 Found 314 hits with Last Name = 'janssen' and Initial = 'c'
Found 314 hits with Last Name = 'janssen' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 1
(RAT) | BDBM86211
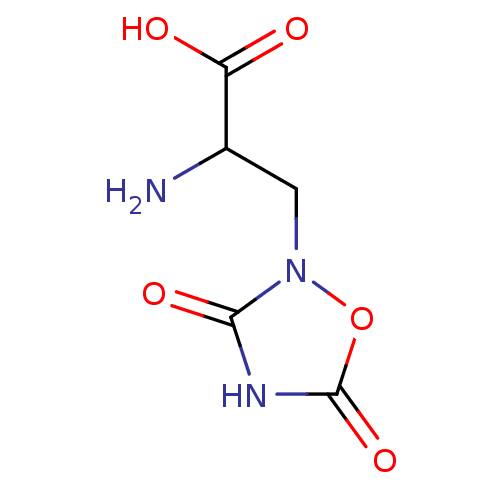
(CAS_52809-07-1 | NSC_40539 | Quisqualate)Show InChI InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50025124
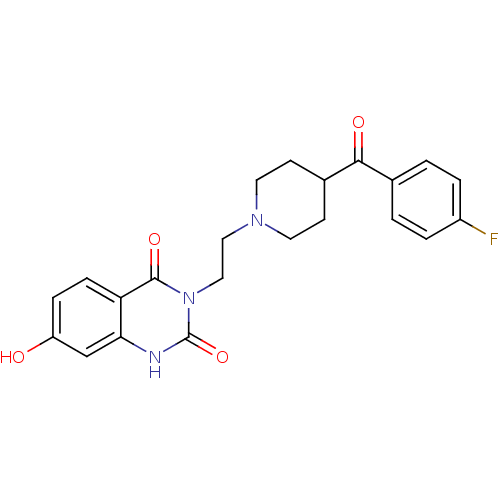
(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding activity against [3H]ketanserin as radioligand for 5-hydroxytryptamine 2 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50002371
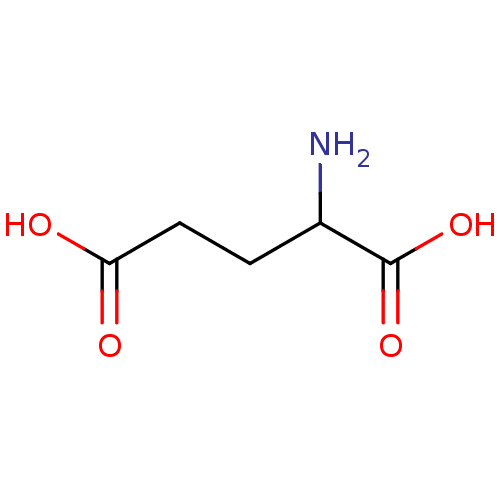
(2-aminopentanedioic acidglutamic acid | CHEMBL2763...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50089896

(4-(Amino-carboxy-methyl)-3-methyl-benzoic acid((+)...)Show InChI InChI=1S/C10H11NO4/c1-5-4-6(9(12)13)2-3-7(5)8(11)10(14)15/h2-4,8H,11H2,1H3,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]pyrilamine as radioligand for Histamine H1 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163606

(1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-ph...)Show InChI InChI=1S/C20H17NO2/c22-19(11-14-5-2-1-3-6-14)15-8-9-18-17(12-15)13-16-7-4-10-23-20(16)21-18/h1-3,5-6,8-9,12-13H,4,7,10-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50231744
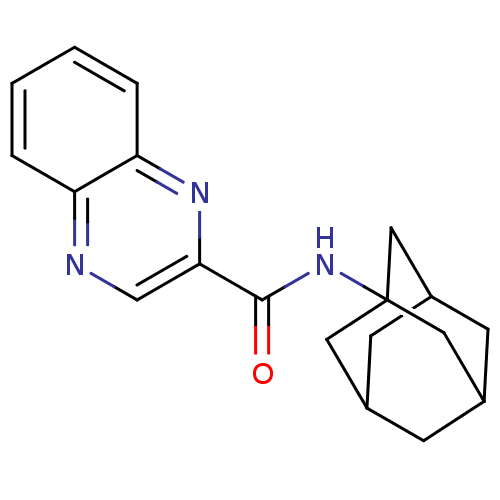
(CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)c1cnc2ccccc2n1 |TLB:2:3:6:10.8.9,THB:8:7:4:10.9.11,8:9:6.7.12:4,11:9:6:12.3.4,11:3:6:10.8.9| Show InChI InChI=1S/C19H21N3O/c23-18(17-11-20-15-3-1-2-4-16(15)21-17)22-19-8-12-5-13(9-19)7-14(6-12)10-19/h1-4,11-14H,5-10H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030628
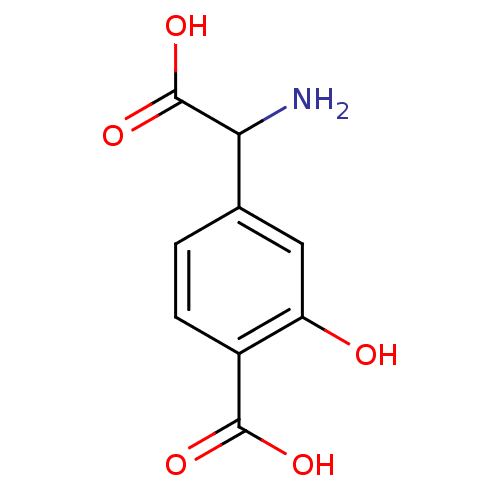
((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...)Show InChI InChI=1S/C9H9NO5/c10-7(9(14)15)4-1-2-5(8(12)13)6(11)3-4/h1-3,7,11H,10H2,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM86212
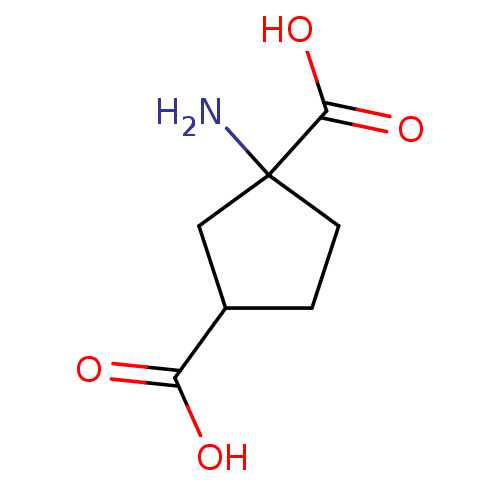
(1S, 3R-ACPD | CAS_104766 | NSC_104766)Show InChI InChI=1S/C7H11NO4/c8-7(6(11)12)2-1-4(3-7)5(9)10/h4H,1-3,8H2,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030629
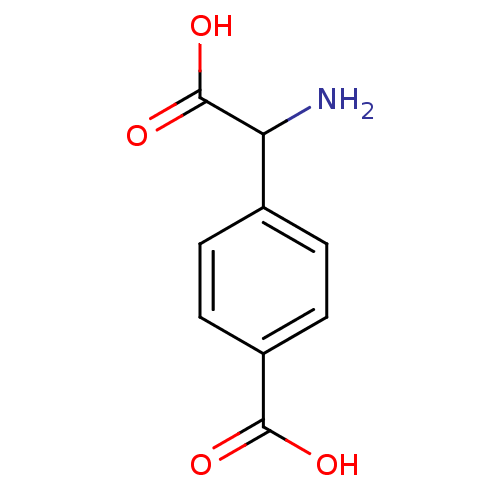
((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...)Show InChI InChI=1S/C9H9NO4/c10-7(9(13)14)5-1-3-6(4-2-5)8(11)12/h1-4,7H,10H2,(H,11,12)(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]-WB- 4101 as radioligand for alpha-1 adrenergic receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50212323
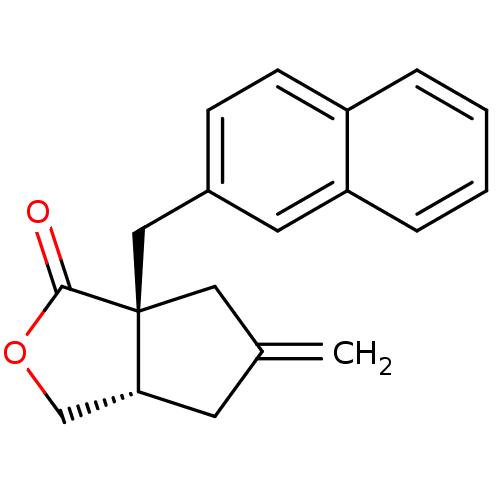
((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...)Show SMILES C=C1C[C@@H]2COC(=O)[C@]2(Cc2ccc3ccccc3c2)C1 Show InChI InChI=1S/C19H18O2/c1-13-8-17-12-21-18(20)19(17,10-13)11-14-6-7-15-4-2-3-5-16(15)9-14/h2-7,9,17H,1,8,10-12H2/t17-,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030630
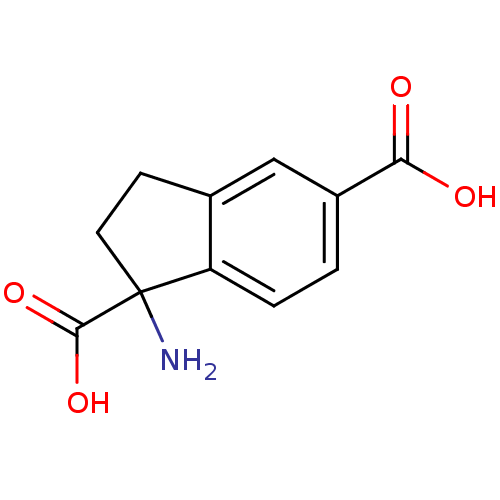
((RS)-1-aminoindan-1,5-dicarboxylic acid | 1-Amino-...)Show InChI InChI=1S/C11H11NO4/c12-11(10(15)16)4-3-6-5-7(9(13)14)1-2-8(6)11/h1-2,5H,3-4,12H2,(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 98.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]-haloperidol as radioligand for Dopamine receptor D2 |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030627
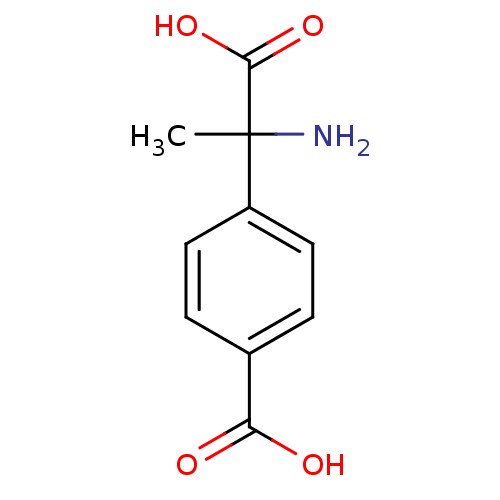
((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...)Show InChI InChI=1S/C10H11NO4/c1-10(11,9(14)15)7-4-2-6(3-5-7)8(12)13/h2-5H,11H2,1H3,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(RAT) | BDBM86213
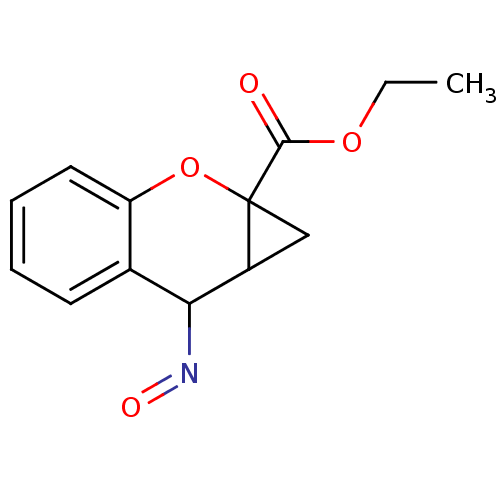
(CAS_5126051 | CHEMBL327783 | CPCCOEt | NSC_5126051)Show InChI InChI=1S/C13H13NO4/c1-2-17-12(15)13-7-9(13)11(14-16)8-5-3-4-6-10(8)18-13/h3-6,9,11H,2,7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50212323

((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...)Show SMILES C=C1C[C@@H]2COC(=O)[C@]2(Cc2ccc3ccccc3c2)C1 Show InChI InChI=1S/C19H18O2/c1-13-8-17-12-21-18(20)19(17,10-13)11-14-6-7-15-4-2-3-5-16(15)9-14/h2-7,9,17H,1,8,10-12H2/t17-,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50231744

(CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)c1cnc2ccccc2n1 |TLB:2:3:6:10.8.9,THB:8:7:4:10.9.11,8:9:6.7.12:4,11:9:6:12.3.4,11:3:6:10.8.9| Show InChI InChI=1S/C19H21N3O/c23-18(17-11-20-15-3-1-2-4-16(15)21-17)22-19-8-12-5-13(9-19)7-14(6-12)10-19/h1-4,11-14H,5-10H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163606

(1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-ph...)Show InChI InChI=1S/C20H17NO2/c22-19(11-14-5-2-1-3-6-14)15-8-9-18-17(12-15)13-16-7-4-10-23-20(16)21-18/h1-3,5-6,8-9,12-13H,4,7,10-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]haloperidol as radioligand for Dopamine receptor D2 |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM86213

(CAS_5126051 | CHEMBL327783 | CPCCOEt | NSC_5126051)Show InChI InChI=1S/C13H13NO4/c1-2-17-12(15)13-7-9(13)11(14-16)8-5-3-4-6-10(8)18-13/h3-6,9,11H,2,7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM86211

(CAS_52809-07-1 | NSC_40539 | Quisqualate)Show InChI InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(RAT) | BDBM50089896

(4-(Amino-carboxy-methyl)-3-methyl-benzoic acid((+)...)Show InChI InChI=1S/C10H11NO4/c1-5-4-6(9(12)13)2-3-7(5)8(11)10(14)15/h2-4,8H,11H2,1H3,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50002371

(2-aminopentanedioic acidglutamic acid | CHEMBL2763...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030627

((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...)Show InChI InChI=1S/C10H11NO4/c1-10(11,9(14)15)7-4-2-6(3-5-7)8(12)13/h2-5H,11H2,1H3,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(RAT) | BDBM50079183
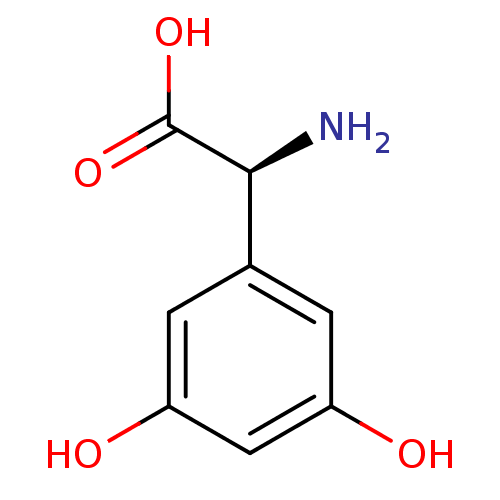
((2S)-amino(3,5-dihydroxyphenyl)ethanoic acid | (S)...)Show InChI InChI=1S/C8H9NO4/c9-7(8(12)13)4-1-5(10)3-6(11)2-4/h1-3,7,10-11H,9H2,(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030628

((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...)Show InChI InChI=1S/C9H9NO5/c10-7(9(14)15)4-1-2-5(8(12)13)6(11)3-4/h1-3,7,11H,10H2,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030629

((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...)Show InChI InChI=1S/C9H9NO4/c10-7(9(13)14)5-1-3-6(4-2-5)8(11)12/h1-4,7H,10H2,(H,11,12)(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM86212

(1S, 3R-ACPD | CAS_104766 | NSC_104766)Show InChI InChI=1S/C7H11NO4/c8-7(6(11)12)2-1-4(3-7)5(9)10/h4H,1-3,8H2,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50030630

((RS)-1-aminoindan-1,5-dicarboxylic acid | 1-Amino-...)Show InChI InChI=1S/C11H11NO4/c12-11(10(15)16)4-3-6-5-7(9(13)14)1-2-8(6)11/h1-2,5H,3-4,12H2,(H,13,14)(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding activity against [3H]ketanserin as radioligand for 5-hydroxytryptamine 2 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]pyrilamine as radioligand for Histamine H1 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]pyrilamine as radioligand for Histamine H1 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]haloperidol as radioligand for Dopamine receptor D2 |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]sufentanil opiate receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]clonidine as radioligand for alpha-2 adrenergic receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]dexetimide as radioligand for muscarinic-acetylcholine receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]dihydroalprenolol as radioligand for beta adrenergic receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Bombyx mori) | BDBM50488465
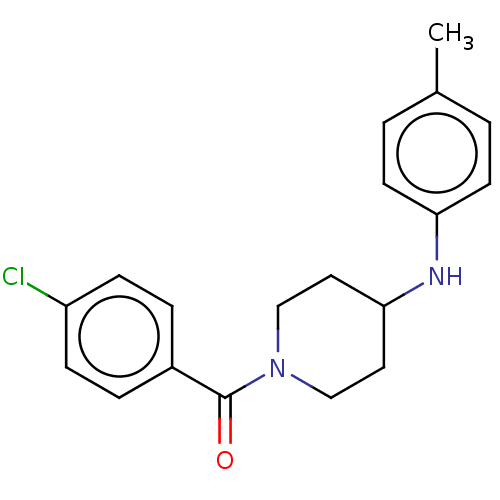
(CHEMBL2286413)Show InChI InChI=1S/C19H21ClN2O/c1-14-2-8-17(9-3-14)21-18-10-12-22(13-11-18)19(23)15-4-6-16(20)7-5-15/h2-9,18,21H,10-13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293171
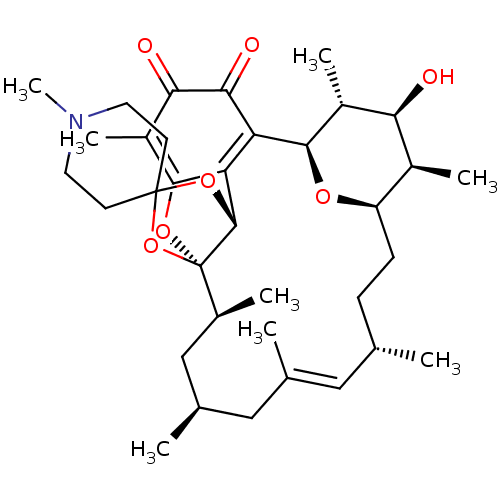
((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC1(CCN(C)CC1)O4 |r,c:7,18,33| Show InChI InChI=1S/C35H51NO7/c1-18-9-10-25-22(5)28(37)23(6)31(40-25)26-27-32(24(7)29(38)30(26)39)41-35(21(4)17-20(3)16-19(2)15-18)33(27)42-34(43-35)11-13-36(8)14-12-34/h15,18,20-23,25,28,31,33,37H,9-14,16-17H2,1-8H3/b19-15+/t18-,20+,21-,22-,23+,25+,28-,31+,33+,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50025124

(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]-5-HT as radioligand for serotonin S1 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Bombyx mori) | BDBM50488464
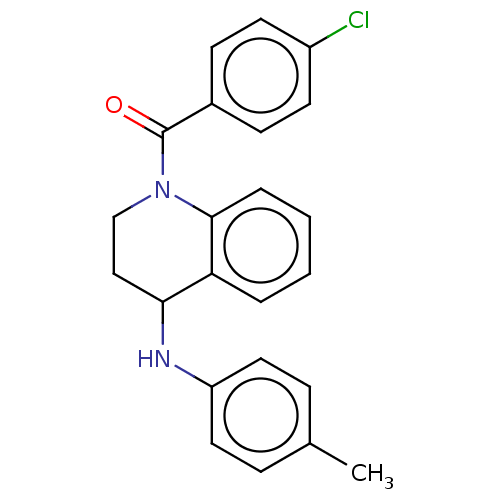
(CHEMBL2286419)Show SMILES Cc1ccc(NC2CCN(C(=O)c3ccc(Cl)cc3)c3ccccc23)cc1 Show InChI InChI=1S/C23H21ClN2O/c1-16-6-12-19(13-7-16)25-21-14-15-26(22-5-3-2-4-20(21)22)23(27)17-8-10-18(24)11-9-17/h2-13,21,25H,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293172
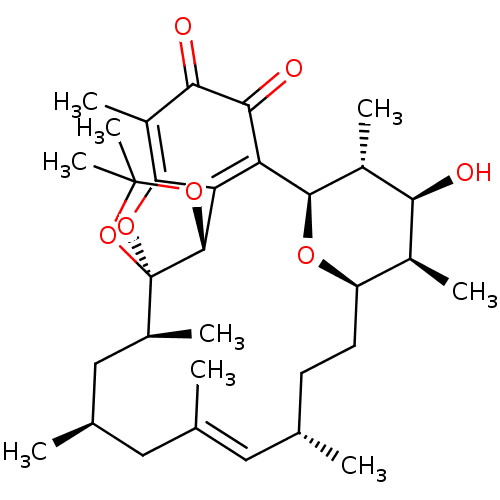
((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC(C)(C)O4 |r,c:7,18,33| Show InChI InChI=1S/C32H46O7/c1-15-10-11-22-19(5)25(33)20(6)28(36-22)23-24-29(21(7)26(34)27(23)35)37-32(30(24)38-31(8,9)39-32)18(4)14-17(3)13-16(2)12-15/h12,15,17-20,22,25,28,30,33H,10-11,13-14H2,1-9H3/b16-12+/t15-,17+,18-,19-,20+,22+,25-,28+,30+,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293169
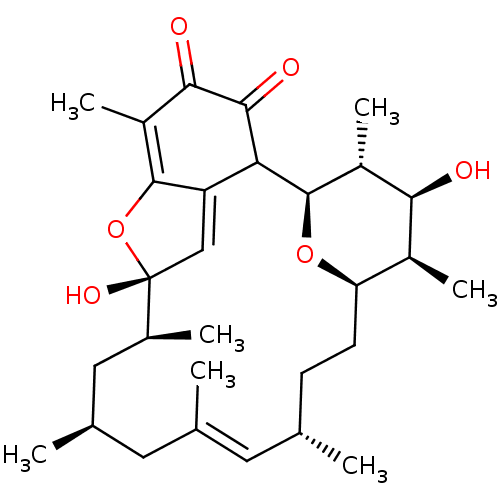
(CHEMBL523927 | kendomycin)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]3(O)OC4=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)C4=C3 |r,c:7,19,36| Show InChI InChI=1S/C29H42O6/c1-14-8-9-22-18(5)24(30)19(6)28(34-22)23-21-13-29(33,17(4)12-16(3)11-15(2)10-14)35-27(21)20(7)25(31)26(23)32/h10,13-14,16-19,22-24,28,30,33H,8-9,11-12H2,1-7H3/b15-10+/t14-,16+,17-,18-,19+,22+,23?,24-,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Spodoptera littoralis) | BDBM50488463
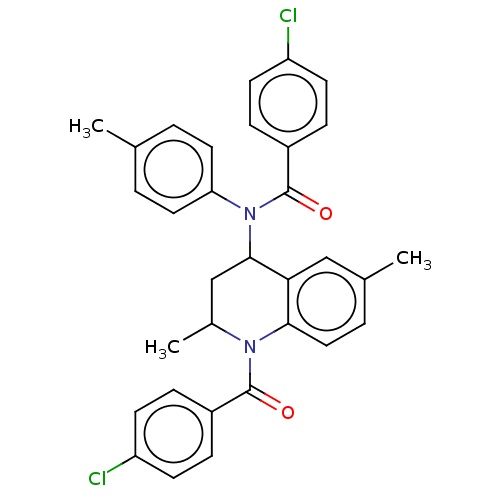
(CHEMBL2285681)Show SMILES CC1CC(N(C(=O)c2ccc(Cl)cc2)c2ccc(C)cc2)c2cc(C)ccc2N1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C32H28Cl2N2O2/c1-20-4-15-27(16-5-20)36(32(38)24-9-13-26(34)14-10-24)30-19-22(3)35(29-17-6-21(2)18-28(29)30)31(37)23-7-11-25(33)12-8-23/h4-18,22,30H,19H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Bombyx mori) | BDBM50488463

(CHEMBL2285681)Show SMILES CC1CC(N(C(=O)c2ccc(Cl)cc2)c2ccc(C)cc2)c2cc(C)ccc2N1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C32H28Cl2N2O2/c1-20-4-15-27(16-5-20)36(32(38)24-9-13-26(34)14-10-24)30-19-22(3)35(29-17-6-21(2)18-28(29)30)31(37)23-7-11-25(33)12-8-23/h4-18,22,30H,19H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Bombyx mori) | BDBM50488437

(CHEMBL2286416)Show SMILES CC1CC(Nc2ccc(C)cc2)c2cc(C)ccc2N1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H25ClN2O/c1-16-4-11-21(12-5-16)27-23-15-18(3)28(24-13-6-17(2)14-22(23)24)25(29)19-7-9-20(26)10-8-19/h4-14,18,23,27H,15H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Spodoptera littoralis) | BDBM50488465

(CHEMBL2286413)Show InChI InChI=1S/C19H21ClN2O/c1-14-2-8-17(9-3-14)21-18-10-12-22(13-11-18)19(23)15-4-6-16(20)7-5-15/h2-9,18,21H,10-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Spodoptera littoralis) | BDBM50488447
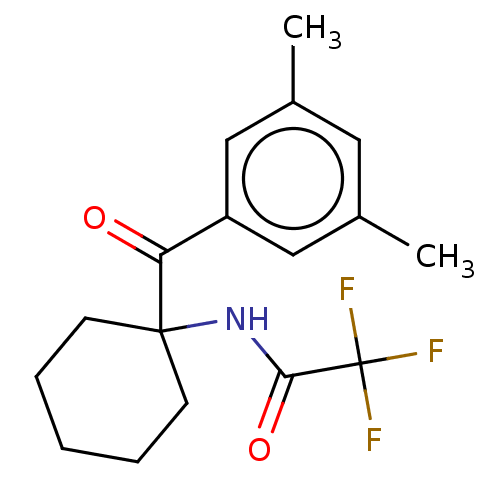
(CHEMBL2286412)Show SMILES Cc1cc(C)cc(c1)C(=O)C1(CCCCC1)NC(=O)C(F)(F)F Show InChI InChI=1S/C17H20F3NO2/c1-11-8-12(2)10-13(9-11)14(22)16(6-4-3-5-7-16)21-15(23)17(18,19)20/h8-10H,3-7H2,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |
Ecdysone receptor
(Spodoptera littoralis) | BDBM50488448
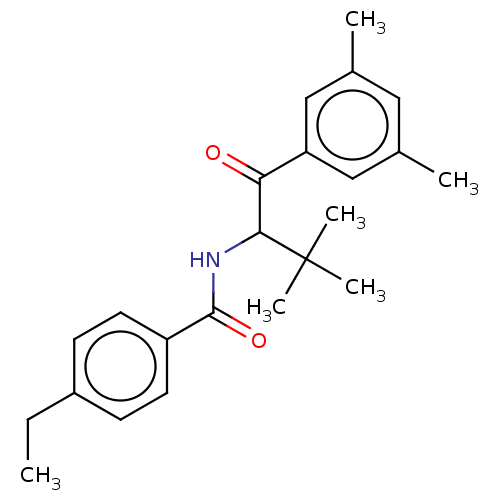
(CHEMBL2286411)Show SMILES CCc1ccc(cc1)C(=O)NC(C(=O)c1cc(C)cc(C)c1)C(C)(C)C Show InChI InChI=1S/C23H29NO2/c1-7-17-8-10-18(11-9-17)22(26)24-21(23(4,5)6)20(25)19-13-15(2)12-16(3)14-19/h8-14,21H,7H2,1-6H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... |
Pest Manag Sci 66: 526-35 (2010)
Article DOI: 10.1002/ps.1903
BindingDB Entry DOI: 10.7270/Q2KK9FPH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data