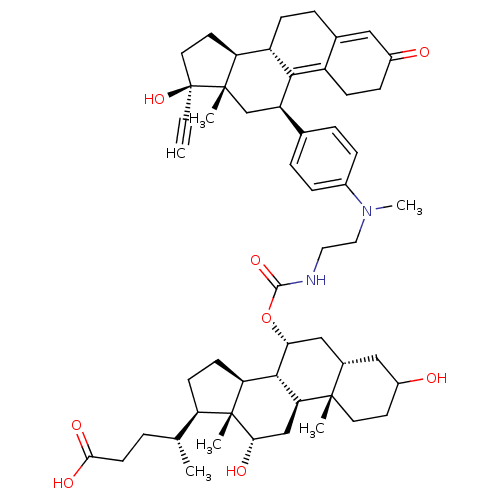
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](C[C@@H]4CC(O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-45-28-34-27-37(58)20-22-51(34,3)44-29-46(59)53(5)41(17-18-43(53)49(44)45)31(2)8-19-47(60)61/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151073
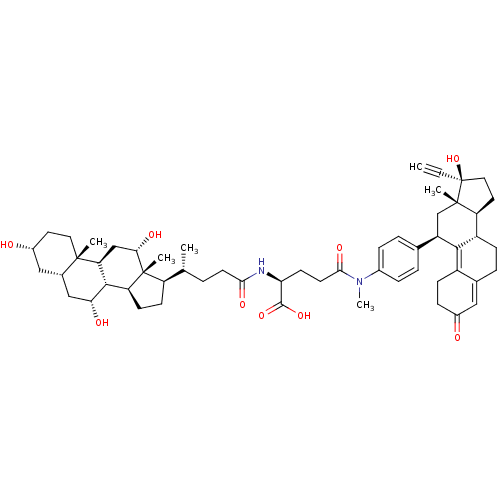
((2S)-4-({4-[(10S,11S,14R,15S,17R)-14-ethynyl-14-hy...)Show SMILES C[C@H](CCC(=O)N[C@@H](CCC(=O)N(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12)C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C |t:36,43| Show InChI InChI=1S/C56H76N2O9/c1-7-56(67)25-23-42-39-15-11-33-26-36(59)14-16-38(33)50(39)40(30-54(42,56)4)32-9-12-35(13-10-32)58(6)49(64)21-19-45(52(65)66)57-48(63)20-8-31(2)41-17-18-43-51-44(29-47(62)55(41,43)5)53(3)24-22-37(60)27-34(53)28-46(51)61/h1,9-10,12-13,26,31,34,37,39-47,51,60-62,67H,8,11,14-25,27-30H2,2-6H3,(H,57,63)(H,65,66)/t31-,34+,37-,39+,40-,41-,42+,43+,44+,45+,46-,47+,51+,53+,54+,55-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151074

(CHEMBL364368 | N-[4-((2R,8S,13S,14S,17R)-17-Ethyny...)Show SMILES CN(C(C)=O)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:27,34| Show InChI InChI=1S/C29H33NO3/c1-5-29(33)15-14-26-24-12-8-20-16-22(32)11-13-23(20)27(24)25(17-28(26,29)3)19-6-9-21(10-7-19)30(4)18(2)31/h1,6-7,9-10,16,24-26,33H,8,11-15,17H2,2-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151069

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50410187

(CHEMBL2096804)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39-,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151065

((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9,16-dih...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)S(=O)(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:64,71| Show InChI InChI=1S/C55H78N2O8S/c1-8-55(63)25-23-44-41-17-13-35-28-39(58)16-18-40(35)50(41)42(32-53(44,55)4)34-11-14-37(15-12-34)56(6)26-9-27-66(64,65)57(7)38-22-24-52(3)36(29-38)30-47(59)51-45-20-19-43(33(2)10-21-49(61)62)54(45,5)48(60)31-46(51)52/h1,11-12,14-15,28,33,36,38,41-48,51,59-60,63H,9-10,13,16-27,29-32H2,2-7H3,(H,61,62)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data